Introduction
Pulmonary artery sarcoma (PAS) is a rare malignant
tumor originating from the pulmonary blood vessels (1). Since it was initially reported by
Mandelstamm (2) in 1923, <300
cases have been reported in the literature (3,4).
Clinically, PAS can easily be misdiagnosed as a pulmonary
thromboembolic disease, such as pulmonary thromboembolism (PTE) or
chronic thromboembolic pulmonary hypertension (CTEPH) (5–7). In such
instances, patients lose the opportunity for early diagnosis and
treatment (8,9). In the present study, the clinical data
of three patients with PAS, as confirmed by surgery at the Beijing
Anzhen Hospital (Beijing, China), are reported and analyzed, in
order to raise awareness and improve the diagnosis of PAS.
Case report
Case 1: Female patient, 36 years
old
The patient was hospitalized on the 26th October
2008 having experienced shortness of breath and chest tightness for
50 days and four syncopes. In September 2007, the patient had
suddenly fainted during agricultural work and had experienced loss
of consciousness and incontinence for ~10 min; however, no
abnormalities were detected by the local hospital upon waking.
Thereafter, the patient experienced recurrent shortness of breath,
chest tightness and difficulty breathing, and discontinuous
syncopes occurred three times in the absence of chest pain,
hemoptysis and convulsions. A chest X-ray obtained from a local
hospital showed no abnormalities, although a chest computed
tomography pulmonary angiography (CTPA) performed in September 2007
showed a suspected pulmonary embolism. The patient was transferred
to the Beijing Anzhen Hospital to confirm the diagnosis and provide
effective treatment. Prior to these events, the patient had been
healthy, with no history of a rash, joint pain, lower extremity
swelling or trauma surgery, and no history of oral contraceptive
use.
The results of a physical examination were as
follows: Blood pressure (Bp), 110/66 mmHg (normal range,
90–140/60–90 mmHg); respiratory rate (R), 20 times/min (normal
range, 16–20 times/min); and heart rate (HR), 79 beats/min (normal
range, 60–100 beats/min). In addition, the lung breath sounds were
clear, the patient's breath did not smell and there were neither
wet nor dry rales. Furthermore, the heart rhythm was regular and
pulmonary valve section two (P2) heart sound hyperthyroidism and
bilateral lower extremity edema were observed. The results of the
laboratory tests (Automatic Blood Cell Analyzer; Sysmex XE2100;
Sysmex Medical Electronics Co., Ltd., Kobe, Japan) were as follows:
White blood cells (WBC), 7.1×109/l (normal range,
3.5–9.5×109/l); red blood cells (RBC),
5.70×1012/l (normal range, 3.8–5.1×1012/l);
hemoglobin (Hb), 146 g/l (normal range, 115–150 g/l); platelets
(BPC), 134×109/l (normal range,
125–350×109/l); and D-dimer, 270 mg/l (normal range,
0–230 mg/l; Dimertest Latex kit; Shanghai Boatman Biotech Co.,
Ltd., Shanghai, China). Normal results were obtained in the routine
