Introduction
Periodontitis is a chronic inflammatory disease in
which destruction of the tooth-supporting connective tissue and
cementum, leukocyte infiltration, bone resorption and the formation
of periodontal pockets occur (1).
The pathogenesis of periodontitis involves the presence of
bacterial plaque, which initiates a local inflammatory reaction
(2). The inflammatory response can
comprise edema, the infiltration of leukocytes and the release of
inflammatory mediators, with subsequent periodontal pocket
formation, detachment of connective tissue and alveolar bone
resorption, ultimately leading to tooth loss (3,4).
Periodontitis is most frequently caused by bacteria, with the
toxins, enzymes and metabolites associated with the bacteria in
dental plaque playing an important role in the initiation of the
inflammatory process (5). Recently,
the involvement of nitric oxide activities and oxidative stresses
in the pathogenesis of periodontitis has been revealed (6), and numerous antioxidants have shown
favorable effects on periodontitis and associated alveolar bone
loss (7–9).
Natural products are of increasing interest to
pharmaceutical industry and potential sources of new bioactive
molecules (10). Herbs, medicinal
plants and their extracts contain antioxidants that may be useful
in the treatment of various diseases (11). Persicariae Rhizoma (PR) is dried stem
parts of Persicaria tinctoria H. Gross (Polygonaceae), and
has been traditionally used as anti-inflammatory and detoxifying
agent in Korea (12). PR contains
two biologically active anti-inflammatory and antioxidative dyes,
namely purple indirubin and blue indigo (13). Indirubin, a 3,2′-bisindole isomer of
indigo, was initially identified as the active ingredient of a
traditional Chinese medicine preparation, Danggui Longhui Wan,
which is used to treat various chronic diseases (14). Indirubin derivatives exhibit strong
anti-inflammatory and anti-leukemic activities (15). It has previously been shown that
indirubin is a potent inhibitor of wide range of kinases, but, in
particular, it strongly suppresses the activation of
cyclin-dependent kinases (16).
Herbal extracts containing indigo or its derivatives also have been
shown to have potent antibacterial (17), antitumor (18), anti-inflammatory (19) and antioxidant (20) activities. Accordingly, PR is a
promising candidate for the treatment of periodontal diseases.
However, there have been no studies examining the
effects of PR on experimental periodontitis (EPD) or related
alveolar bone loss. Thus, the present comparative study of PR
aqueous extracts and indomethacin on ligature-induced EPD and
alveolar bone loss in rats was conducted.
Materials and methods
Animals
In total, 48 healthy male Sprague-Dawley (Slc:SD)
rats (Japan SLC, Inc., Shizuoka, Japan), aged 6 weeks and weighing
170–190 g, were used after acclimatization for 10 days. The rats
were housed four per polycarbonate cage in a room with controlled
temperature (20–25°C) and humidity (50–55%). The light:dark cycle
was 12 h:12 h, and standard rodent chow (Samyang Feed Co., Seoul,
South Korea) and water were supplied ad libitum. All animals
were treated according to international regulations for the usage
and welfare of laboratory animals, and approved was obtained from
the Institutional Animal Care and Use Committee of Daegu Haany
University (Gyeongsan, South Korea) prior to animal
experimentation. The rats were subdivided into six groups,
comprising two control groups (intact and EPD control) and four
treatment groups (indomethacin 5 mg/kg, and PR extracts 50, 100 and
200 mg/kg).
Preparations and administration of
test materials
Aqueous PR extracts (yield, 12.00%) were prepared by
routine methods using a rotary vacuum evaporator (Eyela; Tokyo
Rikakikai Co., Ltd., Tokyo, Japan) and programmable freeze dryer
(Operon Co., Ltd., Kimpo, South Korea) from dried stem parts of
Persicaria tinctoria H. Gross (Omniherb, Yeongcheon, South
Korea). The voucher specimens documenting this purchase were
deposited in the herbarium of the Medical Research Center for
Globalization of Herbal Formulation, Daegu Haany University.
Aqueous PR was boiled at 80°C for 3 h and then, evaporated and
lyophilized. Indomethacin (Sigma-Aldrich, St. Louis, MO, USA) was
used as a reference.
One day after ligation placement, 50, 100 or 200
mg/kg PR extracts or 5 mg/kg indomethacin was orally administered,
in a volume of 5 ml/kg dissolved in distilled water (DW), once a
day for 10 days, respectively. In the intact and EPD controls, same
volume of DW was orally administered.
Measurement of indigo and indirubin
contents in PR extracts
Standard stock solutions of indigo and indirubin
(Sigma-Aldrich) were prepared by dissolving at a concentration of 1
µg/ml in 1 ml dimethyl sulfoxide (DMSO; Sigma-Aldrich). For
preparation of samples, the appropriate amounts of PR extracts were
weighed, and dissolved in 1:1 DMSO and acetonitrile mixtures. Prior
to analysis by high-performance liquid chromatography (HPLC), the
samples were filtered. A Waters Alliance HPLC system (Waters
Corporation, Milford, MA, USA), equipped with a Waters 2489
UV/Visible detector was used for analysis. The Empower Data System
was used for recording the output signal of the detector. A Waters
YMC-Pack Pro C-18 column (1.7 µm, 2.1×100 mm) was used for
separation. The mobile phase comprised 0.1% formic acid water and
0.1% formic acid acetonitrile (Sigma-Aldrich) with the gradient
elution system at a flow rate of 1.0 ml/min. The injection volume
was 10 µl. The detection UV wavelength was set at 540 nm, and the
column temperature was room temperature.
