Introduction
Human infections involving Aspergillus
species are being characterized with growing frequency in
immunocompromised hosts; Aspergillus fumigatus causes ~80%
of invasive aspergillosis, and the second most common pathogenic
species is Aspergillus flavus, followed by Aspergillus
niger and Aspergillus terreus (1). A. flavus is a mold that exists
worldwide. Environment and geographical conditions are significant
determinants of the local frequency of A. flavus infections
(2). The identification of A.
flavus is not simple because of its similarities with species
that are closely related (3).
Variability exists in the phenotype of A.
flavus, for example, isolates with the potential to produce
aflatoxin have been reported (4).
Therefore, the ability to distinguish between different strains of
A. flavus is valuable for diagnosis. The genomic analysis of
DNA using polymerase chain reaction (PCR)-based methods is a
sensitive, fast and reliable approach for the determination of
genetic connections between microorganisms (5,6). The
internal transcribed spacer (ITS) region is an effective target for
phylogenetic analysis in fungi (7);
the ITS region is frequently variable between different isolates of
the same species (8,9).
The development of molecular methods for the genetic
differentiation of fungal species has advanced their taxonomy as a
result of increased sensitivity and specificity. PCR amplification
of ITS regions of ribosomal DNA (rDNA) (10,11),
combined with the sequencing of amplified regions and the analysis
of these by comparing them with sequences that are deposited in
GenBank, has been commonly employed for the detection of fungal
species (11). However, variations
in a sequence of DNA could be recognized using restriction fragment
length polymorphism (RFLP), which can distinguish minor differences
in nucleotides that may not be expressed at the protein level. RFLP
may be able to identify changes in noncoding regions of DNA,
recognize closely related organisms using DNA fingerprints and
infer phylogenetic relations.
In the current study, the genetic variability among
A. flavus isolates was analyzed. The samples included were
reference strains, and clinical and environmental isolates. RFLP of
the PCR fragments of the ITS region were used to analyze the
isolates. The primary aim of the present study was to genetically
distinguish numerous strains of A. flavus, isolated from
various sources, using PCR and RFLP.
Materials and methods
Isolates of A. flavus
A total of 62 A. flavus isolates were used in
the present study. Ten reference strains, including A.
flavus PFCC101, PFCC123, PFCC124, PFCC125, PFCC126, PFCC159,
PFCC209, PFCC170, PFCC173 and PFCC106-139, and 25 clinical and 27
environmental isolates of A. flavus were included. Reference
strains were obtained from the Pasteur Institute of Iran (Tehran,
Iran). The clinical isolates were kindly provided by Dr Hossein
Zarrinfar (Mashhad University of Medical Sciences, Mashhsad, Iran),
Dr Sadegh Khodavaisi (Tehran University of Medical Sciences,
Tehran, Iran) and Dr Parvin Dehghan (Isfahan University of Medical
Sciences, Isfahan, Iran). The environmental isolates were obtained
from soil or air samples collected in Ahvaz, Iran. The isolates
were kept on Sabouraud dextrose agar (Merck KGaA, Darmstadt,
Germany) at room temperature. All A. flavus isolates were
identified by morphology. Isolates were subcultured three times to
obtain a pure culture and stained with lactophenol aniline blue.
The conidial arrangement, philiades, vesicles and conidiophores
were observed under a light microscope for morphological
characterization.
DNA extraction
Thick spore suspension (1 ml) from each isolate was
transferred to an Erlenmeyer flask with 50 ml yeast extract peptone
dextrose medium (Merck KGaA). Following inoculation, the flasks
were kept at 200 rpm under agitation at 37°C for 48 h in order to
allow for mycelia growth. The mycelia were harvested with filters,
washed with 0.5 M ethylenediamine tetraacetic acid (EDTA) and
sterile distilled water (dH2O) and freeze-dried at −70°C
for DNA extraction. The mycelia were then ground into a fine powder
using a pestle and mortar. The powder (~100 mg) was then
transferred into a 1.5-ml sterile tube, and 400 µl lysis buffer
(100 mM Tris-HCl, pH 8.0, 30 mM EDTA, pH 8.0 and sodium dodecyl
sulfate 5% w/v) was added.