examination of stools and urine. The following results were
obtained in the arterial blood gas analysis (FlexQ ABL800;
Radiometer ApS, Copenhagen, Denmark): pH 7.36 (normal range, pH
7.35–7.45); arterial partial pressure of carbon dioxide
(PaCO2), 36.5 mmHg (normal range, 35–45 mmHg); and
arterial partial pressure of oxygen (PaO2), 80.7 mmHg
(normal range, 83–108 mmHg). The results of biochemical tests
(Automatic Biochemical Analysis System; Beckman Coulter AU5421;
Beckman Coulter, Inc., Bream CA, USA) were: Alanine
aminotransferase (ALT), 50 U/l (normal range, 7–45 U/l); aspartate
aminotransferase (AST), 32 U/l (normal range, 13–40 g/l); total
protein (TP), 68 g/l (normal range, 65–85 g/l); albumin, 38 g/l
(normal range, 40–55 g/l); urea nitrogen, 4.5 mmol/l (normal range,
2.8–7.2 mmol/l); creatinine, 79.6 µmol/l (normal range, 41–81
µmol/l); lactate dehydrogenase, 233 U/l (normal range, 140–271
U/l); creatine kinase, 37 U/l (normal range, 26–140 U/l); creatine
kinase-MB, 12 U/l (normal range, 0–25 U/l); and blood glucose, 5.9
mmol/l (normal range, 3.9–6.1 mmol/l). In addition, lipid blood
levels were as follows: Total cholesterol, 4.2 mmol/l (normal
range, 3.1–5.2 mmol/l); low-density lipoprotein, 2.19 mmol/l
(normal range, <3.12 mmol/l in healthy individuals;
<2.59mmol/L for high-risk individuals); triglyceride, 1.1 mmol/l
(normal range, 0–1.7 mmol/l). Furthermore, tests for antinuclear
antibodies (ANA) and anti-extractable nuclear antigen antibodies
(ENA) were performed as previously described (10), and were negative. An
electrocardiogram (ECG) showed an S1Q3T3 pattern and V1-4 T-wave
inversion, and a Color Doppler vascular ultrasound, and ultrasound
examinations of the liver, gall bladder, spleen and kidneys, showed
that the inferior vena cava and pelvic and abdominal regions were
normal. However, a Color Doppler echocardiography (UCG) showed that
the diameter of the right ventricle was at the upper limit of
normal (normal range, 10–20 mm) due to tricuspid aortic valve
insufficiency. The pulmonary artery systolic pressure (SPAP) was 66
mmHg (normal range, <35 mmHg) and the diameter of the main
pulmonary artery was 33.7 mm (normal range, <27 mm). A mass-like
echo, with an irregular shape and uneven internal echo, was
detected in the main pulmonary artery and the left pulmonary
artery. The left ventricular posterior wall of the pericardial
fluid areas had a depth of ~8.0 mm. A pulmonary
ventilation/perfusion scan (lung V/Q) showed that all the segments
of the left and right upper lung lobe possessed perfusion defects,
but the ventilation was normal. Dual-color Doppler ultrasound
examination revealed no abnormalities in the deep veins of the two
legs. A second CTPA performed in October 2008 detected a large
filling defect in the left and the right pulmonary arteries, right
pulmonary artery subtotal occlusion, lobulated margins, and the
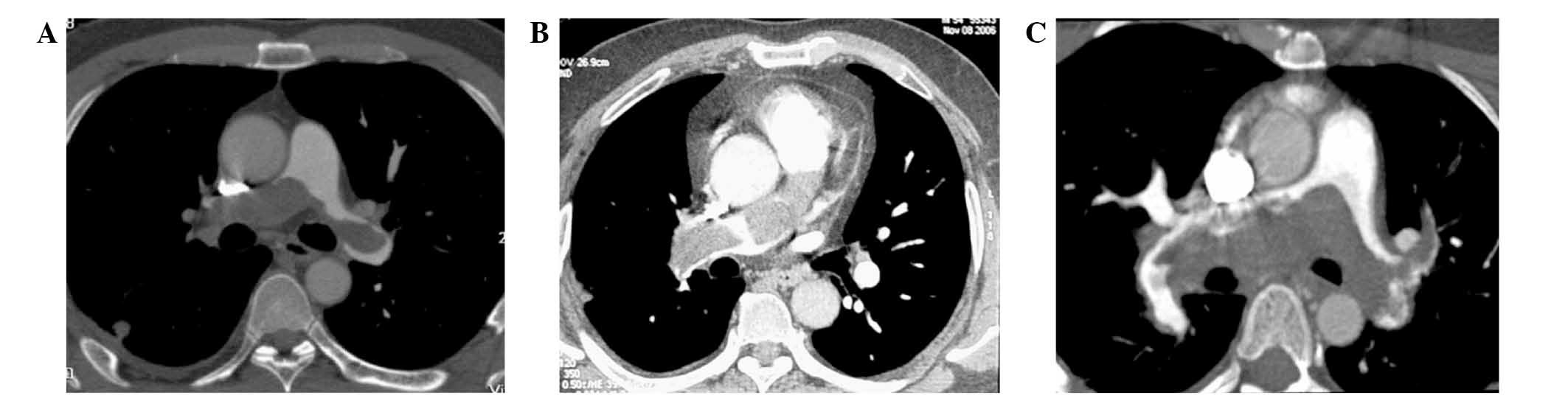
pulmonary wall was less clearly observed (Fig. 1A).