Induction of EPD
EPD was induced by placing a sterilized nylon (3-0)
thread ligature around the cervix of the upper left incisor teeth
of the rats, under anesthetization with a 25-mg/kg intraperitoneal
injection of Zoletile 50 (Virbac Laboratories, Paris, France)
(8). The ligature was knotted on the
buccal side of the tooth, resulting in a subgingival position
palatinally and a supragingival position buccally. In intact
vehicle control rats, the cervix of the upper left incisor tooth
was identified only, instead of ligation placement.
Measurements of body weights
Changes of body weight were measured, once a day
from 1 day prior to ligature placement and throughout the
experimental period. To reduce individual differences, the body
weight gains after 10 days of administrations were also calculated
by subtracting the body weight at the start of administration from
the body weights at sacrifice.
Measurements of alveolar bone
loss
The rats were sacrificed via an overdose of zoletile
anesthesia (50 mg/kg) 10-days after the first administration, and
maxillary bone containing the ligature placement site were excised.
The horizontal alveolar bone loss, the distance between the cusp
tip and the alveolar bone, was measured using a modification of the
methods of Crawford et al (21) as described by Samejima et al
(3). Measurements were made along
the axis of root of the upper left incisor teeth, in units of
mm/rat (2).
Microbiological analysis
The buccal gingival tissues surrounding the upper
left incisor teeth were removed, and placed in 0.3 ml brain heart
infusion broth (BD Biosciences, Cockeysville, MD, USA). Immediately
afterwards, the collected fragment was homogenized, plated in
dilutions of 1:100 and 1:1,000 into blood agar (brain heart
infusion agar supplemented with 5% defibrinated sheep blood and
henin/menadione 10 µg/ml; BD Biosciences), and incubated at 37°C,
48 h under 5% CO2 aerobic conditions. After incubation,
formed colony numbers were counted in units of ×105
CFU/g tissue.
Measurement of myeloperoxidase (MPO)
activity
The buccal gingival tissues surrounding the left
incisor teeth were removed. The material was suspended in 0.5%
hexadecyltrimethyl-ammonium bromide (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in 50 mM potassium phosphate
buffer, pH 6.0, to solubilize MPO. After homogenization in an ice
bath for 15 sec, the samples were freeze-thawed twice. Additional
buffer was added to the test tube to reach 400 µl buffer per 15 mg
tissue for 12 min. Following centrifugation at 1,000 × g for 12
min, 0.1 ml supernatant was added to 2 ml phosphate buffer,
containing 0.167 mg/ml o-dianisidine dihydrochloride
(Sigma-Aldrich), DW and 0.0005% hydrogen peroxide to give a final
volume of 2.1 ml per tube. The absorbance of the supernatant was
measured using a spectrophotometer (Mecasys Co., Ltd., Daejeon,
South Korea) at 460 nm.
Detection of interleukin (IL)-1β and
tumor necrosis factor (TNF)-α in rat maxillary gingival tissue
The buccal gingival tissue collected was homogenized
and processed as described by Safieh-Garabedian et al
(22) and Botelho et al
(2). TNF-α and IL-1β concentrations
were determined by enzyme linked immunosorbent assay kits (ab46070
and ab100768 respectively; Abcam, Cambridge, UK) according to the
manufacturer's protocol. Enzymatic coloration reaction was stopped
with H2SO4 and the absorbance was measured
using a microplate reader (Tecan, Männedorf, Switzerland) at 490
nm.
Malondialdehyde (MDA) measurement
Buccal gingival tissues were placed into a
homogenization buffer comprising 50 mM Tris-HCl, 0.1 mM ethylene
glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid (EGTA) and
1 mM phenylmethylsulfonyl fluoride (pH 7.4) and then homogenized.
An aliquot of the homogenate was added to a reaction mixture
containing 8.1% (w/v) sodium dodecyl sulfate (Sigma-Aldrich), 20%
(v/v) acetic acid (pH 3.5), 0.8% (w/v) thiobarbituric acid
(Sigma-Aldrich) and DW. Samples were heated for 1 h at 95°C, then
centrifuged at 3,000 × g for 10 min, and finally the absorption was
measured at 650 nm.
Inducible nitric oxide synthase (iNOS)
activity measurement
Buccal gingival tissue homogenate was incubated in
the presence of L-[3H]-arginine (10 mM, 5 kBq/tube),
NADPH (1 mM), calmodulin (30 nM), tetrahydrobiopterin (5 mM) and
calcium (2 mM) for 30 min at 22°C. Reactions were stopped by
dilution with 0.5 ml ice-cold HEPES buffer (pH 5.5) containing EGTA
(2 mM) and EDTA (2 mM). Experiments performed in the absence of
NADPH determined the extent of L-[3H]-citrulline
formation independent of a specific NOS activity. Experiments in
the presence of NADH, without calcium, and in the presence of EGTA
(5 mM) determined the calcium-independent NOS activity. Reaction
mixtures were applied to Dowex 50W (Na/form) columns and the eluted
L-[3H]-citrulline activity was measured using a liquid
scintillation counter (Wallac; PerkinElmer, Annapolis, MD,
USA).
Histopathology
Tissue from the maxillary area was fixed in 10%
neutral buffered formalin., and then decalcified using decalcifying
solution (24.4% formic acid and 0.5 N sodium hydroxide) for 5 days.