The microtubes were kept at 100°C for 20 min, and
150 µl 3 M acetate potassium was added to each tube. The suspension
was kept at −20°C for 10 min, and centrifuged at 14,000 × g and 4°C
for 10 min. Following transfer of the supernatant to a 1.5-ml
Eppendorf tube, 250 µl phenol-chloroform-isoamyl alcohol (25:24:1,
v/v) was added, and the solution was briefly was vortexed and
centrifuged at 14,000 × g for 10 min. The upper aqueous phase was
transferred to a new 1.5 ml microtube and 250 µl chloroform-isoamyl
alcohol (24:1) was added. The samples were then briefly vortexed
and centrifuged at 4°C and 14,000 × g for 10 min. The supernatant
was transferred to another microtube, an equal volume of iced-cold
2-propanol was added, and samples were kept in −20°C for 10 min and
then centrifuged at 14,000 × g for 10 min. The upper aqueous phase
was discarded and the pellet was washed with 300 µl 70% ethanol.
Following the removal of ethanol, DNA pellets were air dried and
dissolved in 50 µl dH2O.
PCR amplification
Molecular identification of the ITS region of each
A. flavus isolate was performed using the ITS1
(5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′)
primers. PCR reactions were performed using a final volume of 50
µl, containing reaction buffer, 2.2 mM MgCl2, 200 µM
each dNTP (dATP, dCTP, dGTP and dTTP), 2.5 units Taq DNA
polymerase (all CinnaGen, Tehran, Iran), 100 ng template DNA and 50
pmol of each primer. The amplification conditions were as follows:
Initial denaturation at 94°C for 5 min; 35 cycles of denaturation
at 94°C for 2 min, annealing at 53°C for 2 min and extension at
72°C for 2 min; and final extension at 72°C for 30 min. The PCR
products were separated by 1.2% agarose gel electrophoresis in a
Tris base, acetic acid and EDTA buffer, and stained with ethidium
bromide. PCR amplification of the ITS region yielded a 595-bp
band.
Restriction site analysis of PCR
products
Following amplification, the PCR products were
digested with the restriction endonucleases HaeIII,
EcoRI and TagI (Fermentas; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The reaction for each enzyme was performed
in a total volume of 20 µl containing 10 units enzyme, 2 µl buffer
(500 mM KCl and Tris-HCl, pH 8.4), 8 µl PCR product and ultrapure
water. The fragments were separated on a 1.2% agarose gel by
electrophoresis and stained with ethidium bromide.
A number of amplicons were submitted for direct
sequencing (Bioneer Corporation, Daejeon, South Korea). The
obtained sequences were searched for in the NCBI database
(http://www.ncbi.nlm.nih.gov/). The
sequences had 100% identity with A. flavus sequences
deposited in the NCBI database. The computer software package MEGA5
(http://www.megasoftware.net) was used
for alignment of sequences.
Results
Molecular variation analysis of A.
flavus isolates
Using ITS1 and ITS4 primers, a unique band of ~595
bp was obtained for all tested A. flavus isolates (Fig. 1). The results following digestion
with restriction enzymes indicate that A. flavus isolates
vary in the ITS region. The results suggest the existence of
variation among A. flavus isolates. The pattern of the
ITS-RFLP bands obtained following the cleavage of the PCR products
with the restriction enzymes EcoRI, HaeIII and
TaqI showed genetic variability among the isolates that
varied in the size and number of fragments (Figs. 2–5).
