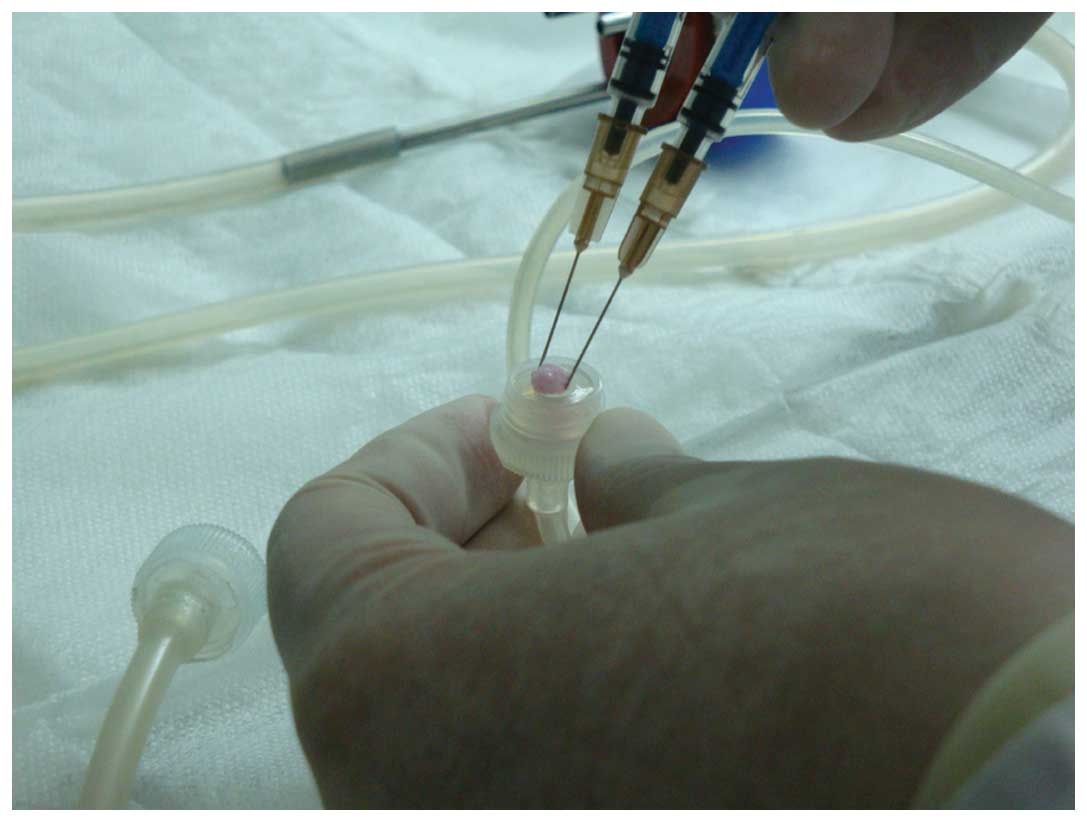
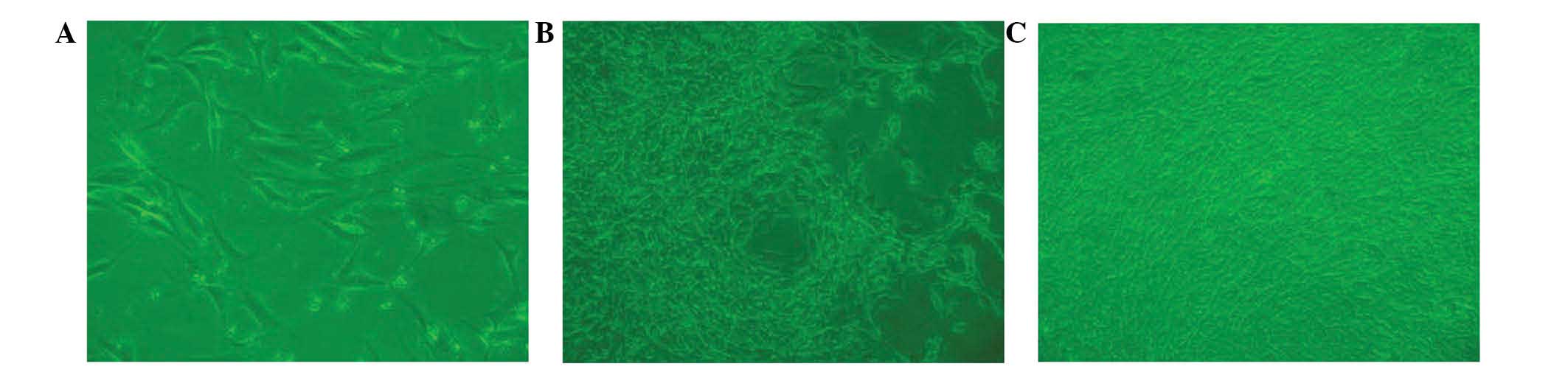
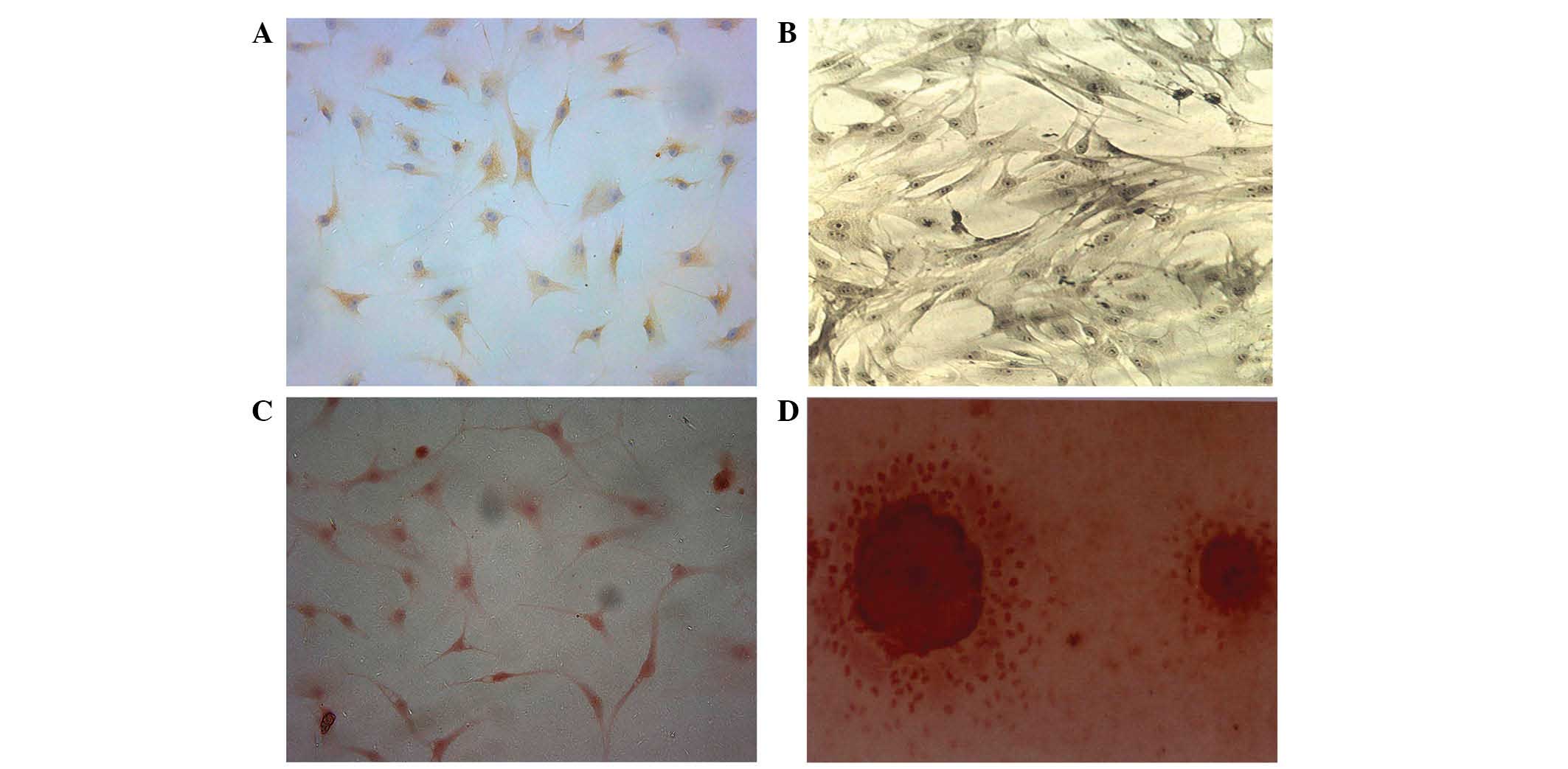
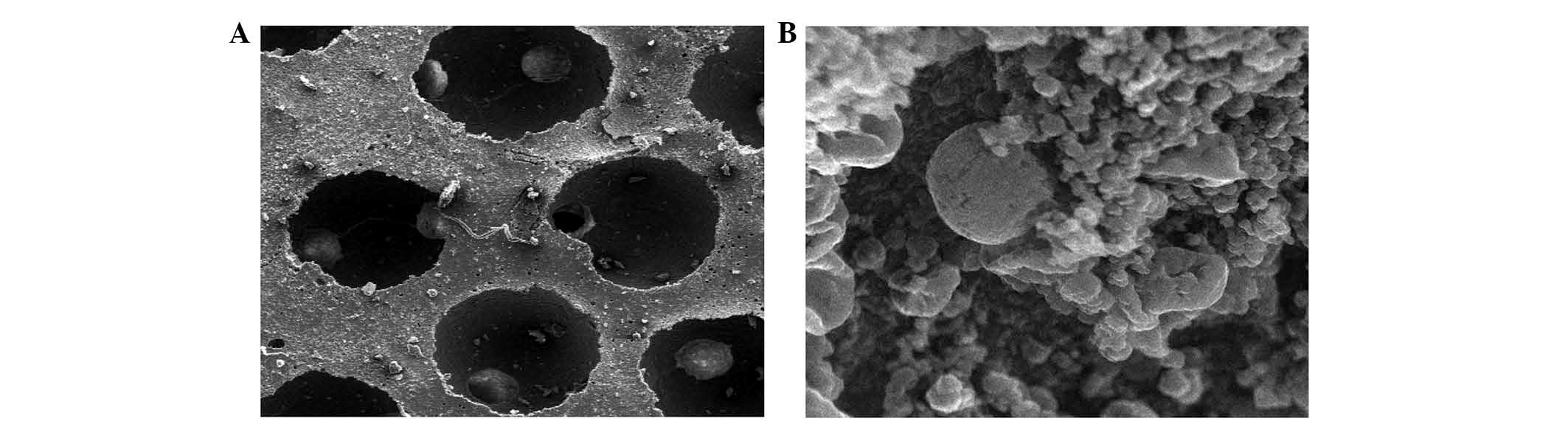
|
1
|
Vacanti CA and Upton J: Tissue-engineered
morphogenesis of cartilage and bone by means of cell
transplantation using synthetic biodegradable polymer matrices.
Clin Plast Surg. 21:445–62. 1994.PubMed/NCBI
|
|
2
|
Liao J, Shi K, Ding Q, Qu Y, Luo F and
Qian Z: Recent developments in scaffold-guided cartilage tissue
regeneration. J Biomed Nanotechnol. 10:3085–3104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng L, Wu HEL, Wang D, Feng F, Dong Y,
Liu H and Wang L: Effects of vascular endothelial growth factor 165
on bone tissue engineering. PLoS One. 8:e829452013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou D, Zhang Z, Ye D, Tang A, Deng L, Han
W, Zhao J, Wang S, Zhang W, Zhu C, et al: Repair of critical-sized
rat calvarial defects using genetically engineered bone
marrow-derived mesenchymal stem cells overexpressing
hypoxia-inducible factor-1α. Stem Cells. 29:1380–1390.
