Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor in children and adolescents (1). OS arises predominantly in the long bone
metaphysis, particularly in the distal femur, proximal tibia and
proximal humerus (2). Previously,
the majority of patients with OS were treated by amputation;
however, the 5-year survival rate was <20%, primarily due to
lung metastases (3). With the
introduction of chemotherapy into multi-modal treatment for OS, its
prognosis has improved (2). In the
past 20 years, although numerous international research groups have
conducted a large quantity of research on OS, its survival rate
remains unchanged (4–6).
The concept of immunogenic cell death (ICD) of tumor
cells has emerged in recent years, which is a cell death modality
that is able to stimulate an immune response against homologous
tumor cells (7,8). This concept was first proposed in the
context of anticancer chemotherapy, and was based on animal
experiments that indicated that tumor specific immune responses
could determine the efficacy of anticancer therapies (9). ICD is characterized by the early
surface exposure of calreticulin (CRT) (10).
To date, only a small number of conventional
cytotoxic anticancer therapeutics are able to induce ICD (11); therefore it is of clinical
significance to identify novel chemicals that can induce ICD.
Capsaicin, a homovanillic acid derivative and the spicy component
of chili pepper, has been shown to have cytotoxicity towards cancer
cells and immunomodulatory functions, suggesting its potential
application in tumor therapy (12).
It has been reported that intratumoral administration of capsaicin
elicits a T cell-mediated antitumor immune response, resulting in
the regression of advanced preexisting solid tumors (12). In addition, research by D'Eliseo
et al (13) indicates that
capsaicin can stimulate anticancer immunity.
In the present study, the effects of capsaicin were
evaluated for its abilities in inducing CRT membrane translocation
and mediating ICD in a human MG-63 OS cell line. As it has been
reported that cisplatin could not induce ICD in tumor cells, the
present study used cisplatin as a control. The present results
indicated that capsaicin induced a rapid membrane translocation of
CRT. Furthermore, apoptotic MG-63 cells induced by capsaicin could
be engulfed more efficiently by phagocytes and these phagocytes
loaded with apoptotic MG-63 cells had the stronger ability in
activating tumor-specific T-cells which could secrete IFN-γ. These
data demonstrate that capsaicin can induce ICD in human OS
cells.
Materials and methods
Cell line
The human OS cell line MG-63 was purchased from the
Cell Bank of China (Wuhan, China). The cells were maintained at
37°C in 5% CO2 and Dulbecco's modified Eagle medium
(DMEM), which contains 10% heat-inactivated fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
µg/ml streptomycin and 100 units/ml penicillin.
Materials
Capsaicin, DMEM, 3,3′-dihexyloxacarbocyanine iodide
(DiOC6)(3) and MTT were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rabbit-anti-human CRT polyclonal antibody was purchased from
Stressgen (Victoria, BC, Canada; cat. no. SPC-122B). Mouse
anti-human phycoerythrin (PE)-conjugated CD11c monoclonal antibody
was purchased from eBioscience (San Diego, CA, USA; cat. no.
12-0116-42). Rabbit anti-B-cell lymphoma 2 (Bcl-2) and rabbit
anti-Bcl-2-associated X protein (Bax) monoclonal antibodies, and
horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA;
cat. nos. sc-492, sc-6236 and sc-516087, respectively).
PE-conjugated goat anti-rabbit IgG polyclonal antibody was obtained
from R&D Systems, Inc. (Minneapolis, MN, USA; cat. no. IC108P).
Recombinant human interleukin (IL)-2, IL-4 and
granulocyte-macrophage colony-stimulating factor (GM-CSF) were
purchased from PeproTech (Rocky Hill, NJ, USA). Lymphocyte
separation medium was purchased from Tianjin Haoyang Biological
Manufacture, Co., Ltd. (Tianjin, China). IL-4 and IFN-γ
enzyme-linked immunosorbent assay (ELISA) kits were purchased from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China).
MTT assay
MG-63 cells were seeded onto 96-well tissue culture
plates at a density of 2×103 cells/100 µl per well, and
incubated for 24 h at 37°C. The next day, the media were replaced
with 100 µl fresh complete medium containing capsaicin (0, 12.5,
25, 50, 100, 200 and 400 µM) and cisplatin (0, 4, 8, 16, 32, 64 and
128 µg/ml). The cells treated with equal quantities of normal
medium or solvent, instead of drugs, served as the control. After
24 h incubation, 100 µl MTT solution (0.5 mg/ml) in DMEM without
fetal bovine serum was added to each well and cultured for 4 h at
37°C in a humidified atmosphere. The medium was removed and 150 µl
DMSO was added into each well to dissolve the purple crystals, then
the absorbance (A) at 570 nm was recorded. The cell proliferation
inhibition rate was calculated according to the following formula:
Cell proliferation inhibition rate = (Acontrol -
Adrug)/Acontrol × 100%.
Mitochondrial membrane potential
(MMP)
The lipophilic fluorescence dye DiOC6
(3) (40 nM) was used to assay the
mitochondrial membrane permeabilization. MG-63 cells
(2×106) treated with 200 µM capsaicin or 32 µg/ml
cisplatin were stained with DiOC6 (3) for 15 min at 37°C and analyzed
immediately by flow cytometry (FCM) equipped with a standard 15 mW
argon-ion laser (488 nm) to excite DiOC6 (3). Then, a narrow band filter was used to
collect emissions between 515 and 545 nm. A minimum of 10,000 cells
were analyzed by FCM for each data point.
