Introduction
Androgens, including testosterone, androstenedione
and dihydrotestosterone, have been reported to have roles in female
reproduction; however, their specific functions remain
controversial (1). In a previous
study, a low dose of testosterone was shown to promote the
aggregation, growth and development of follicles, which may occur
due to the ability of testosterone to increase the secretion of
insulin-like growth factor 1 (2).
Conversely, the apoptosis of preantral follicles and follicular
granulosa cells may be induced by high levels of testosterone,
which has been associated with upregulation of tumor necrosis
factor (TNF)-related apoptosis-inducing ligand (2,3).
Granulosa cells are involved in key physiological
processes, including follicular growth, steroidogenesis and
angiogenesis (4); however, little is
known regarding the role of androgens in the growth and function of
granulosa cells. In previous studies, the secretion of
anti-Müllerian hormone (AMH) was increased in granulosa cells
derived from preantral and small antral follicles (4,5). In
polycystic ovaries, increased serum expression levels of vascular
endothelial growth factor (VEGF) have been associated with
increased ovarian blood flow (6);
however, the underlying mechanism, in particular the intra-ovarian
autoregulatory mechanism, remains unknown.
Hypoxia-inducible factor-1α (HIF-1α) is a
transcription factor that is sensitive to low oxygen tension, and
is able to prevent fatal depletion of oxygen and subsequent cell
death (7). Furthermore, there exists
an inverse correlation between HIF-1α expression levels and
available oxygen, thus suggesting that HIF-1α may be involved in
determining oocyte developmental competence (8). Considering these findings (9–11), the
present study aimed to investigate the effects of testosterone on
the morphology of mouse granulosa cells, and the mRNA expression
levels and secretion of AMH, VEGF and HIF-1α.
Materials and methods
Mice
Immature (5-week-old; n=20) female Kunming mice were
obtained from the Jinzhou Medical University (Jinzhou, China), and
were housed in an air-conditioned environment under a 12-h
light-dark cycle, with ad libitum access to a standard diet
and water. The mice were injected with pregnant mare serum
gonadotropin (10 IU; Ningbo Second Hormone Factory, Hangzhou,
China), in order to stimulate folliculogenesis. After 48 h, the
mice were sacrificed using 45 mg/kg pentobarbital (2%). All
treatments and procedures were conducted in accordance with the
Standards of Human Animal Care, as outlined in the National
Institutes of Health (NIH) Guide for the Care and Use of Laboratory
Animals (NIH, Bethesda, MA, USA). The mice handling protocols were
approved by the Institutional Animal Care and Use Committee at
Liaoning Medical University.
Cell culture and treatment
Following sacrifice, the ovaries of the mice were
removed, dissected and the granulosa cells were collected, as
described in a previous study (12).
Purified granulosa cells were cultured in McCoy's 5A complete
medium, supplemented with 1% follicle-stimulating hormone receptor
(FSHR) antibiotics and 15% fetal bovine serum, at 37°C in an
atmosphere containing 5% CO2. In addition, the cells
were cultured with testosterone (10−7 or 10−6
M), or in the absence of testosterone (control) for 24 or 48 h. All
reagents were purchased from GE Healthcare Life Sciences (Logan,
UT, USA).
Granulosa cell identification
The purity of the granulosa cells was verified using
hematoxylin and eosin (HE; Zhongshan Golden Bridge Biotechnology
Co., Ltd., Beijing, China) and immunofluorescence staining. FSHR
staining was used to identify and confirm the purity of the
granulosa cells, as FSHR is specifically expressed on the surface
of granulosa cells. The cells were counted using trypan blue
staining (Sigma-Aldrich, St. Louis, MO, USA). The Olympus BX61
microscope (Olympus Corp., Tokyo, Japan) was used in these
experiments.
Methyl thiazoyl tetrazolium (MTT)
assay for granulosa cell viability
Isolated granulosa cells were resuspended in
Dulbecco's modified Eagle's medium/F12 medium (Hyclone; GE
Healthcare Life Sciences), and were adjusted to a concentration of
5.0×105 cells/ml prior to seeding into 96-well plates
for culturing at 37°C in a 5% CO2 incubator for 3–5
days. Four testosterone concentrations (10−8,
10−7, 10−6 and 10−5 M) were used
to treat the experimental groups. The cells were cultured for 48 h,
after which 20 µl MTT (5 mg/ml; Sigma-Aldrich) was added to each
well and incubated for 4 h in darkness. Subsequently, 150 µl
dimethyl sulfoxide was added to each well, followed by agitation
for 15 min. The absorbance was measured at 570 nm using Multiskan
MK3 microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and optical density (OD) values were calculated.