After that, the tissue was longitudinally trimmed and embedded in
paraffin, sectioned (3–4 µm) and stained with hematoxylin and eosin
(H&E) according to established methods (7). The areas between the left and right
incisor teeth were analyzed under light microscopy using on a 0–3
score grade, considering the inflammatory cell influx, and alveolar
bone and cementum integrity, as described previously (4). In addition, the numbers of infiltrated
inflammatory cells (numbers/mm2 of gingival tissues) and
collagen-occupied regions (%/mm2 of gingival tissues) on
the gingival areas between the first and second molars were
measured using histomorphometrical analyses of prepared
longitudinally trimmed samples using a computer-assisted image
analysis program, iSolution FL version 9.1 (IMT i-solution Inc.,
Vancouver, Canada). In addition, alveolar bone volumes
(%/mm2 alveolar bone areas), osteoclast cell numbers
(numbers/mm2 of alveolar bone surface) and their
occupied percentages (%/mm2 of alveolar bone surface)
were also measured on the alveolar bone regions between the right
and left incisor. The histopathologist was blinded to the group
distribution when this analysis was conducted.
Statistical analyses
Multiple comparison tests for different dose groups
were conducted. If the Levene test indicated no significant
deviations from variance homogeneity, the obtained data were
analyzed by one-way analysis of variance testing followed by
least-significant differences multi-comparison tests. In cases
where significant deviations from variance homogeneity was observed
in the Levene test, a Kruskal-Wallis H test was conducted. When a
significant difference was observed in the Kruskal-Wallis H test,
the Mann-Whitney U test was conducted to determine the specific
pairs of group that were significantly different. Statistical
analyses were conducted using SPSS for Windows (14.0 Korean
edition; IBM SPSS, Inc., Armonk, NY, USA), and P<0.05 was
considered to indicate a statistically significant difference.
Results
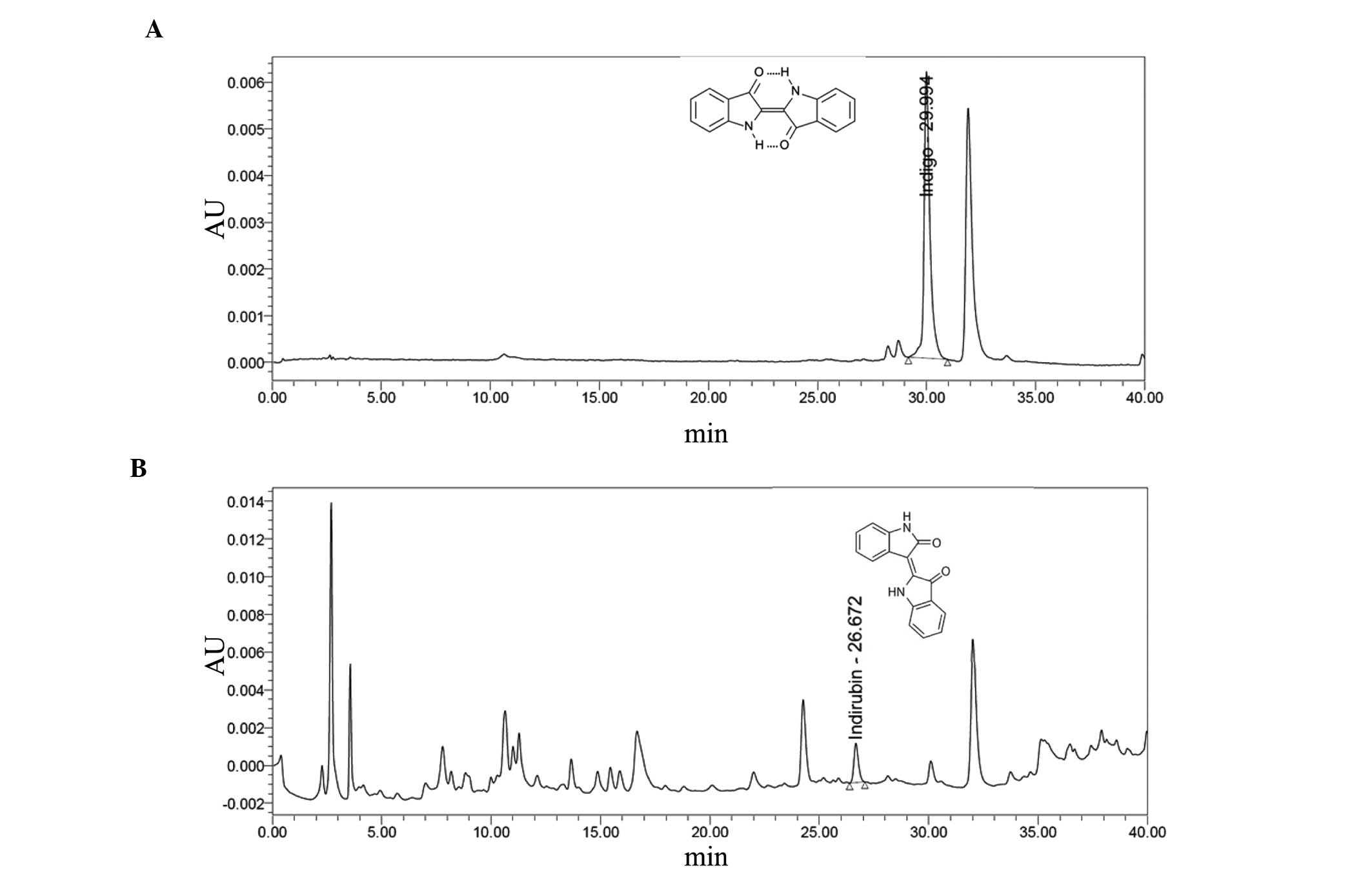
Indigo and indirubin contents in PR
extracts
Contents of indigo and indirubin were calculated
from the calibration curves of the standards. Validation of the
method verified its reliability and stability. Use of the method
indicated that the lyophilized aqueous extracts of PR contain
0.043% indigo and 0.009% indirubin (Fig.