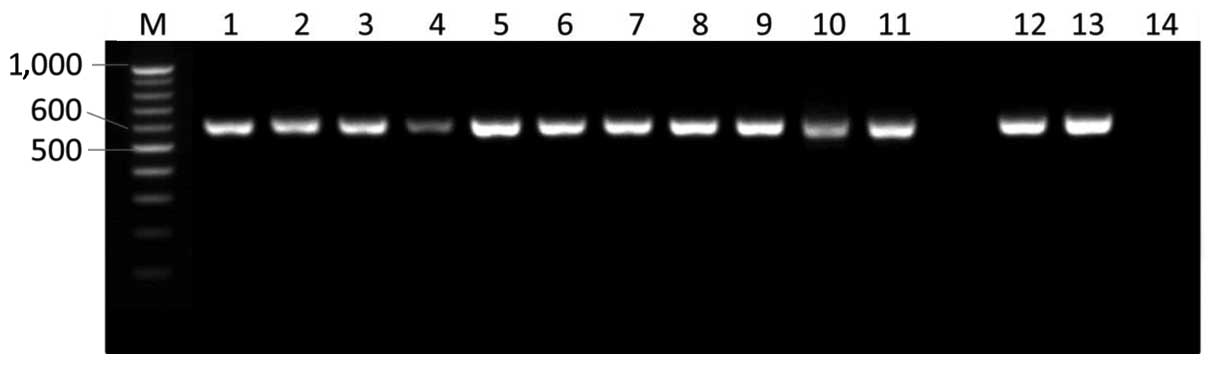 | Figure 1.Internal transcribed spacer (ITS)
regions of Aspergillus flavus isolates were amplified by
polymerase chain reaction using ITS1 and ITS4 primers and the
products were separated by agarose gel electrophoresis. M, 100 bp
ladder; lane 1, Kh4 isolate; lane 2, Kh5 isolate; lane 3, Kh6
isolate; lane 4, Kh9 isolate; lane 5, Kh10 isolate; lane 6, Kh11
isolate; lane 7, M25 isolate; lane 8, M26 isolate; lane 9, M27
isolate; lane 10, M28 isolate; lane 11, M29 isolate; lane 12, M32
isolate; lane 13, M33 isolate; lane 14, no template control. |
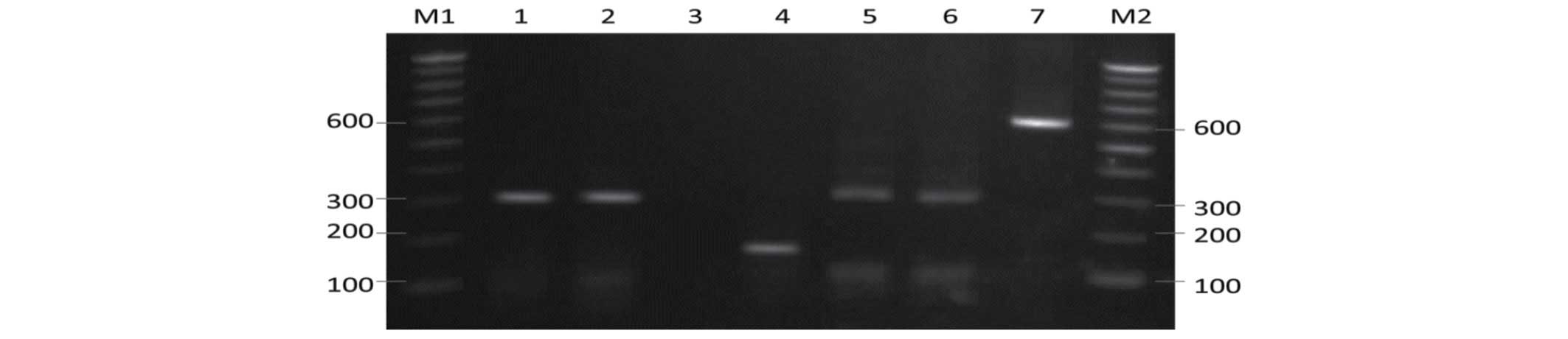
 | Figure 2.Restriction fragment pattern of
internal transcribed spacer (ITS) polymerase chain reaction (PCR)
products of Aspergillus flavus digested with EcoRI.
Lane M1, 100 bp ladder; lane 1, Z7 isolate; lane 2, Z8 isolate;
lane 3, Z9 isolate; lane 4, Z10 isolate; lane 5, PFCC101 isolate;
lane 6, PFCC126 isolate; lane 7, PFCC159 isolate; lane 8, PFCC209
isolate; lane 9, PFCC170 isolate; lane 10, PFCC173 isolate; lane
11, undigested ITS PCR product; lane M2, 1 kb ladder. |
Digestion of the ITS amplicons with EcoRI
produced the expected 300-bp fragment for 59 of the 62 isolates
(Fig. 2). Three clinical isolates
did not present any fragments following digestion with
EcoRI.