Western blot analysis
MG-63 cells (2×106) were cultured in DMEM
medium containing 200 µM capsaicin or 32 µg/ml cisplatin for 12 or
24 h and harvested. Cells were then lysed with cell lysis buffer
(Tris 50 mM, NaCl 150 mM, SDS 0.1%, Sodium Deoxycholate 0.5% and 1%
Triton X-100) for 20 min on ice. The protein concentrations were
determined using the Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.), after which the proteins in the lysate (45 µg)
were separated by 10% SDS-PAGE and transferred onto a PVDF
membranes. The membranes were blocked with fat-free milk solution
(5%, w/v) for 12 h and then incubated with rabbit anti-Bcl-2 and
anti-Bax monoclonal antibodies (1:1,000) or anti-CRT polyclonal
antibody (1:1,000) at 4°C for 12 h. After washing 3 times with
TBST, the membrane was incubated with an HRP-conjugated anti-rabbit
IgG antibody (1:4,000) at room temperature for 1 h and developed
using electrochemiluminescence.
Detection of CRT on the cell surface
by FCM
MG-63 cells were cultured in DMEM medium containing
200 µM capsaicin or 32 µg/ml cisplatin for 12 h and harvested.
After washing once with phosphate-buffered saline (PBS), the cells
were incubated with rabbit anti-CRT polyclonal antibody (1:1,000)
at room temperature for 1 h, following by washing and incubation
with PE-conjugated goat anti-rabbit IgG polyclonal antibody (1:500)
at room temperature for 2 h (avoiding light). After washing with
PBS, the cells were analyzed by FCM to identify CRT on the cell
surface.
In vitro phagocytosis assays
Peripheral blood mononuclear cells (PBMCs) were
isolated from human blood using lymphocyte separation medium
(density, 1.077 g/ml) according to the manufacturer's instructions.
Following the last wash, PBMCs (5×106) were resuspended
in RPMI-1640 and plated in 6-well culture plates. After 3 h
incubation, the monocytes were purified by removing the
non-adherent cells in the supernatant. Purified monocytes were
cultured in a medium supplemented with 30 ng/ml recombinant human
GM-CSF and 10 ng/ml recombinant human IL-4 in 6-well plates
(1×106 cells/well). After 7 days, the non-adherent and
loosely adherent cells were harvested as the dendritic cells (DCs)
and were used as the effector cells for the phagocytosis assay. The
MG-63 cells (1×106) incubated with 200 µM capsaicin or
32 µg/ml cisplatin for 12 h were labeled with the green dye
carboxyfluorescein diacetate succinimidyl ester (1 µM) for 10 min
and were used as the target cells. The effector and target cells
were co-cultured at 37°C for 2 h at a 1:1 effector/target ratio.
After 3 washes, anti-human CD11c PE was added to the cell mixture
for 30 min at room temperature (in the dark) to label the effector
cells. The cells were washed with PBS and analyzed by FCM. The
phagocytotic efficiency was represented by the cell ratio of the
double-positive cell number over the total cell number.