1).
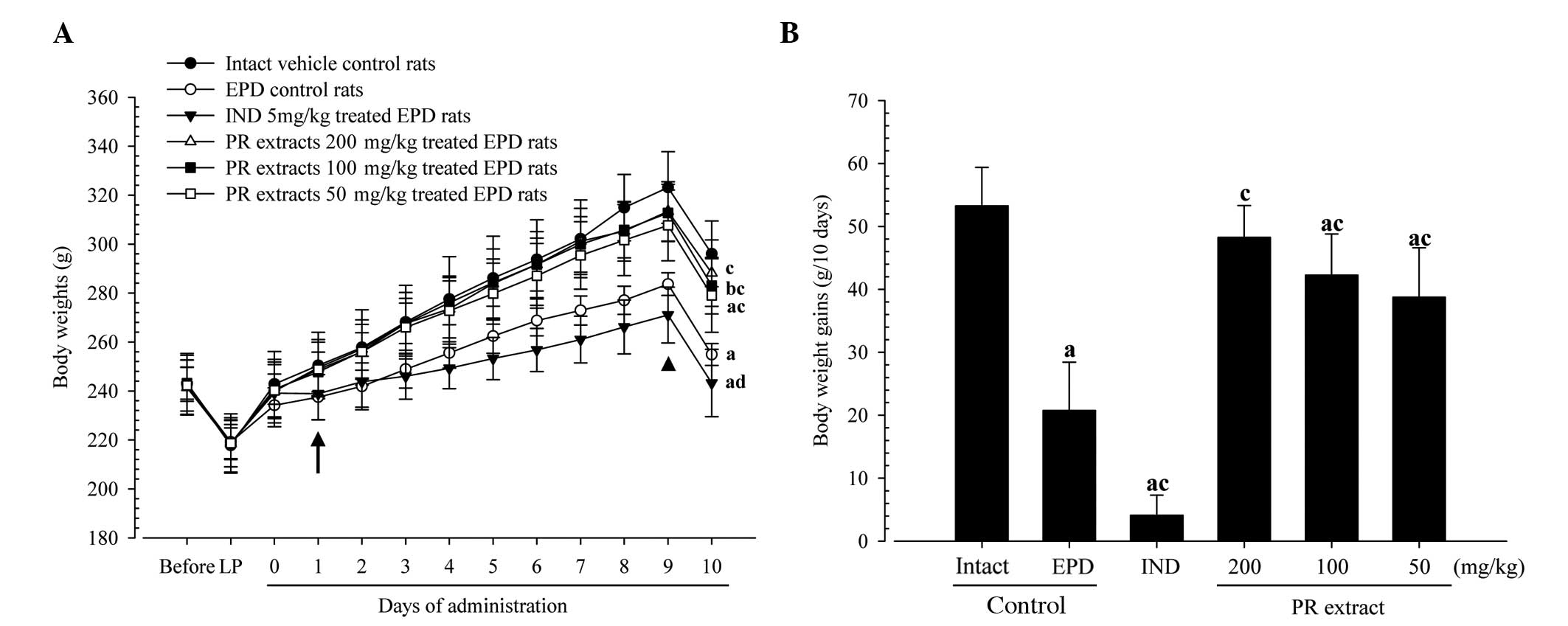
Body weight changes and gains
Rats treated with the three different dosages of PR
extracts showed significantly increased body weights as compared
with the EPD control from 1 day after initial administration, and
the body weight gains during the 10-day administration period were
significantly increased in these PR extract-treated rats,
respectively. By contrast, the rats treated with indomethacin
exhibited significantly lower body weights compared with the EPD
control from 9 days after the initiation of treatment;
consequently, the body weight gain during the 10-days
administration period was also significantly decreased in the
indomethacin group compared with the EPD control (Fig. 2).