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Granulosa cells were seeded into fibronectin-coated
6-well plates at a density of 1×106 cells/well, and were
treated with testosterone (10−7 and 10−6 M)
for 24 or 48 h. Total RNA was isolated using the PureLink RNA Mini
kit (cat. no. 12183018A) and the KingFisher® robot, and
was reverse transcribed into cDNA using the cDNA Reverse
Transcription kit (all Thermo Fisher Scientific, Inc.). The
specific primer sequences (designed by Invitrogen, Thermo Fisher
Scientific Inc., Shanghai, China) were as follows: Mouse AMH,
forward 5′-CCCGCTATTTGGTGCTAACCG-3′, reverse
5′-GGACTCATCCGCGTGAAACAG-3′; mouse VEGF, forward
5′-CCAAAGCCAGCACATAGG-3′, reverse 5′-TCTCCGCTCTGAACAAGG-3′; mouse
HIF-1α, forward 5′-TCCAAGCCCTCCAAGTATG-3′, reverse
5′-GTGCCACTGTATGCTGATG-3′; and mouse glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′-ATCACTGCCACCCAGAAG-3′, reverse
5′-TCCACGACGGACACATTG-3′. qPCR was conducted using a reaction
mixture of 2 µl cDNA, 0.5 µl forward and 0.5 µl reverse primers
(900 nmol/l), and 32.5 µl SYBR Green PCR Master mix, within an ABI
7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The cycling conditions were as follows: 95°C for
10 min, followed by 40 cycles at 95°C for 15 sec, and 60°C for 45
sec. The relative expression levels of the target genes were
normalized to the GAPDH internal control. Data were analyzed using
SDS 1.4 software (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
Granulosa cells (106 cells/well) were
cultured and treated with testosterone (10−7 and
10−6 M). The cell culture medium was collected after 24
and 48 h and stored at −80°C. The protein secretion levels were
analyzed using the mouse AMH, VEGF and HIF-1α ELISA kits, according
to the manufacturer's protocol (DSL Chemicals Co., Ltd., Shanghai,
China). The concentration of the protein was determined by
measuring the absorbance at 450 nm. Each experiment was conducted
in triplicate.
Cytoskeleton F-actin staining
Granulosa cells were seeded into 6-well culture
slides at a density of ~3×104 cells/well in duplicate,
and were treated with testosterone (10−7 and
10−6 M) for 48 h. Subsequently, the cells were subjected
to F-actin and FSHR staining, as demonstrated in a previous study
(12). Briefly, the cells were fixed