IFN-γ and IL-4 expression level
analysis
PBMCs were isolated as described above. Following
the last wash, PBMCs (5×106) were resuspended in
RPMI-1640 and plated in 6-well culture plates. After 3 h
incubation, the non-adherent cells were harvested as lymphocytes,
and DCs were induced as described above. The DCs loaded with
drug-treated MG-63 cells were co-cultured with lymphocytes at a
ratio of 1:4. A volume of 200 U/ml IL-2 was added into the cell
mixture during the culture period. After 24 h, the supernatant was
collected, and the IFN-γ and IL-4 expression levels in the
supernatant were measured by ELISA, according to the manufacturer's
instructions.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Student's t-tests were performed
for the comparison of results between different groups. All of the
tests were performed using SPSS software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Growth inhibition of MG-63 cells by
capsaicin or cisplatin
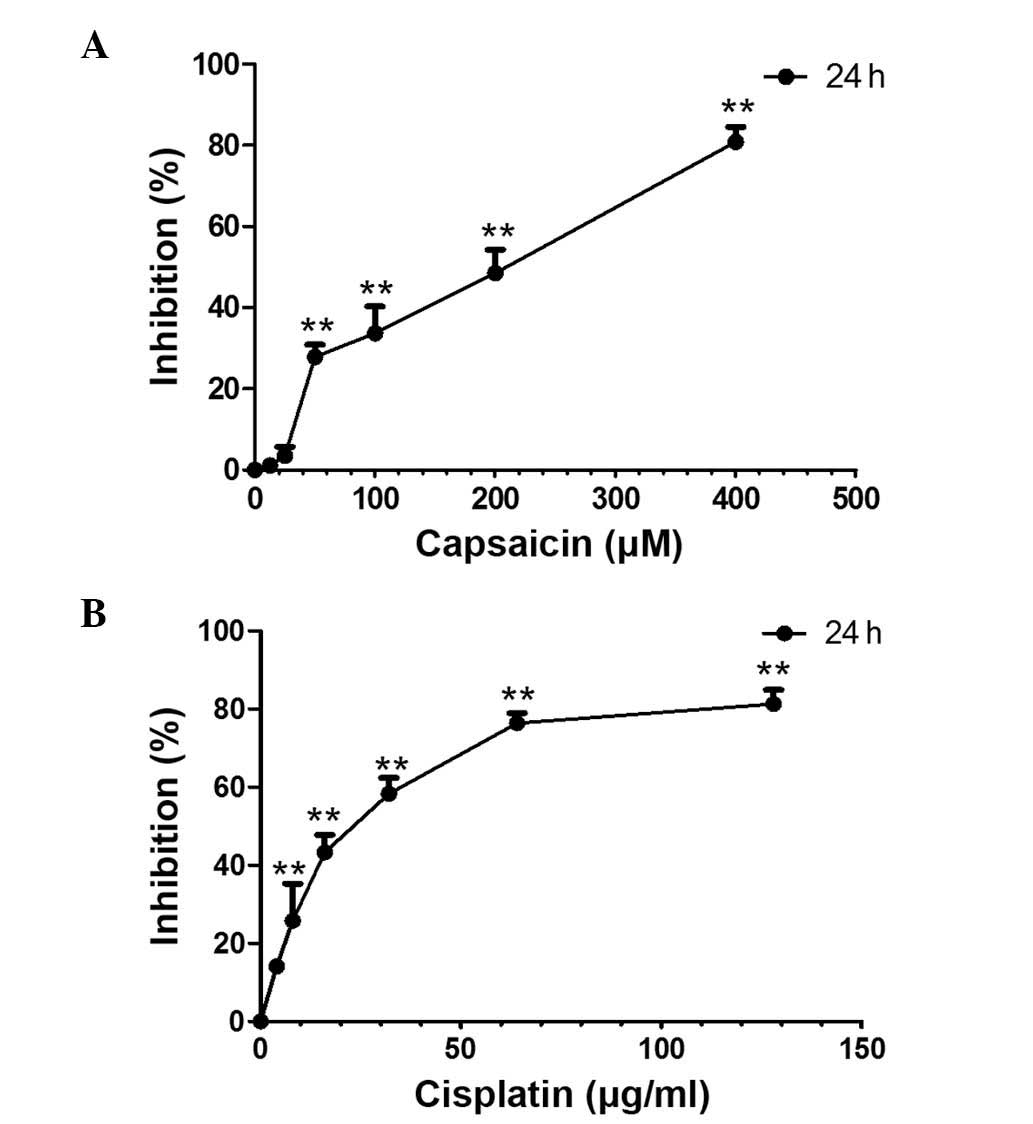
An MTT assay was used to examine the growth
inhibiting effects of capsaicin and cisplatin on MG-63 cells. The
result showed that capsaicin and cisplatin significantly inhibit
the growth of MG-63 cells in a dose-dependent manner (Fig. 1). After 24 h treatment, the
inhibition percentage was 48.5% for 200 µM capsaicin and 58.3% for
32 µg/ml cisplatin. Since these drug concentrations were close to
median lethal doses (capsaicin, 165.7 µM; cisplatin, 16.76 µg/ml),
these concentrations were used to treat cells in the following
experiments.
Alteration of MMP induced by capsaicin
and cisplatin
Decreased MMP is a feature of apoptotic cells. In
order to determine whether capsaicin and cisplatin can induce
apoptosis of MG-63 cells, the effect of the drugs on the MMP was
evaluated by FCM. The cells treated by 200 µM capsaicin or 32 µg/ml
cisplatin were stained with DiOC6 (3), and the MMP was detected by FCM. The
results showed that treatment with both drugs could decrease the
ratio of cells with green fluorescence (Fig. 2). This result suggests that capsaicin
and cisplatin can induce the alteration of MMP in MG-63 cells, and
then initiate endogenous apoptosis.
Effects of capsaicin and cisplatin on
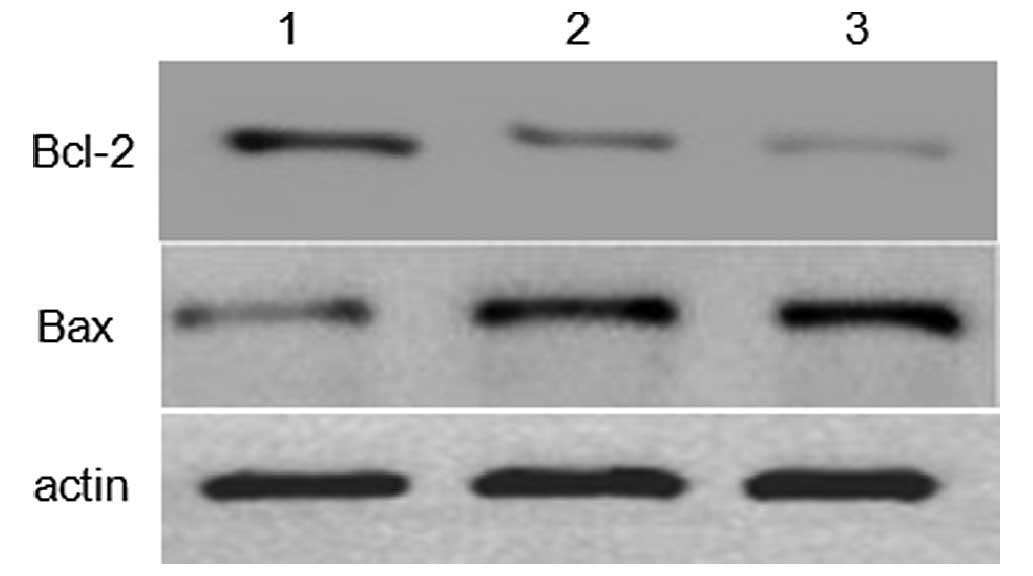
the expression of apoptosis-related proteins
To investigate the molecular mechanism underlying
capsaicin- and cisplatin-induced apoptosis in human MG-63 OS cells,
the expression levels of apoptosis-related proteins Bax and Bcl-2
were investigated using western blot analysis. As shown in Fig. 3, the expression level of Bax was
markedly upregulated, and anti-apoptotic Bcl-2 was markedly
downregulated, when the cells were treated with 200 µM capsaicin or
32 µg/ml cisplatin for 24 h. This suggests that Bcl-2 and Bax
proteins are involved in capsaicin- and cisplatin-induced
apoptosis.