On the basis of all these results, an initial
diagnosis of pulmonary thromboembolism was made. Given that the
patient had a history of pulmonary thromboembolism for >50 days
(the optimum thrombolytic time) and a large embolization, the
clinicians decided to perform surgical resection (pulmonary
thrombus removal surgery and an embolectomy), following a
discussion with the cardiac surgeons. Intraoperatively, it was
observed that the mucinous tumor tissues contained the main
pulmonary artery and the left and right pulmonary arteries. In
addition, lesions in the posterior wall of the right pulmonary
artery had infiltrated to the outside of its posterior wall and
affected the nearby pericardium, and there was hemorrhagic
pericardial effusion. The size (length × diameter) of the resected
tumor tissue was 6.3×2.5 cm2. Histopathological
examination was performed and pathological diagnosis was PAS (type,
pulmonary artery intimal sarcoma) according World Health
Organization Classification of Tumors (11). Specimens were fixed in 4% neutral
formalin, embedded in paraffin, sectioned to a thickness of 4 µm
and stained with hematoxylin and eosin (Beijing Yili Fine Chemical
Co., Ltd., Beijing, China). A microscope (DM3000; Leica
Microsystems, Inc., Buffalo Grove, IL, USA) was used for
histopathologic analysis. Due to a weak body constitution, no
chemotherapy nor radiotherapy was administered and, after 5 months,
the patient succumbed to tumor recurrence and metastasis.
Case 2: Female patient, 41 years
old
The patient was hospitalized on the 6th October 2009
with a 4-year history of intermittent shortness of breath and
palpitations, which were relieved by rest and accompanied by
abdominal distension, lower extremity edema, hemoptysis, chest
pain, fainting, and the absence of joint swelling, pain and fever.
A UCG showed that the right side of the heart was enlarged and
detected pulmonary hypertension, which were considered clinical
manifestations of CTEPH, right ventricular failure and respiratory
failure. After receiving oxygen and anticoagulation treatment,
including 0.6 g Clexane subcutaneously injected every 12 h for 7
days (Sanofi Winthrop Industrie, Paris, France), followed by 4.5 mg
oral administration warfarin sodium (Orion Corporation, Espoo,
Finland) daily for 1 month, these symptoms were marginally
relieved. Following discharge, the patient self-administered oral
warfarin and hydrochlorothiazide (25 mg orally administrated twice
daily for >2 years; TianJin LiSheng Pharmaceutical Co., Ltd.,
Tianjin, China) prior to being transferred to the Beijing Anzhen
Hospital for further treatment due to aggravating and persistent
symptoms, loss of appetite, fatigue and weight loss. Prior to these
events, the patient had been healthy with no history of trauma
surgery nor diet pill or contraceptive use. In addition, the
patient had given birth to a healthy child.
The results of a physical examination were as
follows: T, 36.4°C (normal range, 36.3–37.2°C); Bp, 96/60 mmHg
(normal range, 90–140/60–90 mmHg); HR, 80 beats/min (normal range,
60–100 beats/min); detection of a skin rash and bleeding, the lung
breath sounds were clear, the patient's breath did not smell, and
wet and dry rales were not detected; the heart rhythm was regular;
P2 hyperactivity; the abdomen was soft without tenderness; there
was no swelling of the liver and spleen; and edema of the lower
extremities was observed. The results of laboratory tests were as
follows: WBC, 6.1×109/l; RBC, 6.3×1012/l; Hb,
155 g/l; BPC, 159×109/l; and D-dimer, 350 mg/l. The
results of the arterial blood gas analysis were as follows: pH
7.40; PaCO2, 33 mmHg; PaO2, 61 mmHg; and
N-terminal pro-brain natriuretic peptide (NT-proBNP), 2904 mol/l
(normal, <500 mol/l). An X-ray showed double lung markings,
small streak shadows in the lower lobe of the left lung and
thickening of the right main pulmonary artery. UCG analysis showed
an enlarged right ventricle, tricuspid aortic calve insufficiency
and a SPAP of 90 mmHg (normal range, <35 mmHg). CTPA detected a
large block within the right pulmonary artery filling defects,
lobulated margins and subtotal occlusion of the right pulmonary
artery (Fig. 1B). There was no
significant obstruction observed in the bilateral lower extremity
vein, inferior vena cava and bilateral renal vein by Color Doppler
sonography analysis.