with 3.7% paraformaldehyde at 4°C for 10 min, after which they were
treated with 0.5% Triton-X-100 for 15 min (for F-actin staining) or
3% hydrogen peroxide solution (for FSHR staining) for 5 min. For
F-actin staining, the cells were incubated with 2 U/ml
rhodamine-phalloidin (cat. no. PHDR1; Cytoskeleton, Inc., Denver,
CO, USA) for 1 h at room temperature in the dark, and were mounted
using ProLong® gold antifade reagent (cat. no. p36930;
Thermo Fisher Scientific, Inc.). The cell nuclei were stained using
4′,6-diamidino-2-phenylindole (Invitrogen; Thermo Fisher
Scientific, Inc.), following several washes. For FSHR staining, the
slides were blocked with normal goat serum (cat. no. 36119ES03;
Yeasen, Shanghai, China) for 20 min, after which they were
incubated with rabbit polyclonal anti-FSHR (1:100; cat. no. A3172;
ABclonal Biotechnology, Co., Ltd., Hubei, China) overnight at 4°C,
followed by anti-rabbit fluorescein isothiocyanate-conjugated
immunoglobulin G (1:200; cat. no. bs-0295G; Bioss Co., Beijing,
China) for 60 min. After washing with phosphate-buffered saline,
the slides were mounted using mounting medium, and the slides were
examined using an Olympus BX61 fluorescence microscope (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analyses were conducted using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard error of the mean. The mean values were compared
using one-way analysis of variance, followed by post-hoc tests:
Tukey's honest significant difference test or Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
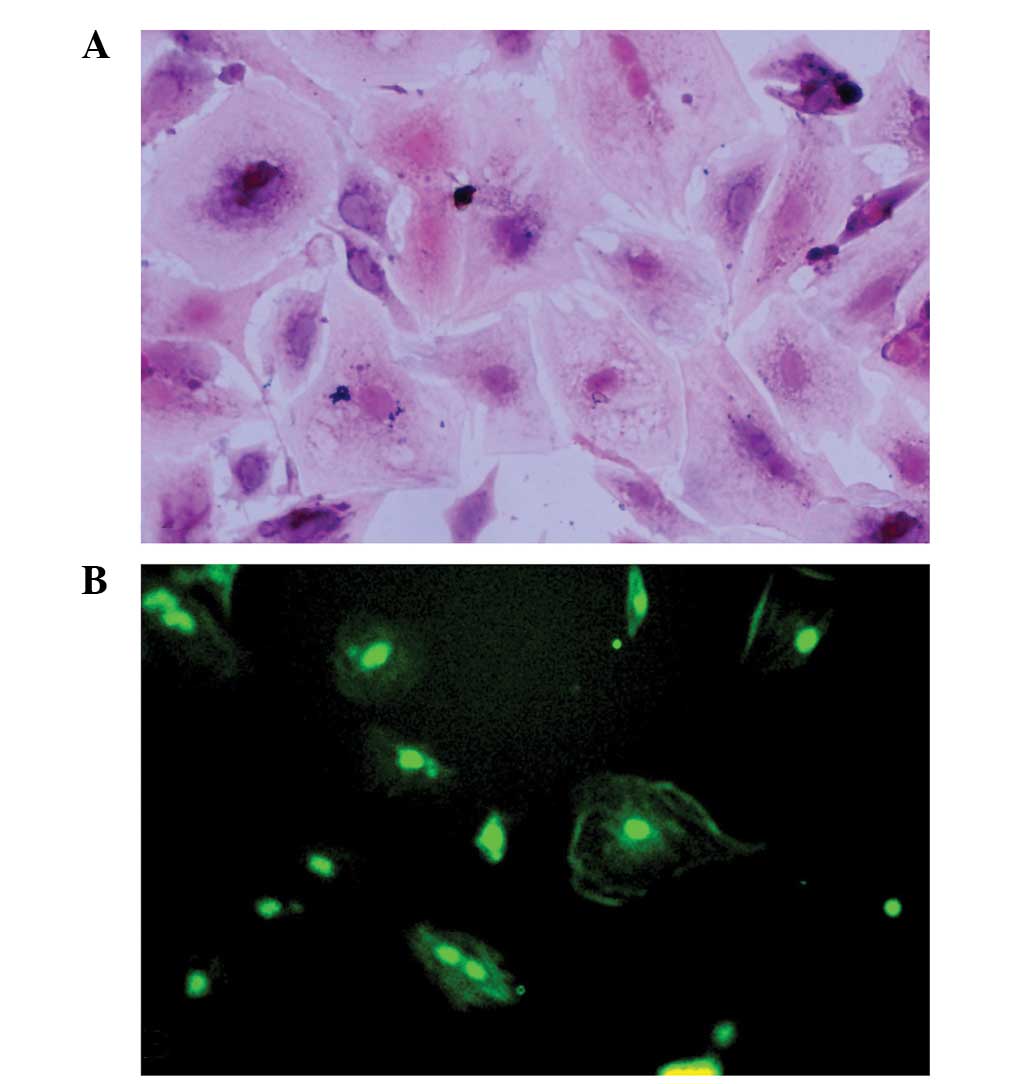
Granulosa cell identification
Granulosa cells were identified using trypan blue
staining, HE staining and FSHR immunostaining. The cell viability
of the granulosa cells was 80–90%, as demonstrated by trypan blue
staining. The purity of the granulosa cells was high, as
demonstrated by the fact that >90% of the cells were
FSHR-positive (Fig. 1).
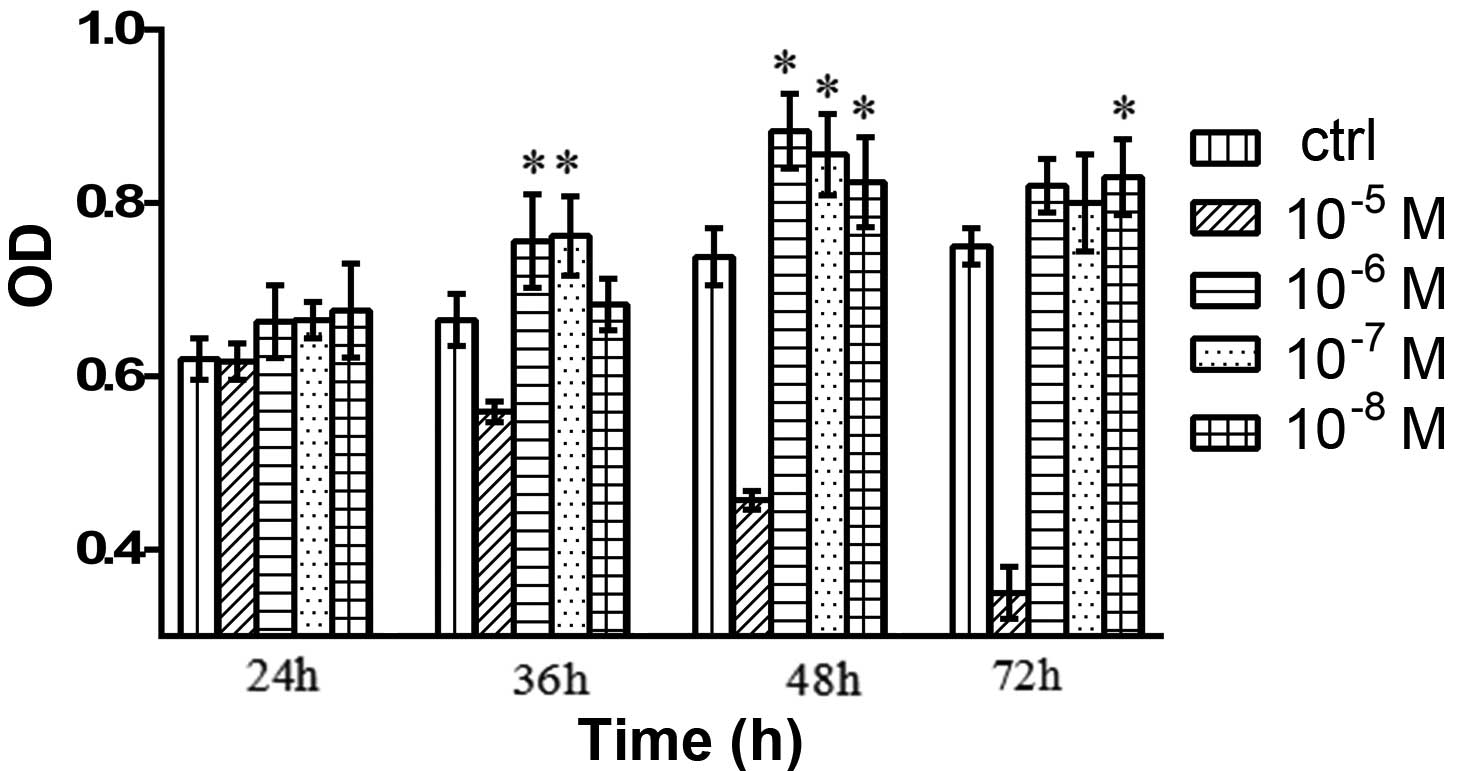
Effects of testosterone on the cell
viability of granulosa cells
As compared with the control group, the cells
treated with 10−8, 10−7, 10−6 and
10−5 M testosterone exhibited markedly increased
viability, and those treated with 10−6 M testosterone
showed the greatest increase in viability (Fig. 2).