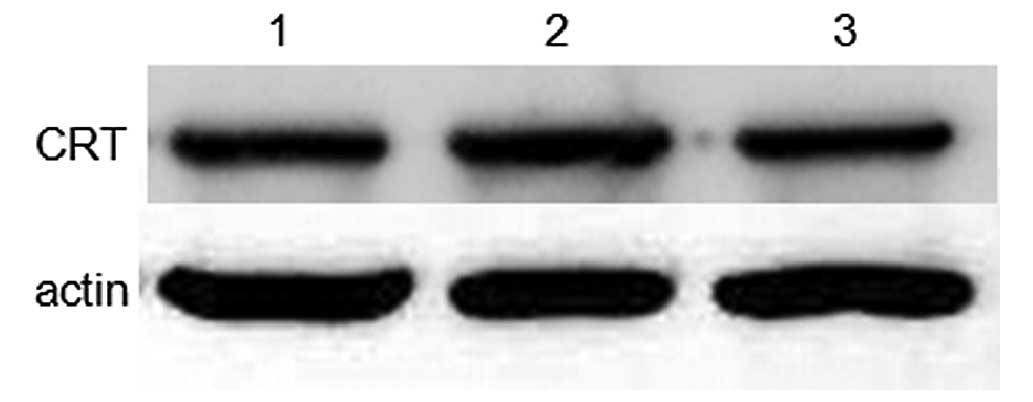
Effect of capsaicin and cisplatin on
the total expression levels of CRT in MG-63 cells
To validate whether capsaicin and cisplatin could
affect CRT expression in MG-63 cells, a western blot assay was used
to analyze CRT protein expression in whole cell lysates. The result
showed that treating cells with capsaicin or cisplatin for 12 h did
not change the total cellular CRT expression level (Fig. 4).
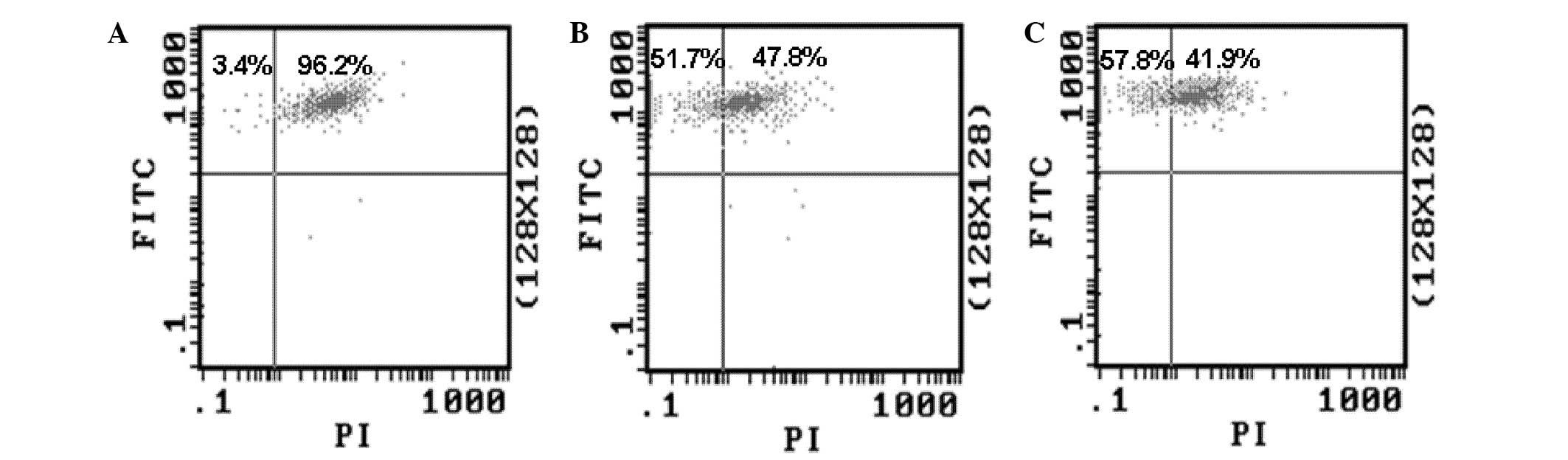
Capsaicin and cisplatin promoted the
translocation of CRT onto the surface of MG-63 cells
To validate whether capsaicin and cisplatin could