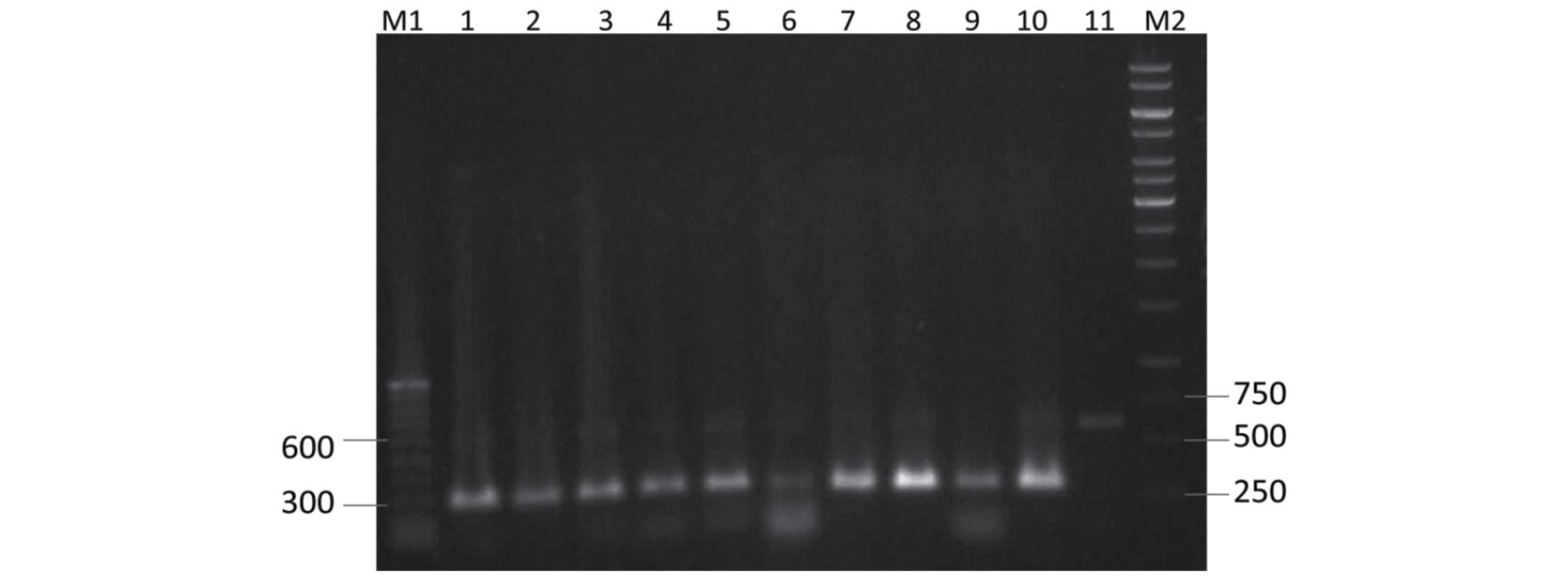
Restriction maps of the PCR product of the ITS
region fragments allowed the identification of a restriction
endonuclease, TaqI, which could be used to differentiate
A. flavus isolates. Following PCR amplification of the ITS
region cut with a TaqI enzyme, the PCR product produced two
fragments, ~150 and 250 bp in size, for 59 of the 62 isolates.
Three isolates, including 2 clinical and 1 environmental isolate,
showed one band, ~150 bp in size (Fig.
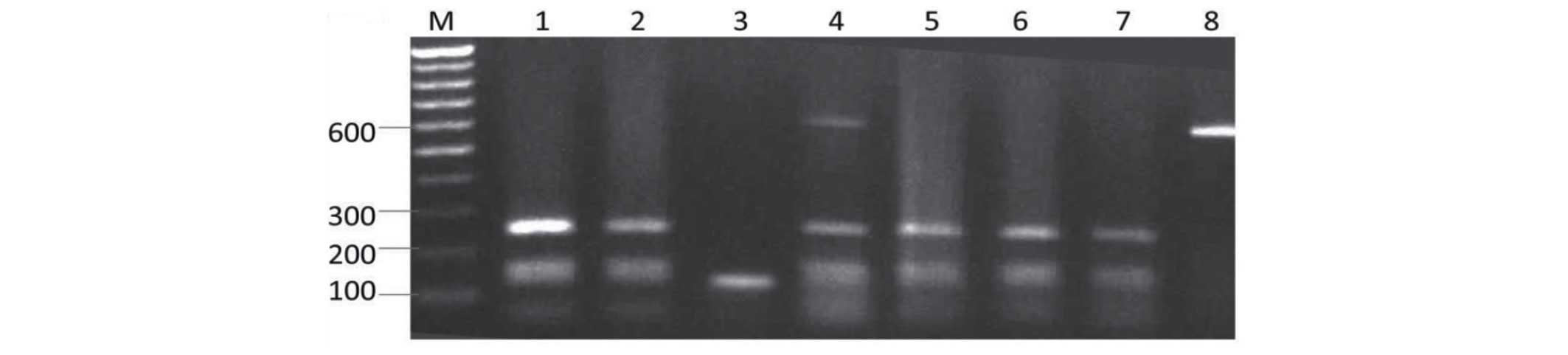
3). Digestion of the ITS amplicons with HaeIII resulted
in more restriction patterns, as compared with TaqI and
EcoRI (Figs. 4 and 5).