The patient was initially diagnosed with CTEPH, and
a pulmonary thromboendarterectomy was performed on the 11th October
2009. Intraoperatively, it was observed that the tumor tissue was
located in the main and right pulmonary arteries; the right upper
lobe pulmonary artery was entirely occluded. The size of the
resected tumor tissue was 5.0×1.2 cm2. The tumor was
diagnosed as PAS (type, leiomyosarcoma) by a pathological
diagnosis. At 1 month after the surgical procedure, the patient
succumbed to septic shock and heart and lung failure in the
hospital.
Case 3: Male patient, 47 years
old
The patient was hospitalized on 15th July 2012 due
to a 3-month history of shortness of breath during activity and
fatigue, in the absence of chest palpitations, chest pain, syncopes
and fever. The patient was diagnosed with a pulmonary embolism in a
local hospital. The patient received local thrombolysis (Urokinase,
4400 IU/kg, injection; Sichuan Bei Ao Biological Pharmaceutical
Co., Ltd., Chengdu, China) via right heart catheterization followed
by anticoagulation treatment (Fraxiparine, 0.4 mg subcutaneously
injected every 12 h for 5 days; nadroparin calcium injection,
GlaxoSmithKline, Maharashtra, India; followed by warfarin sodium,
4.5 mg oral administration daily for 1 month, Orion Corporation).
Following discharge, the patient self-administered oral warfarin (3
mg daily for >2 months) and was transferred to the Beijing
Anzhen Hospital for further treatment due to insignificant
improvement of symptoms. From the onset the patient exhibited
symptoms of anorexia and weight loss. The patient had previously
been healthy, with no history of alcohol addiction, leg swelling,
surgery or a family history of carcinoma.
The results of a physical examination were as
follows: No cyanosis of the lips, clear breath sounds, the
patient's breath did not smell, and wet and dry rales were not
observed; HR, 68 times/min; P2 no hyperthyroidism; no pathological
murmurs in the valve area; a soft abdomen; no swelling of the liver
and spleen; ascites were negative; and no swelling of the lower
limbs. The results of the laboratory tests were as follows: WBC,
8.2×109/l; RBC, 5.8×1012/l; Hb, 140 g/l; BPC,
132×109/l; anti-human immunodeficiency virus (HIV)
antibody, negative; and D-dimer, 170 mg/l. The results of the
arterial blood gas analysis were as follows: pH 7.46;
PaCO2, 43 mmHg; and PaO2, 83 mmHg.
Biochemical tests detected no abnormalities. The prothrombin time
was 35.6 sec (normal range, 11–14 sec). An ECG showed right
ventricular hypertrophy and II, III and aVF lead T-wave inversion.
An X-ray showed heavy markings in the left lung and light markings
in the right lung. UCG showed an enlarged right atrium and right
ventricle, and a SPAP of 53 mmHg. CTPA detected a filling defect in
the main, left and right pulmonary arteries. The lesion was uneven
in density, had lobulated margins and protruded towards the right
ventricular outflow tract. The posterior walls of the right and
left pulmonary arteries were not clearly observed (Fig. 1C). A lung V/Q scan showed the
bilateral iliac veins and two venous imaging and detected no
abnormalities, and mismatch of lung perfusion and ventilation
imaging. An abdominal B ultrasound examination detected the
presence of gallstones but no abnormalities in the remaining organs
of the abdominal cavity.