affect CRT subcellular localization, MG-63 cells were treated with
200 µM capsaicin or 32 µg/ml cisplatin, and FCM was used to assay
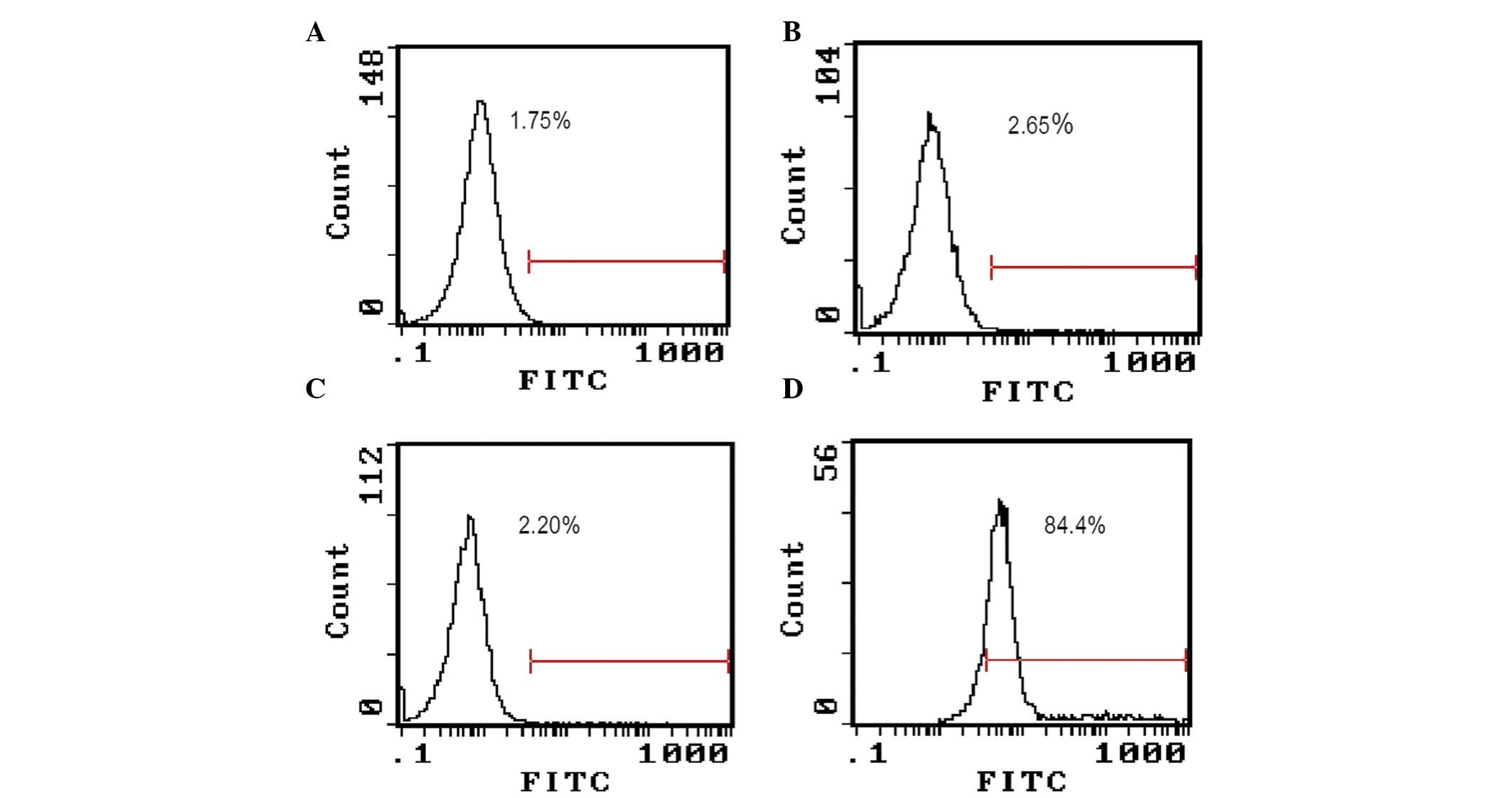
CRT expression on the cell surface. The result (Fig. 5) showed that capsaicin treatment for
12 h significantly increased the expression of CRT on the cell
surface, but no such effect was observed when the cells were
treated with cisplatin. In this experiment, MG-63 cells have been
dyed with fluorescence- labeled secondary antibody alone as a
control, to exclude non-specific fluorescence binding to cells
(Fig. 5).