2011.PubMed/NCBI
|
|
5
|
Sun S, Ren Q, Wang D, Zhang L, Wu S and
Sun XT: Repairing cartilage defects using chondrocyte and
osteoblast composites developed using a bioreactor. Chin Med J
(Engl). 124:758–763. 2011.PubMed/NCBI
|
|
6
|
Fridenshtein AI: Stromal bone marrow cells
and the hematopoietic microenvironment. Arkh Patol. 44:3–11.
1982.(In Russian). PubMed/NCBI
|
|
7
|
Xue K, Qi L, Zhou G and Liu K: A two-step
method of constructing mature cartilage using bone marrow-derived
mesenchymal stem cells. Cells Tissues Organs. 197:484–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Intini G: The use of platelet-rich plasma
in bone reconstruction therapy. Biomaterials. 30:4956–4966. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fréchette JP, Martineau I and Gagnon G:
Platelet-rich plasmas: Growth factor content and roles in wound
healing. J Dent Res. 84:434–439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lacoste E, Martineau I and Gagnon G:
Platelet concentrates: Effects of calcium and thrombin on
endothelial cell proliferation and growth factor release. J
Periodontol. 74:1498–1507. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffman R, Benz EJJ, Shattil SJ, Furie B,
Cohen HJ, Silberstein LE LE and McGlave P: Hematology: Basic
principles and practice. Churchill Livingstone (Philadelphia).
2000.
|
|
12
|
Thiede MA, Smock SL, Petersen DN, Grasser
WA, Thompson DD and Nishimoto SK: Presence of messenger ribonucleic
acid encoding osteocalcin, a marker of bone turnover, in bone
marrow megakaryocytes and peripheral blood platelets.
Endocrinology. 135:929–937. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Landesberg R, Moses M and Karpatkin M:
Risks of using platelet rich plasma gel. J Oral Maxillofac Surg.
56:1116–1117. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie L, Yu H, Deng Y, Yang W, Liao L and
Long Q: Preparation, characterization and in vitro dissolution
behavior of porous biphasic α/β-tricalcium phosphate bioceramics.
Mater Sci Eng C Mater Biol Appl. 59:1007–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Fiqi A, Kim JH and Kim HW:
Osteoinductive fibrous scaffolds of biopolymer/mesoporous bioactive
glass nanocarriers with excellent bioactivity and long-term
delivery of osteogenic drug. ACS Appl Mater Interfaces.
7:1140–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Ye F, Yang R, Lu X, Shi Y, Li L,
Fan H and Bu H: Osteoinduction of hydroxyapatite/beta-tricalcium
phosphate bioceramics in mice with a fractured fibula. Acta
Biomater. 6:1569–1574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marx RE, Carlson ER, Eichstaedt RM,
Schimmele SR, Strauss JE and Georgeff KR: Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:638–646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dwyer SD and Meyers KM: Anesthetics and
anticoagulants used in the preparation of rat platelet-rich-plasma
alter rat platelet aggregation. Thromb Res. 42:139–151. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasten P, Vogel J, Geiger F, Niemeyer P,
Luginbühl R and Szalay K: The effect of platelet-rich plasma on
healing in critical-size long-bone defects. Biomaterials.
29:3983–3992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wiltfang J, Kloss FR, Kessler P, Nkenke E,
Schultze-Mosgau S, Zimmermann R and Schlegel KA: Effects of
platelet-rich plasma on bone healing in combination with autogenous
bone and bone substitutes in critical-size defects. An animal
experiment. Clin Oral Implants Res. 15:187–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dugrillon A and Klüter H: Topical
application of platelets for improved wound healing. Blood Ther
Med. 3:21–26. 2002.
|
|
23
|
Fennis JP, Stoelinga PJ and Jansen JA:
Mandibular reconstruction: A histological and histomorphometric
study on the use of autogenous scaffolds, particulate
cortico-cancellous bone grafts and platelet rich plasma in goats.