From these results, the patient was diagnosed with
CTEPH and gallstone disease. A pulmonary thromboendarterectomy was
performed on the 18th July 2012. Intraoperatively, it was shown
that the tumor tissue was located in the main, left and right
pulmonary arteries. The size of the resected tumor was 7.0×2.7
cm2. The tumor was diagnosed as PAS (type,
leiomyosarcoma) by a pathological diagnosis. The patient was
transferred from Beijing Cancer Hospital (Beijing, China) on the
29th June to the Cancer Hospital for chemotherapy (50 mg
doxorubicin and 5 g ifosfamide every 3 weeks for six cycles).
However, the patient succumbed to tumor recurrence and metastasis
after 18 months.
The present study was conducted in accordance with
the declaration of Helsinki and with approval from the Ethics
Committee of the Beijing Anzhen Hospital. Written informed consent
was obtained from all participants.
Discussion
PAS is a primary tumor that occurs in the pulmonary
valve and/or pulmonary artery (1,12). The
diagnosis of PAS must rule out tumor metastases from other parts of
the body (13). The incidence of PAS
worldwide ranges from 0.001 to 0.03%; however, the underlying
pathogenesis of PAS remains unclear (14). The ages of individuals previously
diagnosed with PAS range from 13–86 years, with an average age of
52-years-old, and the ratio of females to males is 2:1 (15). PAS may be divided into numerous
pathological types, according to 138 cases of pathological
specimens summarized in the literature (14,16,17);
these types include pulmonary artery intimal sarcoma (31.2%),
leiomyosarcoma (15.9%), spindle cell sarcoma (13.8%), malignant
fibrous tissue sarcoma (7.2%), fibrosarcoma (5.1%), fibromyxoid
sarcoma (4.3%), rhabdomyosarcoma (4.3%) and chondrosarcoma (3.6%)
(16). The present study reports the
analysis of three cases of PAS, including one case of pulmonary
artery intimal sarcoma and two cases of leiomyosarcoma. Due to the
small number of reported cases, the incidence of the various
pathological types of PAS in the Chinese population requires
further study.
There are a lack of specific clinical manifestations
for primary pulmonary sarcomas (17,18). The
majority of patients have demonstrated insidious onset,
predominantly involving progressive dyspnea, chest pain, coughing,
hemoptysis, syncopes, fever, fatigue and weight loss (4,6,8,15,16).
X-rays of these patients typically show a prominent hilar shadow,
sparse texture of the peripheral vasculature, lung nodules and
enlargement of the heart. A clinical diagnosis based on a CTPA has
numerous benefits, and it should be used as a routine examination,
which can be expressed as the pulmonary artery dilatation,
intraluminal filling defect and luminal stenosis. It has been
reported that ~90% of PAS lesions affected at least two parts of
the pulmonary arteries, >85% of patients had primary pulmonary
lesions, 71% of patients had right pulmonary artery lesions, 65% of
patients had left pulmonary artery lesions, and 10% of patients had
lesions involving the right ventricular outflow channel (19). In previous studies, a Color Doppler
UCG showed right ventricular enlargement, tricuspid regurgitation
and pulmonary hypertension (15,20). The
Doppler vascular ultrasound examination results of the lower limbs,
inferior vena cava and pelvis were normal. Arterial blood gas
analysis showed decreased PaO2 levels. Clinically, it is
easy to misdiagnose the patients with PTE or CTEPH, since the
clinical manifestations of pulmonary thromboembolic diseases are
similar to PAS (5–7). Therefore, confirmation of the diagnosis
typically requires surgery (19,21).