Capsaicin treatment enhanced the
phagocytosis of MG-63 cells by DCs in vitro
In view of the established role of CRT as an ‘eat
me’ signal, and the results from the experiments described above,
the difference of phagocytotic rates among the two drug-treated
MG-63 cells by DCs was investigated. The DCs (effector cells) and
drug-treated MG-63 cells (target cells) were co-cultured for 2 h in
a 1:1 effector/target ratio, and the phagocytotic rate was
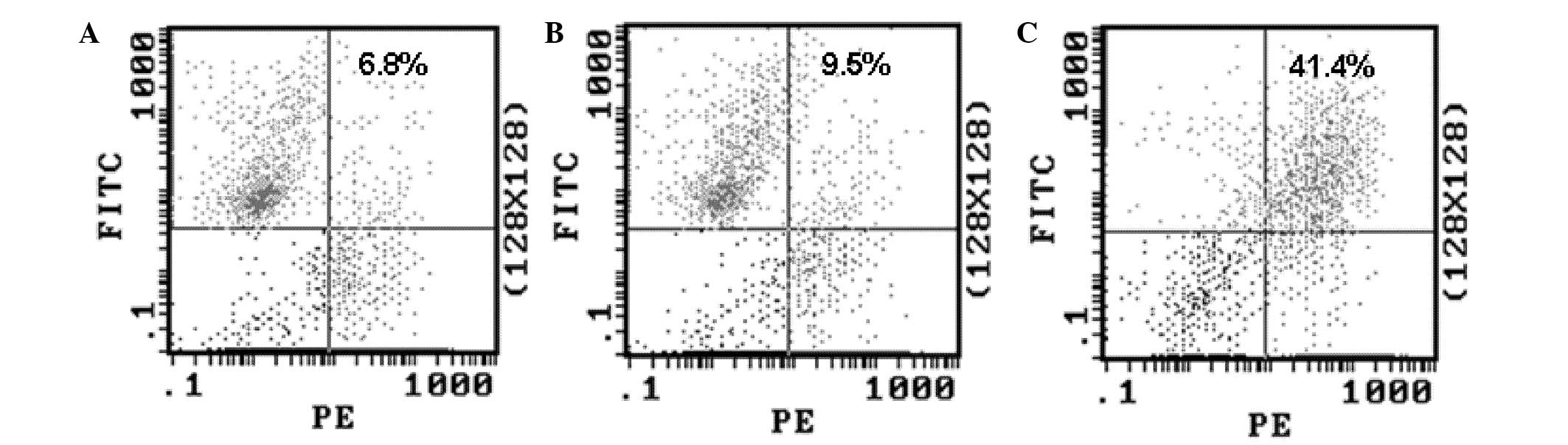
determined by FCM. The results (Fig.
6) showed that MG-63 cells treated with capsaicin were
phagocytosed at a higher rate than the MG-63 cells treated with
cisplatin, indicating that CRT on the cell surface enhanced the
phagocytosis of the human OS cells by DCs.
Activation of lymphocytes by DCs
loaded with capsaicin-treated MG-63 cells in vitro
To investigate whether MG-63 cells with high CRT
expression levels on the cell surface can induce antitumor
immunity, the ability of DCs loaded with MG-63 cells to activate
tumor cell-specific T-cell responses was investigated. The
drug-treated MG-63 cells (target cells) were co-cultured with DCs
(effector cells), then MG-63-loaded DCs were further co-cultured
with lymphocytes for 24 h. The IFN-γ and IL-4 expression levels in
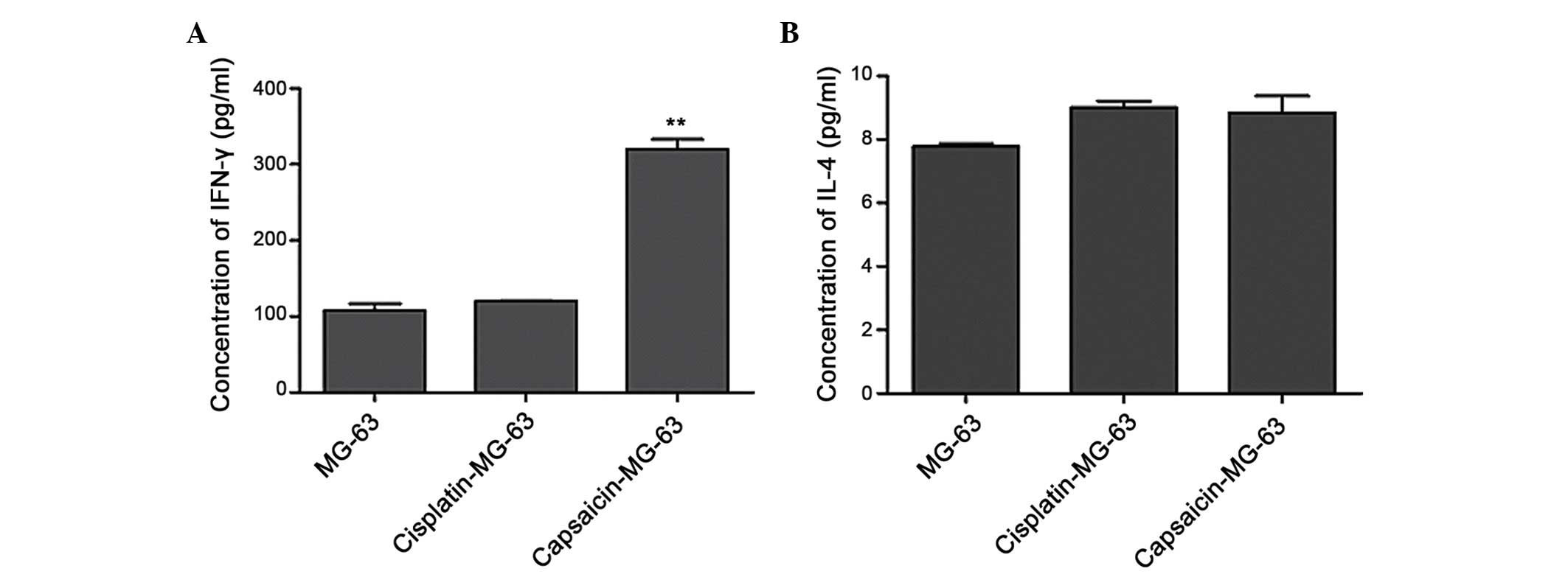
the supernatant were then analyzed by ELISA. The result (Fig. 7) showed that, compared with MG-63
cells treated with cisplatin, capsaicin-treated MG-63 cells
expressed a higher level of IFN-γ (P<0.01) in the supernatant,
but no significant change was observed in the IL-4 expression
level.