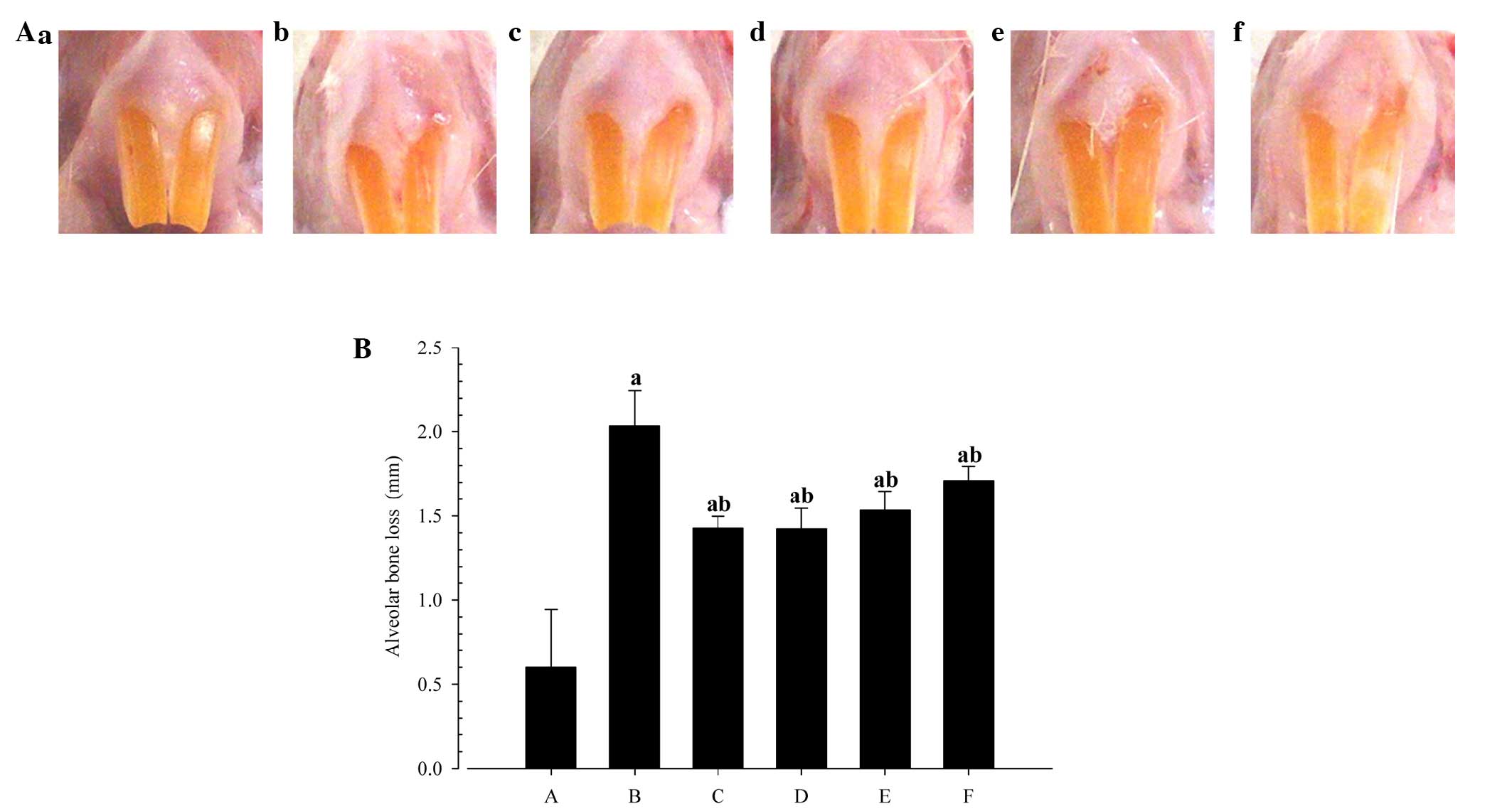
Changes in alveolar bone loss
measurements
Significant reductions in the extent of alveolar
bone loss were detected in the rats treated with indomethacin, or
50, 100 or 200 mg/kg PR extracts as compared with the EPD control
(Fig. 3).
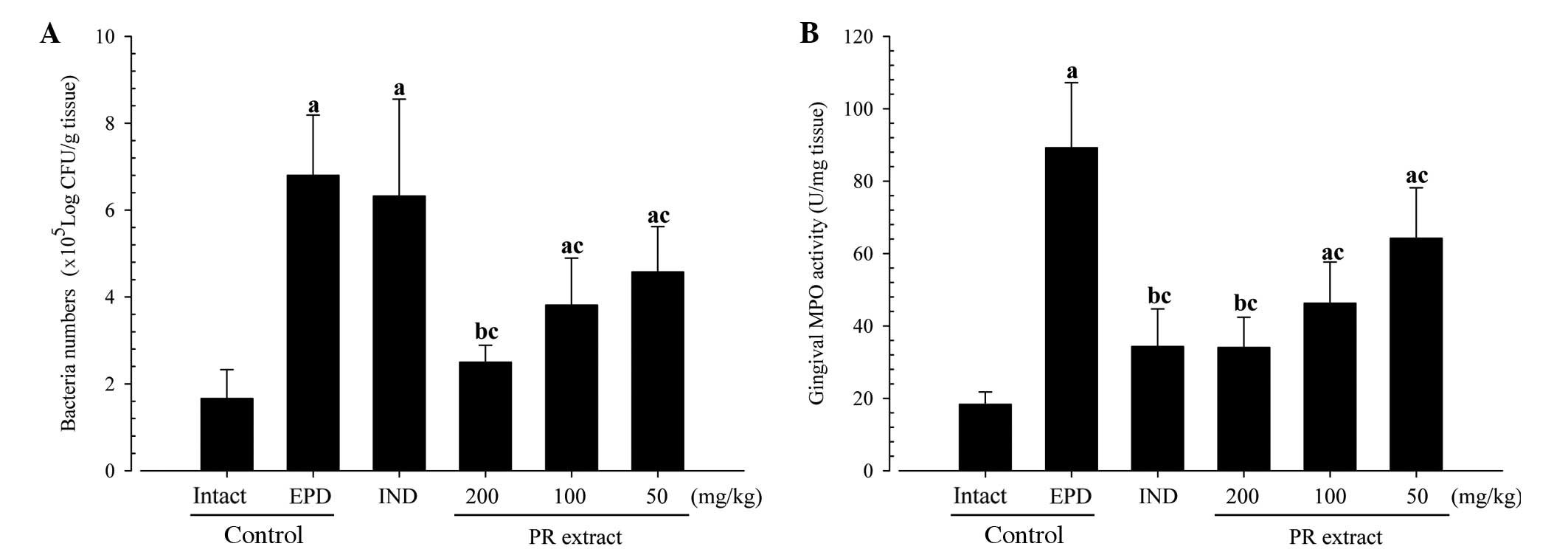
Changes in gingival viable bacteria
counts
Significant and dose-dependent reductions in the
numbers of viable bacteria (colony numbers) were detected in the
three PR extract-treated groups compared with the EPD control. No
significant changes in viable bacteria numbers were demonstrated in
the indomethacin-treated rats as compared with the EPD control in
this experiment (Fig. 4A).