The present study reports three cases of PAS at the
Beijing Anzhen Hospital with symptoms resembling PTE. Arterial
blood gas analyses, ECG characteristics, X-ray scans, and lung V/Q
examination results prior to surgery misdiagnosed the patients with
PTE (Case 1) or CTEPH (Cases 2 and 3). The clinical and imaging
findings of the three patients were carefully analyzed and compared
with the literature. From this, the authors of the present study
propose the following to aid in the differential diagnosis of PAS:
i) PAS exhibits insidious onset and slow progression, as compared
with the rapid onset of PTE; ii) PAS patients typically show signs
of a fever, loss of appetite, weight loss and other systemic
manifestations; iii) PAS patients typically lack clinical
manifestations that may cause pulmonary emboli, including deep vein
thrombosis; iv) CTPA of PAS patients usually detects abnormalities
that are typically absent from PTE patients, including filling
defects in the main pulmonary artery, which may extend to the right
and/or left pulmonary artery, as well as lobulated margins in the
lesion or segregation phenomena, which project in the direction of
blood flow in the right ventricular outflow tract; and v) PAS does
not respond to thrombolysis or anticoagulation therapy, and may
deteriorate after treatment. In addition, previous studies have
recommended that the CT data be used together with
positron-emission tomography (PET)-CT results for the diagnosis of
PAS (4,7–9). The
performance of 18F-fludeoxyglucose (18FDG)
PET-CT in patients with PTE or CTEPH showed no increase in
radiotracer intake, whereas in patients with PAS, 18FDG
PET-CT showed an increase in radiotracer uptake, which may help to
differentiate between the two diseases.
PAS has a poor prognosis; its natural survival
period is ~1.5 months following diagnosis (15). Surgery is the preferred treatment for
PAS, since it can relieve symptoms and prolong the survival period
(9). Kim et al (22) successfully completed surgical
resection on nine patients with PAS; after 1 month, the cardiac
functions of the patients were of grade 1 (New York Heart
Association Classification system) (23) and their exercise capacities were
improved. Furthermore, their postoperative mean survival period was
19.2 months, including one patient who survived for >45 months
(21). In addition, the use of
chemotherapeutics combined with surgery to treat PAS has shown some
efficacy; Xu et al (24)
reported a patient with intimal PAS who was successfully treated
with a vinorelbine-based regimen as second-line chemotherapy and
was stable at 19 months following the onset of postoperative
recurrence. Surgery, combined with chemotherapy and (or)
radiotherapy post-surgery, may increase the survival period to 1–2
years.
In conclusion, PAS is an extremely rare tumor that
occurs in the cardiovascular system. Due to its non-specific
clinical manifestations, PAS is often misdiagnosed as pulmonary
thromboembolic diseases. Therefore, PAS should be suspected in
patients with large ‘mass’ within the pulmonary trunk and its main
branches, and who have insidious onset, no history of venous
thromboembolism and no evidence of positive hypercoagulability,
particularly in those who are unresponsive to current
anticoagulation therapy or in which there is clinical evidence of
neoplasm. These patients should be investigated to confirm the
diagnosis and should undergo surgical intervention as soon as
possible, in order to improve the prognosis of PAS.
References
|
1
|
Travis WD, Brambilla E, Hermelink Müller
HK and Harris CC: World Health Organization Classification of
Tumours. Pathology & Genetics of Tumours of the Lung, Pleura,
Thymus and Heart. IARC Press. (Lyon). 2004.
|
|
2
|
Mandelstamm M: About primary neoplasms of
the heart. Virchows Arch A Pathol Pathol Anat. 245:43–54. 1923.(In
German). View Article : Google Scholar
|
|
3
|
Shehatha J, Saxena P, Clarke B, Dunning J
and Konstantinov IE: Surgical management of extensive pulmonary
artery sarcoma. Ann Thorac Surg. 87:1269–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gan HL, Zhang JQ, Huang XY and Yu W: The
wall eclipsing sign on pulmonary artery computed tomography
angiography is pathognomonic for pulmonary artery sarcoma. PLoS
One. 8:e832002013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Sayed Ahmed MM, Aftab M, Al-Najjar RM,
de la Cruz KI, Benjamin RS and Hallman CH: Pulmonary artery sarcoma
mimicking pulmonary embolism. Tex Heart Inst J. 41:515–517. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Renilla A, Fernández-Vega I, Martín M and
Weinsaft JW: Pulmonary artery sarcoma mimicking a pulmonary
embolism. Eur Heart J Cardiovasc Imaging. 14:10252013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sandhu A, Yates TJ and Kuriakose P:
Pulmonary artery sarcoma mimicking a pulmonary embolism. Indian J
Cancer. 45:27–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Attinà D, Niro F, Tchouanté P, Mineo G,
Russo V, Palazzini M, Galiè N, Fanti S, Lovato L and Zompatori M:
Pulmonary artery intimal sarcoma. Problems in the differential
diagnosis. Radiol Med. 118:1259–1268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhagwat K, Hallam J, Antippa P and
Larobina M: Diagnostic enigma: Primary pulmonary artery sarcoma.