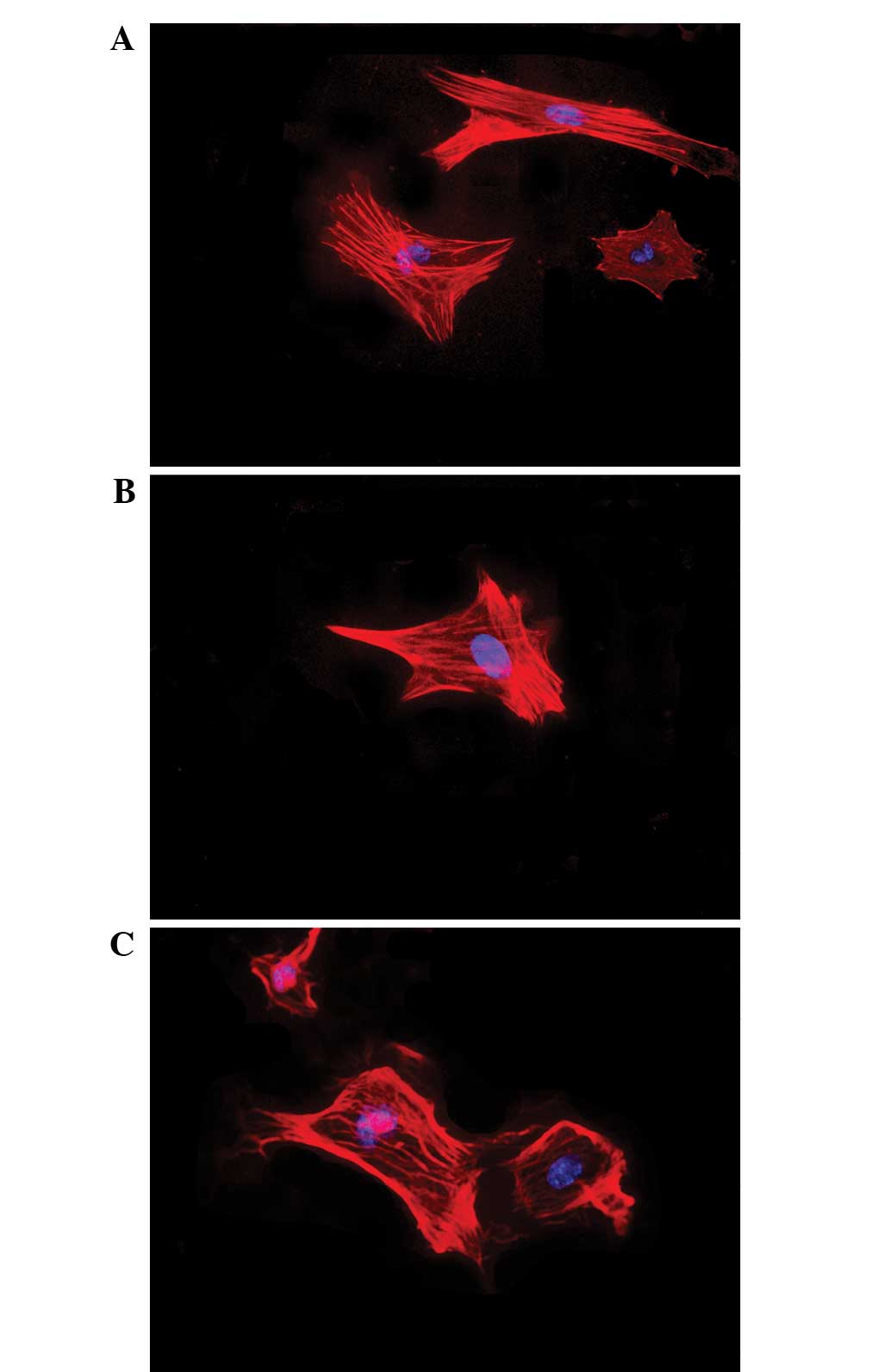
Effects of testosterone on cell
morphology
The effects of testosterone on the nuclear and
cytoskeletal morphology of granulosa cells are presented in
Fig. 3. Following 48 h of exposure
to testosterone (10−7 or 10−6 M), the
morphology of the granulosa cells remained unaltered, consisting of
large oval-shaped nuclei and well-organized F-actin fibers.