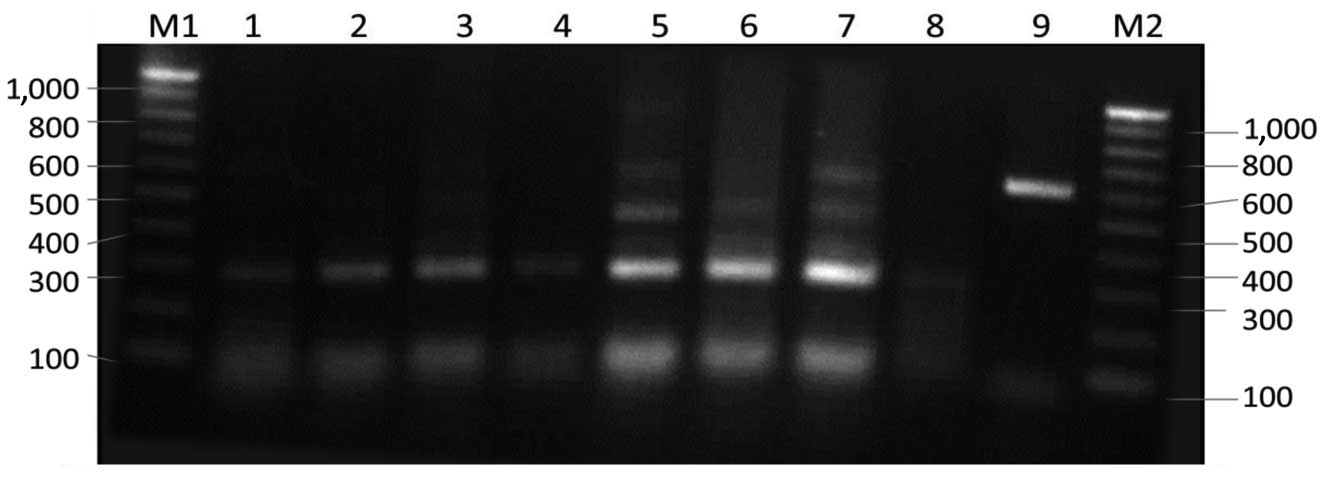 | Figure 4.Restriction fragment pattern of
internal transcribed spacer (ITS) polymerase chain reaction (PCR)
products of Aspergillus flavus digested with HaeIII.
Lane M1, 100 bp ladder; lane 1, M2 isolate; lane 2, M4 isolate;
lane 3, Kh1 isolate; lane 4, Kh2 isolate; lane 5, Kh4 isolate; lane
6, Kh5 isolate; lane 7, Kh6 isolate; lane 8, Kh8 isolate; lane 9,
undigested ITS PCR product; lane M2, 100 bp ladder. |
The PCR products of the ITS region of 3 isolates
were sequenced and aligned with references in the NCBI database.
The sequences had 100% identity with A. flavus sequences
deposited in the NCBI database.
Discussion
Methods in molecular biology have been efficiently
employed for the rapid identification of microorganisms, and for
overcoming the limitations associated with conventional direct
culture analysis (12). Fungal rDNA
has been demonstrated to include regions that are variable within
genera. The ITS region of nuclear rDNA, including the intervening
5.8S rRNA gene, ITS1 and ITS2, has been extensively used to
investigate the variability in fungal species and subspecies.