Int J Oral Maxillofac Surg. 33:48–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gandhi A, Dumas C, O'Connor JP, Parsons JR
and Lin SS: The effects of local platelet rich plasma delivery on
diabetic fracture healing. Bone. 38:540–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kawase T, Okuda K, Wolff LF and Yoshie H:
Platelet-rich plasma-derived fibrin clot formation stimulates
collagen synthesis in periodontal ligament and osteoblastic cells
in vitro. J Periodontol. 74:858–864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada Y, Ueda M, Naiki T, Takahashi M,
Hata K and Nagasaka T: Autogenous injectable bone for regeneration
with mesenchymal stem cells and plateletrich plasma:
Tissue-engineered bone regeneration. Tissue Eng. 10:955–964. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marx RE: Platelet-rich plasma: Evidence to
support its use. J Oral Maxillofac Surg. 62:489–496. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freymiller EG and Aghaloo TL:
Platelet-rich plasma: Ready or not? J Oral Maxillofac Surg.
62:484–488. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ouyang XY and Qiao J: Effect of
platelet-rich plasma in the treatment of periodontal intrabony
defects in humans. Chin Med J (Engl). 119:1511–1521.
2006.PubMed/NCBI
|
|
30
|
Man D, Plosker H and Winland-Brown JE: The
use of autologous platelet-rich plasma (platelet gel) and
autologous platelet-poor plasma (fibrin glue) in cosmetic surgery.
Plast Reconstr Surg. 107:229–237; discussion 238–239. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mazor Z, Peleg M, Garg AK and Luboshitz J:
Platelet-rich plasma for bone graft enhancement in sinus floor
augmentation with simultaneous implant placement: Patient series
study. Implant Dent. 13:65–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kassolis JD, Rosen PS and Reynolds MA:
Alveolar ridge and sinus augmentation utilizing platelet-rich
plasma in combination with freeze-dried bone allograft: Case
series. J Periodontol. 71:1654–1661. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH
and Jung JH: Effect of platelet-rich plasma (PRP) concentration on
the viability and proliferation of alveolar bone cells: An in vitro
study. Int J Oral Maxillofac Surg. 34:420–424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Graziani F, Ivanovski S, Cei S, Ducci F,
Tonetti M and Gabriele M: The in vitro effect of different PRP
concentrations on osteoblasts and fibroblasts. Clin Oral Implants
Res. 17:212–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fennis JP, Stoelinga PJ and Jansen JA:
Mandibular reconstruction: A clinical and radiographic animal study
on the use of autogenous scaffolds and platelet-rich plasma. Int J
Oral Maxillofac Surg. 31:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Intini G, Andreana S, Intini FE, Buhite RJ
and Bobek LA: Calcium sulfate and platelet-rich plasma make a novel
osteoinductive biomaterial for bone regeneration. J Transl Med.
5:132007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cenni E, Perut F, Ciapetti G, Savarino L,
Dallari D, Cenacchi A, Stagni C, Giunti A, Fornasari PM and Baldini
N: In vitro evaluation of freeze-dried bone allografts combined
with platelet rich plasma and human bone marrow stromal cells for
tissue engineering. J Mater Sci Mater Med. 20:45–50. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lieberman JR, Daluiski A and Einhorn TA:
The role of growth factors in the repair of bone. Biology
andclinical applications. J BoneJoint Surg Am. 84:1032–1044.
2002.
|
|
39
|
Froum SJ, Wallace SS, Tarnow DP and Cho
SC: Effect of platelet-rich plasma on bone growth and
osseointegration in human maxillary sinus grafts: Three bilateral
case reports. Int J Periodontics Restorative Dent. 22:45–53.
2002.PubMed/NCBI
|
|
40
|
Bruder SP, Kraus KH, Goldberg VM and
Kadiyala S: The effect of implants loaded with autologous
mesenchymal stem cells on the healing of canine segmental bone
defects. J Bone Joint Surg Am. 80:985–996. 1998.PubMed/NCBI
|
|
41
|
Roldán JC, Jepsen S, Miller J, Freitag S,
Rueger DC, Açil Y and Terheyden H: Bone formation in the presence
of platelet-rich plasma vs. bone morphogenetic protein-7. Bone.