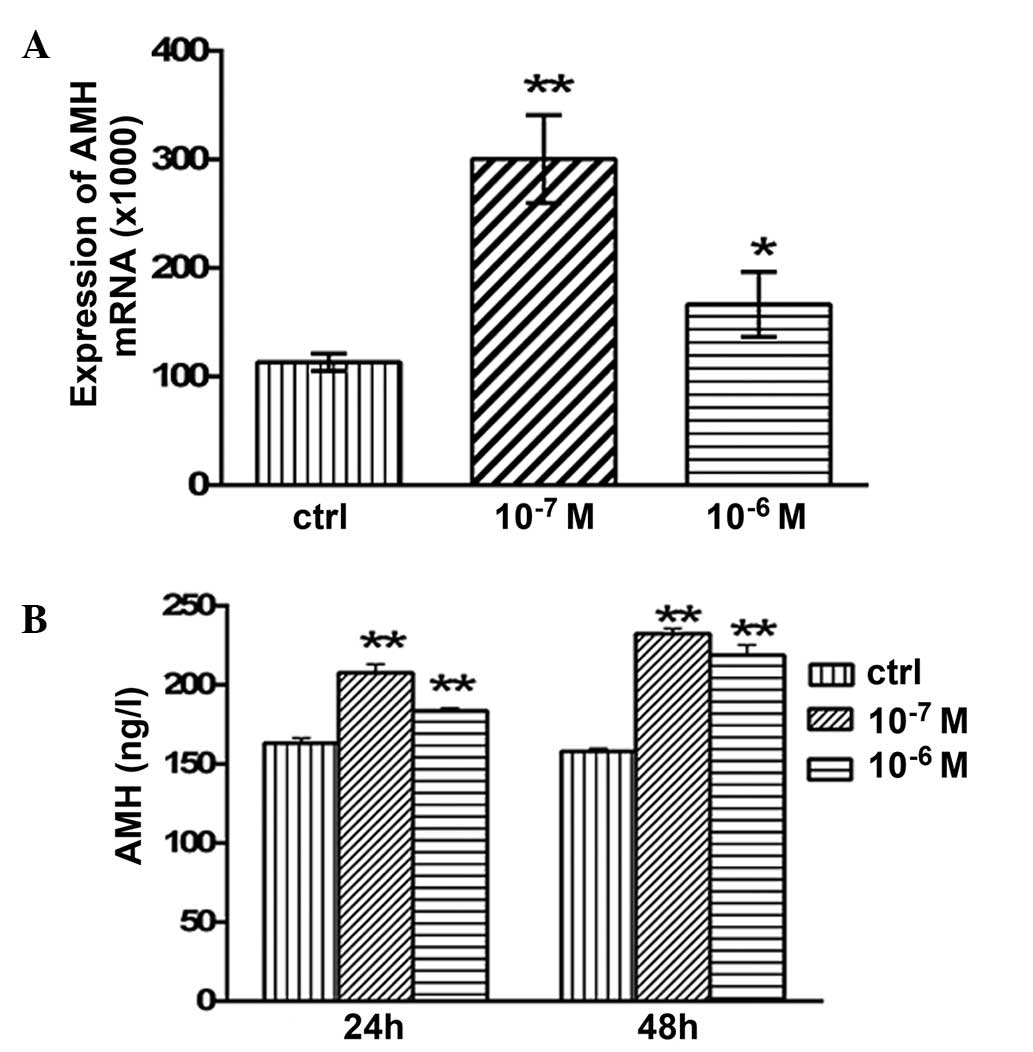
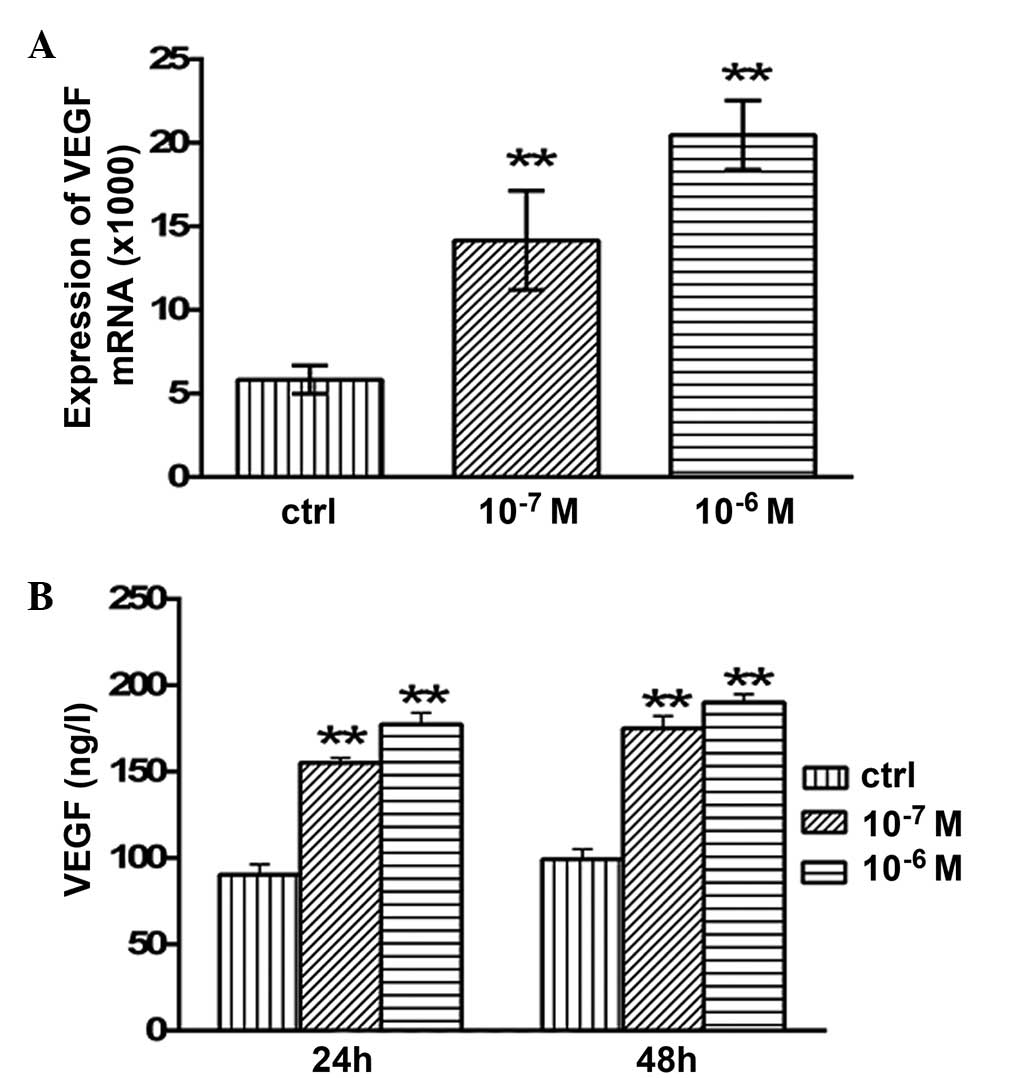
Effects of testosterone on the mRNA
expression levels and secretion of AMH
The mRNA expression levels of AMH in the granulosa
cells are presented in Fig. 4.
Following 48 h of treatment with 10−7 and
10−6 M testosterone, the mRNA expression levels of AMH
were significantly increased (P<0.05 and P<0.01,
respectively; Fig. 4A) compared with
the control group. The concentration of secreted AMH in the
granulosa cell culture medium was examined using ELISA. After 24 h
of treatment with 10−7 and 10−6 M
testosterone, the concentration of secreted AMH was 207.64±5.34
(P<0.01) and 183.64±1.86 ng/l (P<0.01), respectively, which
was significantly higher compared with the control group
(163.2±3.05 ng/l; Fig. 4B). In
addition, after 48 h of treatment with 10−7 and
10−6 M testosterone, the concentration of secreted AMH
was 232.4±3.05 (P<0.01) and 218.93±6.28 ng/l (P<0.01),
respectively, which was significantly higher compared with that of
the control group (157.94±1.94 ng/l; Fig
4B).
Effects of testosterone on the mRNA
expression levels and secretion of VEGF
After 48 h of treatment with 10−7 and
10−6 M testosterone, the mRNA expression levels of VEGF
in the granulosa cells were significantly increased (P<0.01)
compared with the control group (Fig.
5A). In addition, the concentration of secreted VEGF protein
was significantly increased following testosterone treatment. After
24 h of treatment with 10−7 and 10−6 M
testosterone, the concentration of secreted VEGF protein was
155.2±2.91 (P<0.01) and 177.34±6.64 ng/l (P<0.01), which was
significantly increased compared with the control group (90.4±6.01
ng/l; Fig. 5B). After 48 h of
treatment with 10−7 and 10−6 M testosterone,
the concentration of VEGF in the granulosa cell culture medium was
175.13±7.13 (P<0.01) and 190.18±4.79 ng/l (P<0.01),
respectively, which was significantly higher than that of the
control group (99.26±5.89 ng/l; Fig.
5B).