Discussion
The role of the immune system in anticancer therapy
has been long neglected, as chemo- and radiotherapy-induced cell
death frequently occurs through apoptosis, a cell death modality
that was widely considered as immunologically silent, if not
tolerogenic (14–16). In addition, cytotoxic
chemotherapeutics are typically considered to be immunosuppressive
(17,18). Moreover, chemotherapy- induced nausea
is often treated with high dose corticoids, which also induce an
unwarranted immunosuppressive side effects (19).
In recent years, increasing evidence has
demonstrated that a number of chemotherapeutics can induce ICD
(20). Exploring effective ways and
routes that can induce ICD have already become the hot spot of
tumor prevention and therapy (21).
In the process of tumor cell apoptosis induced by
various physical and chemical factors, CRT within endoplasmic
reticulum (ER) is rapidly translocated to the cell surface
(22). In homeostatic conditions,
CRT is localized to the ER, where it functions as a molecular
chaperone and modulates calcium homeostasis (23). However, small quantities of CRT can
be detected in other intracellular compartments, including the
cytosol and nucleus (24,25).
CRT on the cell surface has numerous functions,
including the modulation of cell adhesion and migration (26), and mediating the phagocytosis of
apoptotic tumor cells by professional and non-professional
phagocytes (11,27–29).
During the process of tumor cell apoptosis induced by special
stimulus, CRT is rapidly translocated from the ER to the cell
surface and serves as an ‘eat me’ signal that can be recognized by
phagocytes within hours after the initiation of ICD (11,28,29).
Subsequently, the tumor cells undergoing apoptosis downregulate the
expression of ‘do not eat me’ signals, such as surface CD47, to
facilitate the recognition and engulfment of the tumor cells by
phagocytes, then to induce the anticancer immune response (30).
Only a small quantity of conventional cytotoxic
anticancer therapeutics can induce ICD (11,29);
therefore it is clinically significant to explore novel chemicals
that can induce ICD. In the current study capsaicin and cisplatin
were tested for their ability to induce ICD. Human MG-63 OS cells
were treated with capsaicin or cisplatin, and flow cytometry assay
analysis was used to determine the changes in CRT expression levels
on the cell surface. The result showed that capsaicin and cisplatin
can induce apoptosis of MG-63 cells; however, only capsaicin
treatment increased the expression level of CRT on the cell
surface. Since the total CRT expression levels did not
significantly change in MG-63 cells treated with capsaicin and
cisplatin compared with the control, CRT appearing on the surface
of capsaicin-treated MG-63 cells may be derived from the membrane
translocation of CRT from the ER.
DCs serve important roles in processing and
presenting of antigens, thus the recognition and phagocytosis of
tumour cells by DCs is crucial in ICD (29). In order to evaluate the effect of
capsaicin and cisplatin on the phagocytosis of MG-63 cells by DCs,
the labeled MG-63 cells treated with capsaicin and cisplatin (the
target cells) were co-cultured with the DCs (the effector cells),
and FCM analysis was used to determine the phagocytic rate. The
results showed that capsaicin-treated MG-63 cells were phagocytosed
at a higher rate than MG-63 cells treated with cisplatin,
indicating that CRT on the cell surface can enhance the
phagocytosis of human OC cells by DCs.
Based on the above results, it can be suggested that
capsaicin-treated MG-63 cells can activate lymphocytes through DCs
using their phagocytosis and antigen presenting properties. To
confirm this conjecture, in the current study MG-63 cells treated
with capsaicin were co-cultured with DCs, and these MG-63-loaded
DCs were co-cultured with lymphocytes for 24 h. The IFN-γ
concentration in the supernatant was then analyzed by ELISA. The
result showed that, compared with cisplatin-treated MG-63 cells,
the capsaicin-treated MG-63 cells induced a higher production and
release of IFN-γ from lymphocytes into the culture medium.
In conclusion, the results from the present study
demonstrate that capsaicin can induce the translocation of a large
quantity of CRT from intracellular compartments to the cell surface
in human OS cells. In addition, CRT on the human OS cell surface
can be used as specific signaling molecules to promote the
phagocytosis of tumor cells, thereby mediating tumor cell
immunogenic death. This may be one of the underlying molecular
mechanisms of capsaicin's anti-tumor cytotoxicity. These results
indicate that capsaicin can be explored as a novel drug to treat
OS.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402557).
References
|
1
|
Messerschmitt PJ, Garcia RM, Abdul-Karim
FW, Greenfield EM and Getty PJ: Osteosarcoma. J Am Acad Orthop
Surg. 17:515–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson ME: Update on Survival in
Osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chou AJ, Geller DS and Gorlick R: Therapy
for osteosarcoma: Where do we go from here? Paediatr Drugs.