Changes in gingival MPO
activities
Significant reductions of MPO activities in the
buccal gingival tissues were detected in the four treatment groups
as compared with the EPD control, respectively. Notably, the PR
extracts exhibited a clear dose-dependent inhibitory effect against
the EPD-related elevations of gingival MPO activities in this
experiment (Fig. 4B).
Changes in gingival IL-1β and TNF-α
levels
The elevations in the levels of gingival IL-1β and
TNF-α induced by EPD were demonstrated to be significantly and
dose-dependently inhibited by treatment with 50, 100 and 200 mg/kg
PR extracts (P<0.01). In addition, the rats treated with 5 mg/kg
indomethacin also exhibited significantly decreased gingival IL-1β
and TNF-α levels as compared with the EPD control rats (P<0.01;
Table I).
 | Table I.Gingival IL-1β, TNF-α and MDA levels
and iNOS activities around the ligation site in EPD model rats. |
Table I.
Gingival IL-1β, TNF-α and MDA levels
and iNOS activities around the ligation site in EPD model rats.
|
| Pro-inflammatory
cytokines | Antioxidative
stresses |
|---|
|
|
|
|
|---|
| Group | IL-1β (pg/ml) | TNF-α (pg/ml) | MDA (µM/mg
tissue) | iNOS
(fM/mg/min) |
|---|
| Controls |
|
|
|
|
|
Intact |
22.05±7.88 |
229.25±36.63 |
1.69±0.43 |
25.35±10.97 |
|
EPD |
63.40±9.81a |
824.13±194.75a |
8.96±1.63a |
207.39±56.02a |
| Indomethacin 5
mg/kg |
37.88±4.64a,b |
458.25±142.40a,b |
5.99±0.97a,b |
133.09±26.97a,b |
| PR extracts |
|
|
|
|
| 50
mg/kg |
50.61±7.67a,b |
600.75±78.60a,b |
6.87±1.10a,c |
149.79±9.90a,b |
| 100
mg/kg |
45.93±9.40a,b |
539.63±79.30a,b |
6.34±0.80a,b |
142.99±17.65a,b |
| 200
mg/kg |
49.23±6.33a,b |
456.00±126.53a,b |
5.91±1.27a,b |
135.00±21.85a,b |
Changes in gingival MDA levels and
iNOS activities
Statistically significant reductions in gingival MDA
levels and iNOS activities were observed in each of the four groups
of rats with EPD that were treated with test substances, including
indomethacin, as compared with the EPD control. Notably, PR
extracts were observed to have a clear dose-dependent inhibitory
activity against the elevations of gingival MDA levels and iNOS
activities that were induced by EPD (Table I).
Histopathological changes of maxillary
regions
Marked increases in inflammatory cell infiltrations,
predominantly polymorphneutrophils, were detected in the gingival
tissues between upper left and right incisor teeth in the EPD
control rats with severe edematous changes (loosening of collagen
fibers and loss of compactness). In addition, activation of
osteoclast cells, increases in the number and the percentages of
osteoclast cells occupied regions on the alveolar bone surface
(OS/BS), were also observed in the alveolar bone areas of the EPD
control rats with marked decreases of osteoid alveolar bones; they
were re-confirmed by histomorphometrical analysis. Significant
(P<0.01) increases in the histological scores, infiltrated
inflammatory cell numbers in gingival tissues, and decreased
collagen fiber occupied regions in gingival tissues were
demonstrated in the EPD control rats, along with significant
(P<0.01) decreases in alveolar bone volumes, increased
osteoclast cell numbers and OS/BS, as compared with those of intact
control rats, respectively. However, these histopathological
periodontitis and associated alveolar bone losses were
significantly (P<0.05) and dose-dependently reduced by treatment
of all three different dosages of PR extracts, as compared with EPD
control rats, respectively. In addition, indomethacin was also
observed to significantly ameliorate the EPD-induced periodontitis
and related alveolar bone losses, as revealed by histopathological
inspections in this experiment (Table
II and Fig. 5).