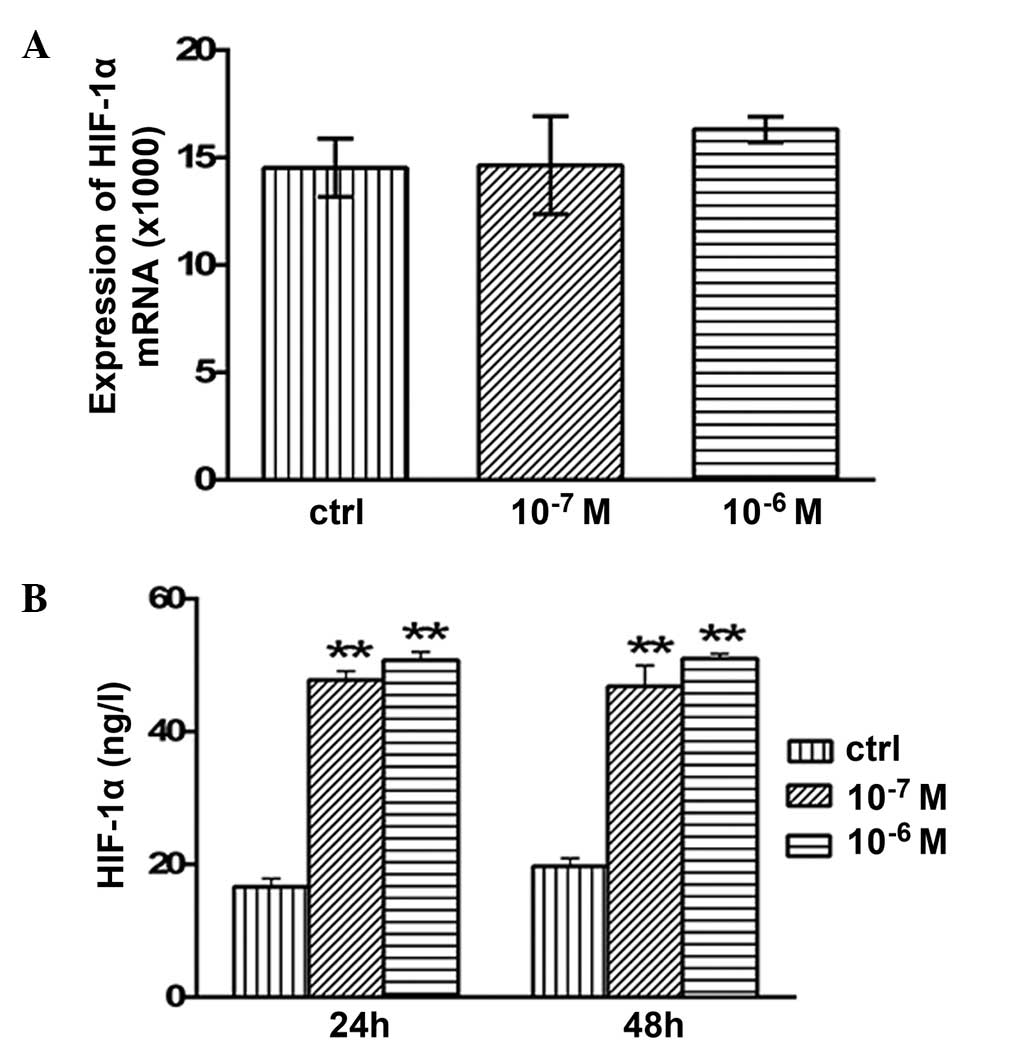
Effects of testosterone on the mRNA
expression levels and secretion of HIF-1α
No significant alterations in the mRNA expression
levels of HIF-1α were detected following 48 h of treatment with
10−7 or 10−6 M testosterone (P>0.05;
Fig. 6A). However, the concentration
of secreted HIF-1α protein was 47.76±1.35 (P<0.01) and
50.77±1.24 ng/l (P<0.01) after 24 h of treatment with
10−7 or 10−6 M testosterone, respectively;
this was significantly higher compared with the concentration in
the control group (16.6±1.29 ng/l; Fig.
6B). In addition, after 48 h of treatment with 10−7
and 10−6 M testosterone, HIF-1α protein secretion was
46.83±3.12 (P<0.01) and 51.0±0.73 ng/l (P<0.01), which was
significantly increased compared with the control group (19.72±1.2
ng/l; Fig. 6B).
Discussion
The results of the present study demonstrated that
testosterone was able to increase the mRNA expression levels and
secretion of AMH, VEGF and HIF-1α in cultured mouse granulosa
cells. In addition, testosterone treatment did not exert
discernible effects on granulosa cell morphology.
A previous study of primates demonstrated that
testosterone may have a role in folliculogenesis (13,14).
Short-term administration of testosterone in rhesus monkeys
increased the number of preantral follicles, and promoted the
proliferation of theca and granulosa cells in ovarian follicles,
whereas it decreased ovarian follicle apoptosis, as compared with a
placebo (13). In addition,
testosterone has been shown to regulate secretion from granulosa
cells, which in turn may alter the follicular microenvironment
(15).
AMH, which is a member of the transforming growth
factor-α family, is able to suppress the cyclical recruitment of
primordial follicles. It is predominantly produced by preantral and
early antral follicles, which are thought to serve as a proxy for
various primordial follicles in the ovaries (16,17). The
present study demonstrated that testosterone was able to promote
the upregulation and secretion of AMH in granulosa cells, thus
suggesting that testosterone was able to affect follicular growth
and development. A previous study reported that prenatal
testosterone treatment was able to decrease the protein expression
levels of AMH in the granulosa cells of preantral follicles,
whereas it was able to increase its expression levels in those of
antral follicles (18). The majority
of the granulosa cells used in the present study were derived from
cumulus granulosa cells and antral follicles. However, the specific
mechanism underlying the regulation of AMH by testosterone requires
further investigation.
VEGF is produced by follicular granulosa and ovarian
theca cells in response to gonadotropin stimulation (19). VEGF was previously shown to initiate
ovarian angiogenesis and to increase the permeability of blood
vessels during the normal ovarian cycle (20). The present study demonstrated that
testosterone was able to increase the mRNA and protein expression
levels of VEGF in granulosa cells, which may serve to improve blood
supply to the ovaries.
HIF-1α is a heterodimeric transcription factor,
which is produced by mammals and humans under anoxic conditions.