34:80–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Damsky CH: Extracellular matrix-integrin
interactions in osteoblast function and tissue remodeling. Bone.
25:95–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garcia AJ and Reyes CD: Bio-adhesive
surfaces to promote osteoblast differentiation and bone formation.
J Dent Res. 84:407–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weiss RE and Reddi AH: Role of fibronectin
in collagenous matrix-induced mesenchymal cell proliferation and
differentiation in vivo. Exp Cell Res. 133:247–254. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moursi AM, Damsky CH, Lull J, Zimmerman D,
Doty SB, Aota S and Globus RK: Fibronectin regulates calvarial
osteoblast differentiation. J Cell Sci. 109:1369–1380.
1996.PubMed/NCBI
|
|
46
|
Zimmerman D, Jin F, Leboy P, Hardy S and
Damsky C: Impaired bone formation in transgenic mice resulting from
altered integrin function in osteoblasts. Dev Biol. 220:2–15. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Weibrich G, Gnoth SH, Otto M, Reichert TE
and Wagner W: Growth stimulation of human osteoblast-like cells by
thrombocyte concentrates in vitro. Mund Kiefer Gesichtschir.
6:168–174. 2002.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Intini G, Andreana S, Margarone JE III,
Bush PJ and Dziak R: Engineering a bioactive matrix by
modifications of calcium sulfate. Tissue Eng. 8:997–1008. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bateman J, Intini G, Margarone J III,
Goodloe S III, Bush P, Lynch SE and Dziak R: Platelet-derived
growth factor enhancement of two alloplastic bone matrices. J
Periodontol. 76:1833–1841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang D, Jiang H, Wang S, Li H, Zhang H,
Zhao L, Peng T, Cao Z and Sun S: Construction of tissue-engineered
bone using a bioreactor and platelet-rich plasma. Exp Ther Med.
8:413–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Klein-Nulend J, van der Plas A, Semeins
CM, Ajubi NE, Frangos JA, Nijweide PJ and Burger EH: Sensitivity of
osteocytes to biomechanical stress in vitro. FASEB J. 9:441–445.
1995.PubMed/NCBI
|
|
52
|
Owan I, Burr DB, Turner CH, Qiu J, Tu Y,
Onyia JE and Duncan RL: Mechanotransduction in bone: Osteoblasts
are more responsive to fluid forces than mechanicalstrain. Am J
Physiol. 273:C8l0–C815. 1997.
|
|
53
|
Bakker AD, Soejima K, Klein-Nulend J and
Burger EH: The production of nitric oxide and prostaglandin E(2) by
primary bone cells is shear stress dependent. J Biomech.
34:671–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang Y, Kim UJ, Blasioli DJ, Kim HJ and
Kaplan DL: In vitro cartilage tissue engineering with 3D porous
aqueous-derived silk scaffolds and mesenchymal stem cells.
Biomaterials. 26:7082–7094. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang W, Itaka K, Ohba S, Nishiyama N,
Chung UI, Yamasaki Y and Kataoka K: 3D spheroid culture system on
micropatterned substrates for improved differentiation efficiency
of multipotent mesenchymal stem cells. Biomaterials. 30:2705–2715.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Song K, Wang H, Zhang B, Lim M, Liu Y and
Liu T: Numerical simulation of fluid field and in vitro
three-dimensional fabrication of tissue-engineered bones in a
rotating bioreactor and in vivo implantation for repairing
segmental bone defects. Cell Stress Chaperones. 18:193–201. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Song Z, Wu C, Sun S, Li H, Wang D, Gong J
and Yan Z: Quantitative analysis of factors influencing
tissue-engineered bone formation by detecting the expression levels
of alkaline phosphatase and bone γ-carboxyglutamate protein 2. Exp
Ther Med. 9:1097–1102. 2015.PubMed/NCBI
|