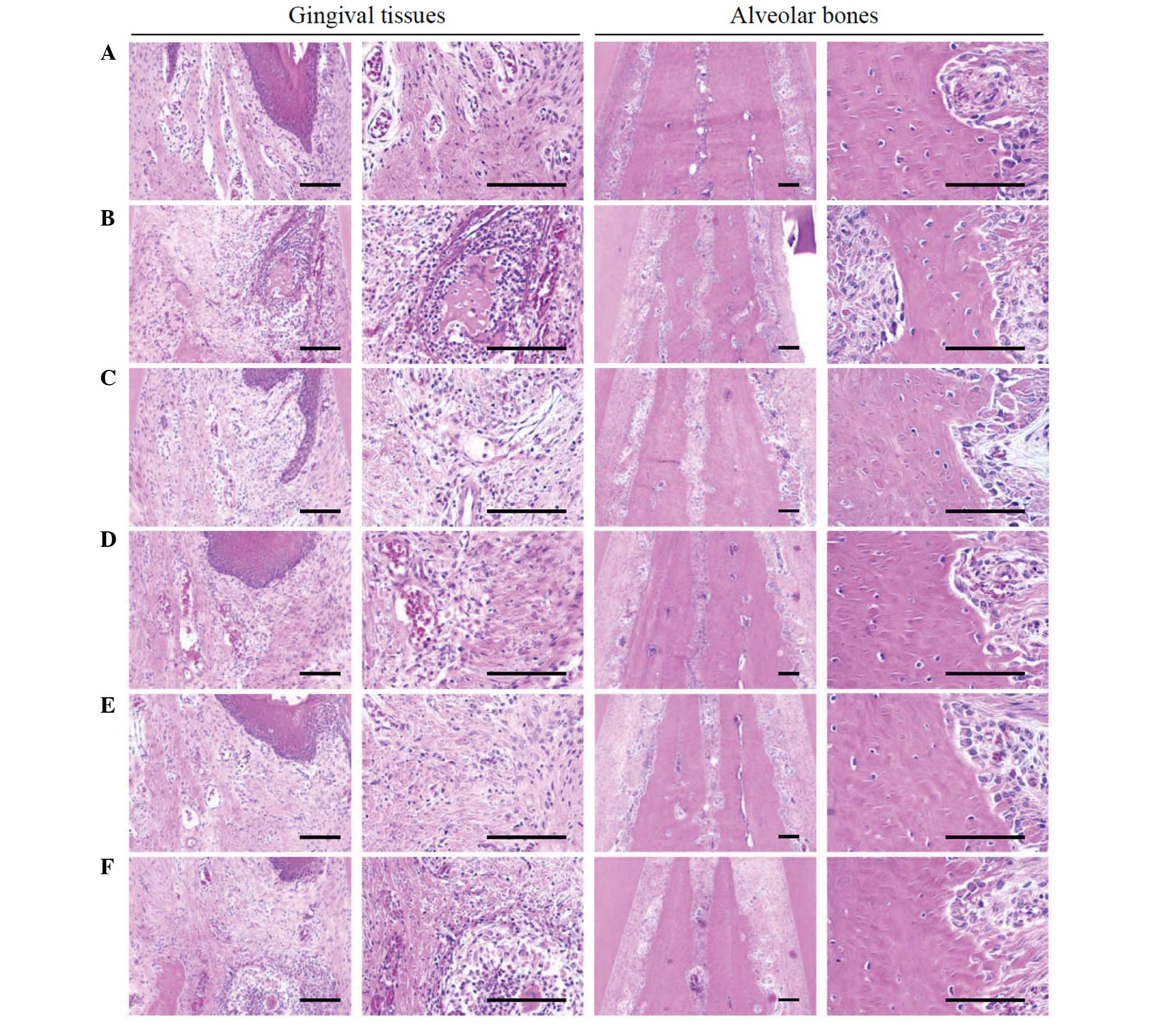 | Figure 5.Representative histological images of
gingival tissues and alveolar bones between upper incisor teeth,
taken from around the upper left incisor teeth of intact or rats
with EPD. (A) Intact control, (B) EPD control, (C) 5 mg/kg
indomethacin-treated EPD rats, (D) 200 mg/kg PR extract-treated EPD
rats, (E) 100 mg/kg PR extract-treated EPD rats and (F) 50 mg/kg PR
extract-treated EPD rats. Marked increases of inflammatory cell
infiltrations were detected in the gingival tissues between the
upper left and right incisor teeth in EPD control rats with severe
edematous changes, with the loosening of collagen fibers and loss
of their compactness. In addition, activation of osteoclast cells,
increases in the number of osteoclast cells and the percentage of
the region they occupy on the alveolar bone surface were also
observed in the alveolar bone areas of the EPD control with marked
reductions of osteoid alveolar bone. However, these
histopathological features of periodontitis and related alveolar
bone loss were obviously and dose-dependently reduced by treatment
with all three different dosages of PR extract as compared with the
EPD control. In addition, indomethacin also markedly ameliorated
the EPD-induced periodontitis and related alveolar bone loss as
revealed by these histopathological inspections. All hematoxylin
and eosin stained; scale bars, 120 µm. |
 | Table II.Histomorphometrical analysis of
maxillary regions around the ligation site - gingival and alveolar
bone areas in EPD model rats. |
Table II.