HIF-1α is the major regulatory factor in mammals for maintaining
the oxygen balance, and oxygen is closely associated with the
utilization of glucose in granulosa cells (7). However, the function of HIF-1α in
granulosa cells has rarely been studied. In the present study,
testosterone treatment increased the secretion of the HIF-1α
protein. These results suggested that testosterone may improve the
oxygen supply in granulosa cells and the energy metabolism in
ovarian follicles, and thus contribute to follicular development. A
previous study demonstrated that inhibition of VEGF in the primate
ovary upregulates HIF-1α expression levels in follicles (7). Consistent with this finding, the
increased HIF-1α secretion in the present study may be associated
with upregulation of VEGF upon testosterone treatment.
Previous studies have suggested that testosterone
was able to improve ovarian activity (21,22);
however, few studies have investigated the association between
testosterone and AMH, VEGF and HIF-1α in granulosa cells. The
results of the present study suggested that testosterone was able
to regulate the expression and secretion of AMH, VEGF and HIF-1α;
however, the underlying mechanisms require further
investigation.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Liaoning Province (no. 201102134) and
the Jinzhou Municipal Science and Technology Program (no. 12A1E35).
The authors would like to thank the Department of Key Laboratory of
Tissue Engineering of the First Affiliated Hospital of Jinzhou
Medical University for technical assistance.
References
|
1
|
Trivax B and Azziz R: Diagnosis of
polycystic ovary syndrome. Clin Obstet Gynecol. 50:168–177. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Azziz R, Carmina E, Dewailly D,
Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen
OE, Legro RS, Norman RJ, Taylor AE and Witchel SF: Androgen Excess
Society: Positions statement: Criteria for defining polycystic
ovary syndrome as a predominantly hyperandrogenic syndrome: An
Androgen Excess Society guideline. J Clin Endocrinol Metab.
91:4237–4245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barber TM, Wass JA, McCarthy MI and Franks
S: Metabolic characteristics of women with polycystic ovaries and
oligo-amenorrhoea but normal androgen levels: Implications for the
management of polycystic ovary syndrome. Clin Endocrinol (Oxf).
66:513–517. 2007.PubMed/NCBI
|
|
4
|
Dokras A, Clifton S, Futterweit W and Wild
R: Increased risk forabnormal depression scores in women with
polycystic ovary syndrome: A systematic review and meta-analysis.
Obstet Gynecol. 117:145–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Solomon CG: The epidemiology of polycystic
ovary syndrome. Prevalence and associated disease risks. Endocrinol
Metab Clin North Am. 28:247–263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schneider JG, Tompkins C, Blumenthal RS
and Mora S: The metabolic syndrome in women. Cardiol Rev.
14:286–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duncan WC, van den Driesche S and Fraser
HM: Inhibition of vascular endothelial growth factor in the primate
ovary up-regulates hypoxia-inducible factor-1alpha in the follicle
and corpus luteum. Endocrinology. 149:3313–3320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011.PubMed/NCBI
|
|
9
|
van Disseldorp J, Faddy MJ, Themmen AP, de
Jong FH, Peeters PH, van der Schouw YT and Broekmans FJ:
Relationship of serum antimüllerian hormone concentration to age at
menopause. J Clin Endocrinol Metab. 93:2129–2134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freeman EW, Gracia CR, Sammel MD, Lin H,
Lim LC and Strauss JF III: Association of anti-mullerian hormone
levels with obesity in late reproductive-age women. Fertil Steril.
87:101–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HX, Li TY and Kidder GM: WNT2
regulates DNA synthesis in mouse granulosa cells through
beta-catenin. Biol Reprod. 82:865–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vendola KA, Zhou J, Adesanya OO, Weil SJ
and Bondy CA: Androgens stimulate early stages of follicular growth
in the primate ovary. J Clin Invest. 101:2622–2629. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weil SJ, Vendola K, Zhou J, Adesanya OO,
Wang J, Okafor J and Bondy CA: Androgen receptor gene expression in
the primate ovary: Cellular localization, regulation, and
functional correlations. J Clin Endocrinol Metab. 83:2479–2485.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duda M: The influence of FSH: LH and
testosterone on steroidsecretion by two subpopulations of porcine
granulosa cells. J Physiol Pharmacol. 48:89–96. 1997.PubMed/NCBI
|
|
16
|
Seifer DB and Maclaughlin DT: Mullerian
inhibiting Substance is an ovarian growth factor of emerging
clinical significance. Fertil Steril. 88:539–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tal R and Seifer DB: Potential mechanisms
for racial and ethnic differences in antimüllerian hormone and
ovarian reserve. Int J Endocrinol. 2013:8189122013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Veiga-Lopez A, Ye W and Padmanabhan V:
Developmental programming: Prenatal testosterone excess disrupts
anti-Müllerian hormone expression in preantral and antral
follicles. Fertil Steril. 97:748–756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fraser HM and Duncan WC: Vascular
morphogenesis in the primate ovary. Angiogenesis. 8:101–116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kisliouk T, Levy N, Hurwitz A and Meidan
R: Presence and regulation of endocrine gland vascular endothelial
growth factor/prokineticin-1 and its receptors in ovarian cells. J
Clin Endocrinol Metab. 88:3700–3707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grzesiak M, Williams L and Luck MR:
Testosterone influences water transport in porcine granulosa cells.
Reprod Domest Anim. 48:e52–e54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanderhyden BC and Macdonald EA: Mouse
oocytes regulate granulosa cell steroidogenesis throughout
follicular development. Biol Reprod. 59:1296–1301. 1998. View Article : Google Scholar : PubMed/NCBI
|