Interact Cardiovasc Thorac Surg. 14:342–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arbuckle MR, McClain MT, Rubertone MV,
Scofield RH, Dennis GJ, James JA and Harley JB: Development of
autoantibodies before the clinical onset of systemic lupus
erythematosus. N Engl J Med. 349:1526–1533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fletcher CDM, Unni KK and Mertens F: World
Health Organization Classification of Tumours. Pathology &
genetics of tumours of soft tissue and bone. IARC Press. (Lyon).
2002.
|
|
12
|
Fletcher CDM, Unni KK and Mertens F: World
Health Organization Classification of Tumours. Pathology &
Genetics of Tumours of Soft Tissue and Bone. IARC Press. (Lyon).
2002.
|
|
13
|
Dimitrakakis G, Zilidis G, Buchalter M and
Von Oppell U: Pulmonary artery sarcoma-a challenging diagnosis: A
case report. Heart Surg Forum. 9:E897–E899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huo L, Moran CA, Fuller GN, Gladish G and
Suster S: Pulmonary artery sarcoma: A clinicopathologic and
immunohistochemical study of 12 cases. Am J Clin Pathol.
125:419–424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parish JM, Rosenow EC III, Swensen SJ and
Crotty TB: Pulmonary artery sarcoma-clinical features. Chest.
110:1480–1488. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cox JE, Chiles C, Aquino SL, Savage P and
Oaks T: Pulmonary artery sarcomas: A review of clinical and
radiologic features. J Comput Assist Tomogr. 21:750–755. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keel SB, Bacha E, Mark EJ, Nielsen GP and
Rosenberg AE: Primary pulmonary sarcoma: A clinicopathologic study
of 26 cases. Mod Pathol. 12:1124–1131. 1999.PubMed/NCBI
|
|
18
|
Pewarchuk JA, Nassaralla CL and Midthun
DE: A 39-year-old woman with cough, chest pressure, and worsening
dyspnea. Chest. 131:934–937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scheffel H, Stolzmann P, Plass A, Weber A,
Prêtre R, Marincek B and Alkadhi H: Primary intimal pulmonary
artery sarcoma: A diagnostic challenge. J Thorac Cardiovasc Surg.
135:949–950. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zurick AO 3rd, De Lenge Rosen V, Tan CD,
Rodriguez ER, Flamm SD and Schoenhagen P: Pulmonary artery intimal
sarcoma masquerading as pulmonary embolism. Circulation.
124:1180–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi CA, Lee KS, Choe YH, Han D, Kwon OJ and
Kim S: Computed tomography in pulmonary artery sarcoma:
Distinguishing features from pulmonary embolic disease. J Comput
Assist Tomogr. 28:34–39. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HK, Choi YS, Kim K, Shim YM, Sung K,
Lee YT, Park PW and Kim J: Surgical treatment for pulmonary artery
sarcoma. Eur J Cardiothorac Surg. 33:712–716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
The Criteria Committee of the New York
Heart Association: Nomenclature and Criteria for Diagnosis of
Diseases of the Heart and Great Vessels (9th). Little, Brown &
Co. Boston, MA: 253–256. 1994.
|
|
24
|
Xu Y, Wang K, Geng Y, Shao Y and Yin Y: A
case of intimal sarcoma of the pulmonary artery successfully
treated with chemotherapy. Int J Clin Oncol. 17:522–527. 2012.
View Article : Google Scholar : PubMed/NCBI
|