Restriction enzyme map analysis of the ITS regions
has been employed to study the genetic diversity among the fungal
isolates of various types (13,14).
Variations in DNA sequences can be identified using PCR-RFLP, which
is able to identify minor differences in nucleotides (11). Henry et al (7) reported that ITS1 and ITS2 are required
to accurately identify the species of Aspergillus. Huang
et al (15) reported
interspecies variability in the ITS2 region, and used this
dissimilarity to design microarray probes for the detection of
pathogenic fungi.
In the present study, isolates of A. flavus
were analyzed using ITS-RFLP to evaluate the genetic variability
among them. A total of 62 A. flavus isolates were tested for
genetic variability in the ITS regions. The primers ITS1 and ITS4
amplified successfully all the ITS region isolates tested using
conventional PCR. Sequence analysis indicates that the restriction
enzymes EcoRI, HaeIII and TaqI can cleave the
PCR products into fragments that are useful tools for the detection
of specific strains.
Numerous techniques have been developed for the
systematic investigation of fungi, including random amplified
polymorphic DNA, and diagnosis based on specific PCR primers
(16) and sequencing (17,18).
However, the techniques used are frequently based on rRNA (or rDNA)
gene analysis sequences that are universal and include conserved
and variable regions, and permit the discrimination of fungi at
different taxonomic levels (18,19).
Analysis of PCR-amplified rDNA sequences with
restriction enzymes has been shown to be an appropriate approach
for taxonomic studies in several Aspergillus and
Fusarium species (20–23).
RFLP analysis of ITS regions has demonstrated that the quantity of
the carcinogenic metabolite aflatoxin B1 produced by
isolates of A. flavus ranges between 1.9 and 206.6 ng/ml,
with the variability being suggested to be due to differences in
genetic composition (24).
In conclusion, the present study demonstrated that
restriction fragments of the amplified ITS regions of A.
flavus isolates are effective for the identification of
different strains. The restriction enzyme found to be the most
effective in the discrimination of isolates in the current study
was HaeIII, followed by TaqI and EcoRI.
Acknowledgements
The present study is based on an MSc thesis by Mrs.
Maryam Erfaninejad, which was supported by the Health Research
Institute, Infectious and Tropical Diseases Research Center,
Jundishapur University of Medical Sciences, Ahvaz, Iran (grant no.
92104).
References
|
1
|
Krishnan S, Manavathu EK and Chandrasekar
PH: Aspergillus flavus: An emerging non-fumigatus
Aspergillus species of significance. Mycoses. 52:206–222.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pasqualotto AC: Differences in
pathogenicity and clinical syndromes due to Aspergillus
fumigatus and Aspergillus flavus. Med Mycol. 47(Suppl
1): S261–S270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ehrlich KC and Mack BM: Comparison of
Expression of Secondary Metabolite Biosynthesis Cluster Genes in
Aspergillus flavus, A. parasiticus, and A.
oryzae. Toxins (Basel). 6:1916–1928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karthikeyan M, Sandosskumar R,
Mathiyazhagan S, Mohankumar M, Valluvaparidasan V, Kumar S and
Velazhahan R: Genetic variability and aflatoxigenic potential of
Aspergillus flavus isolates from maize. Arch Phytopathol
Plant Protect. 42:83–91. 2009. View Article : Google Scholar
|
|
5
|
Zhang ZG, Zhang JY, Zheng XB, Yang YW and
Ko WH: Molecular distinctions between Phytophthora capsici
and P. tropicalis based on ITS sequences of ribosomal DNA. J
Phytopathol. 152:358–364. 2004. View Article : Google Scholar
|
|
6
|
Khoodoo MHR and Jaufeerally-Fakim Y:
RAPD-PCR fingerprinting and southern analysis of Xanthomonas
axonopodis pv. Dieffenbachiae strains isolated from
different aroid hosts and locations. Plant Dis. 88:980–988. 2004.
View Article : Google Scholar
|
|
7
|
Henry T, Iwen PC and Hinrichs SH:
Identification of Aspergillus species using internal
transcribed spacer regions 1 and 2. J Clin Microbiol. 38:1510–1515.