10:315–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siclari VA and Qin L: Targeting the
osteosarcoma cancer stem cell. J Orthop Surg Res. 5:782010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuijjer ML, Hogendoorn PC and
Cleton-Jansen AM: Genome-wide analyses on high-grade osteosarcoma:
Making sense of a genomically most unstable tumor. Int J Cancer.
133:2512–2521. 2013.PubMed/NCBI
|
|
6
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kroemer G, Galluzzi L, Kepp O and Zitvogel
L: Immunogenic cell death in cancer therapy. Annu Rev Immunol.
31:51–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krysko DV, Garg AD, Kaczmarek A, Krysko O,
Agostinis P and Vandenabeele P: Immunogenic cell death and DAMPs in
cancer therapy. Nat Rev Cancer. 12:860–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Casares N, Pequignot MO, Tesniere A,
Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs
S, Obeid M, et al: Caspase-dependent immunogenicity of
doxorubicin-induced tumor cell death. J Exp Med. 202:1691–1701.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pol J, Vacchelli E, Aranda F, Castoldi F,
Eggermont A, Cremer I, Sautès-Fridman C, Fucikova J, Galon J,
Spisek R, et al: Trial Watch: Immunogenic cell death inducers for
anticancer chemotherapy. Oncoimmunology. 4:e10088662015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beltran J, Ghosh AK and Basu S:
Immunotherapy of tumors with neuroimmune ligand capsaicin. J
Immunol. 178:3260–3264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
D'Eliseo D, Manzi L and Velotti F:
Capsaicin as an inducer of damage-associated molecular patterns
(DAMPs) of immunogenic cell death (ICD) in human bladder cancer
cells. Cell Stress Chaperones. 18:801–808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savill J and Fadok V: Corpse clearance
defines the meaning of cell death. Nature. 407:784–788. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matzinger P: The danger model: A renewed
sense of self. Science. 296:301–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri S, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Nomenclature Committee on Cell Death
2009: Classification of cell death: Recommendations of the
Nomenclature Committee on Cell Death 2009. Cell Death Differ.
16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou W: Regulatory T cells, tumour immunity
and immunotherapy. Nat Rev Immunol. 6:295–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zitvogel L, Apetoh L, Ghiringhelli F,
André F, Tesniere A and Kroemer G: The anticancer immune response:
Indispensable for therapeutic success? J Clin Invest.
118:1991–2001. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han HS, Shim YK, Kim JE, Jeon HJ, Lim SN,
Oh TK, Lee KH and Kim ST: A pilot study of adrenal suppression
after dexamethasone therapy as an antiemetic in cancer patients.
Support Care Cancer. 20:1565–1572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garg AD and Agostinis P: Editorial:
Immunogenic Cell Death in Cancer: From Benchside Research to
Bedside Reality. Front Immunol. 7:1102016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song KH, Jung SY, Kang SM, Kim MH, Ahn J,
Hwang SG, Lee JH, Lim DS, Nam SY and Song JY: Induction of
immunogenic cell death by radiation-upregulated karyopherin alpha 2
in vitro. Eur J Cell Biol. Apr 11–2016.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fučíková J, Bartůňková J and Špíšek R: The
Concept of Immunogenic Cell Death in Antitumor Immunity. Klin
Onkol. 4:4S48–4S55. 2015.(In Czech). View Article : Google Scholar
|
|
23
|
Krause KH and Michalak M: Calreticulin.
Cell. 88:439–443. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson S, Michalak M, Opas M and Eggleton
P: The ins and outs of calreticulin: From the ER lumen to the
extracellular space. Trends Cell Biol. 11:122–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bedard K, Szabo E, Michalak M and Opas M:
Cellular functions of endoplasmic reticulum chaperones
calreticulin, calnexin, and ERp57. Int Rev Cytol. 245:91–121. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gold LI, Eggleton P, Sweetwyne MT, Van
Duyn LB, Greives MR, Naylor SM, Michalak M and Murphy-Ullrich JE:
Calreticulin: Non-endoplasmic reticulum functions in physiology and
disease. FASEB J. 24:665–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu H, Han Y, Qin Y, Cao C, Xia Y, Liu C
and Wang Y: Whole-cell vaccine coated with recombinant calreticulin
enhances activation of dendritic cells and inducestumour-specific
immune responses. Oncol Rep. 2:529–534. 2013.
|
|
28
|
Tesniere A, Panaretakis T, Kepp O, Apetoh
L, Ghiringhelli F, Zitvogel L and Kroemer G: Molecular
characteristics of immunogenic cancer cell death. Cell Death
Differ. 1:3–12. 2008. View Article : Google Scholar
|
|
29
|
Son KJ, Choi KR, Lee SJ and Lee H:
Immunogenic Cell Death Induced by Ginsenoside Rg3: Significance in
Dendritic Cell-based Anti-tumor Immunotherapy. Immune Netw.
1:75–84. 2016. View Article : Google Scholar
|
|
30
|
Chao MP, Jaiswal S, Weissman-Tsukamoto R,
Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB,
Raveh T, Park CY, Majeti R and Weissman IL: Calreticulin is the
dominant pro-phagocytic signal on multiple human cancers and is
counterbalanced by CD47. Sci Transl Med. 2:63ra942010. View Article : Google Scholar : PubMed/NCBI
|