2000.PubMed/NCBI
|
|
8
|
Gomes EA, Maria Kasuya CM, de Barros EG,
Borges AC and Araújo EF: Polymorphism in the internal transcribed
spacer (ITS) of the ribosomal DNA of 26 isolates of ectomycorrhizal
fungi. Genet Mol Biol. 25:477–483. 2002. View Article : Google Scholar
|
|
9
|
Krimitzas A, Pyrri I, Kouvelis VN,
Kapsanaki-Gotsi E and Typas MA: A phylogenetic analysis of Greek
isolates of Aspergillus species based on morphology and
nuclear and mitochondrial gene sequences. Biomed Res Int.
2013:2603952013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Criseo G, Bagnara A and Bisignano G:
Differentiation of aflatoxin producing and non-producing strains of
Aspergillus flavus group. Lett Appl Microbiol. 33:291–295.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen RS, Tsay JG, Huang YF and Chiou RY:
Polymerase chain reaction-mediated characterization of molds
belonging to the Aspergillus flavus group and detection of
Aspergillus parasiticus in peanut kernels by multiplex
polymerase chain reaction. J Food Prot. 65:840–844. 2002.PubMed/NCBI
|
|
12
|
Toju H, Tanabe AS, Yamamoto S and Sato H:
High-coverage ITS primers for the DNA-based identification of
ascomycetes and basidiomycetes in environmental samples. PLoS One.
7:e408632012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diguta CF, Vincent B, Guilloux-Benatier M,
Alexandre H and Rousseaux S: PCR ITS-RFLP: A useful method for
identifying filamentous fungi isolates on grapes. Food Microbiol.
28:1145–1154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pereira F, Carneiro J and Amorim A:
Identification of species with DNA-based technology: Current
progress and challenges. Recent Pat DNA Gene Seq. 2:187–199. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang A, Li JW, Shen ZQ, Wang XW and Jin
M: High-throughput identification of clinical pathogenic fungi by
hybridization to an oligonucleotide microarray. J Clin Microbiol.
44:3299–3305. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nicholson P, Simpson DR, Weston G,
Rezanoor HN, Lees AK, Parry DW and Joyce D: Detection and
quantification of Fusarium culmorum and Fusarium
graminearum in cereals using PCR assays. Physiol Mol Plant
Pathol. 53:17–37. 1998. View Article : Google Scholar
|
|
17
|
O'Donnell K, Cigelnik E and Nirenberg HI:
Molecular systematics and phylogeography of the Gibberella
fujikuroi species complex. Mycologia. 90:465–493. 1998.
View Article : Google Scholar
|
|
18
|
Paterson RRM: Identification and
quantification of mycotoxigenic fungi by PCR. Process Biochem.
71:1467–1474. 2006. View Article : Google Scholar
|
|
19
|
Ferrer C, Colom F, Frasés S, Mulet E, Abad
JL and Alió JL: Detection and identification of fungal pathogens by
PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections.
J Clin Microbiol. 39:2873–2879. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mirete S, Patiño B, Vázquez C, Jiménez M,
Hinojo MJ, Soldevilla C and González-Jaén MT: Fumonisin production
by Gibberella fujikuroi strains from Pinus species.
Int J Food Microbiol. 89:213–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
González-Salgado A, Patño B, Vázquez C and
González-Jaén MT: Discrimination of Aspergillus niger and
other Aspergillus species belonging to section Nigri
by PCR assays. FEMS Microbiol Lett. 245:353–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kizis D, Natskoulis P, Nychas GJ and
Panagou EZ: Biodiversity and ITS-RFLP characterisation of
Aspergillus section Nigri isolates in grapes from
four traditional grape-producing areas in Greece. PLoS One.
9:e939232014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dubey SC, Tripathi A and Singh SR:
ITS-RFLP fingerprinting and molecular marker for detection of
Fusarium oxysporum f.sp. ciceris. Folia Microbiol (Praha).
55:629–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohankumar M, Vijayasamundeeswari A,
Karthikeyan M, Mathiyazhagan S, Paranidharan V and Velazhahan R:
Analysis of molecular variability among isolates of Aspergillus
flavus by PCR-RFLP of the its regions of rDNA. J Plant Prot
Res. 50:446–451. 2010. View Article : Google Scholar
|