Histomorphometrical analysis of
maxillary regions around the ligation site - gingival and alveolar
bone areas in EPD model rats.
|
|
| Gingival areas | Alveolar bone
areas |
|---|
|
|
|
|
|
|---|
| Group | Histological
score | Inflammatory cells
(cells/mm2) | Collagen
(%/mm2) | Alveolar bone
volume (%) | Osteoclast cells
(cells/mm) | OS/BS (%) |
|---|
| Controls |
|
|
Intact |
0.75±0.46 |
9.88±3.80 |
72.32±10.79 |
75.92±7.03 |
6.50±1.93 |
1.56±0.93 |
|
EPD |
2.88±0.35a |
1,010.38±171.56a |
28.60±11.30a |
37.95±5.28a |
43.63±12.39a |
40.60±10.58a |
| Indomethacin |
|
| 5 mg/kg |
1.75±0.46a,b |
263.50±83.94a,b |
51.27±11.47a,b |
52.13±4.45a,b |
24.88±8.97a.b |
17.99±6.59a.b |
| PR extracts |
|
| 50
mg/kg |
2.13±0.64a,b |
780.13±106.57a,c |
41.80±7.11a,c |
47.83±7.53a,c |
27.38±4.66a,c |
23.70±4.33a,b |
| 100
mg/kg |
2.00±0.53a,b |
313.13±100.50a,b |
45.36±10.38a,b |
50.51±7.22a,b |
25.75±7.17a,b |
19.97±3.58a,b |
| 200
mg/kg |
1.88±0.35a,b |
259.50±41.29a,b |
51.14±9.13a,b |
56.88±12.04a,b |
22.25±3.92a,b |
14.26±4.73a,b |
Discussion
The present study demonstrated that PR has effective
inhibitory effects against ligation-induced EPD and associated
alveolar bone loss, which are mediated by antibacterial,
antioxidative and anti-inflammatory activities.
Periodontitis and the alveolar bone loss associated
with it in EPD directly induce mal-mastication, which results in
marked loss of body weight (2).
Therefore, the inhibition of these EPD-related body weight
reductions can be considered to be indirect evidence that a
treatment ameliorates periodontitis and alveolar bone loss. In the
present study, marked increases in body weight and gains in body
weight over the 10-day treatment period were detected in the rats
treated with PR extracts, as compared with the EPD control, and
these increases were dose-dependent.
Measuring alveolar bone loss on the basis of the
exposure of tooth roots from alveolar sockets is a generally used
macroscopical evaluation method for alveolar bone loss (2,23).
Significant reductions in the alveolar bone loss measurements by
indomethacin, and 50, 100 or 200 mg/kg PR extracts provides direct
evidences that these treatments ameliorated EPD-related alveolar
bone loss.
Bacteria are considered to be the primary etiologic
agents of periodontal disease (24).
Bacterial plaque is considered to be involved in the pathogenesis
of periodontitis, and may initiate a local inflammatory reaction
(2), leading to edema, leukocyte
infiltration and the release of inflammatory mediators, which may
cause the formation of periodontal pockets, detachment of
connective tissue and resorption of alveolar bone, ultimately
leading to tooth loss (3,4). Previous studies have indicated that
periodontal microbial flora change during periodontal disease in
rats following the cervical ligation of teeth, with anaerobic
gram-negative bacilli becoming predominant (2,4). The
present study demonstrated that marked increases in viable total
bacteria numbers occurred in gingival tissues around ligature-bound
incisor teeth. However, significant reductions in viable bacteria
numbers were dose-dependently detected in PR extract-treated rats,
but not in those treated with indomethacin, suggesting the
existence of different mechanisms of action between PR extracts and
indomethacin. It is suggested that indomethacin is a representative
anti-inflammatory agent whose actions are mediated by
cyclooxygenase inhibition, whereas PR extracts contain
antibacterial dyes, namely indigo and indirubin (17).
The importance of acute inflammatory cells,
particularly polymorphonuclear neutrophil (PMN) infiltrations, on
gingival tissue in the evolution of periodontal disease has been
demonstrated previously (4).
Although, inflammatory cells play key roles in eliminating the
causes of inflammations (25),
activated PMNs also generate oxygen metabolites (26). MPO is an activating cytotoxic enzyme
released from PMNs (27), and its
levels are markedly increased in periodontal diseases (2,28). In
the present study, significant increase in gingival MPO levels were
detected in the EPD control; however, PR extracts significantly
inhibited these increases, suggesting that PR extracts suppressed
the cytotoxic effects of PMNs.
Pro-inflammatory cytokines, particularly TNF-α and
IL-1β, have been shown to play a significant role in periodontal
disease (29). The cytokine TNF-α,
which is produced by a variety of cell types, including
splenocytes, has been found to be associated with critical events
leading to T-lineage commitment and differentiation (30). Periodontitis may be potentiated by
the TNF-stimulated release of eicosanoids and other cytokines, such
as TNF-α and IL-1. IL-1 activates neutrophils and macrophages, and
thereby induces the production and release of reaction oxygen
species and nitric oxide, which has been implicated to be a cause
of local tissue damage (31). In the
present study, significant decreases of gingival TNF-α and IL-1β
levels were detected in all test substance-treated rats as compared
with the EPD control, providing direct evidence that their
anti-inflammatory effects are sufficient to ameliorate the
periodontitis induced by ligation placement.
MDA is an index of lipid peroxidation (32) and its levels are increased in
periodontal diseases (33). iNOS a
distinct isoform of NOS that can be induced by proinflammatory
agents such as endotoxin, IL-1β, TNF-α and interferon-γ in a
variety of cells. Increased production of NO following the
induction of iNOS has been implicated in the pathogenesis of shock
and inflammation (34). In
periodontal diseases, activation of iNOS and associated increases
in NO production have been found to occur, and accordingly, this
induces damage of the surrounding tissues, particularly the
alveolar bones (33,35). In the present study, the increments
of MDA levels and iNOS activities were significantly decreased by
treatment with both PR extracts and indomethacin, providing direct
evidence that they exhibit antioxidant effects on the periodontal
tissue damage caused by increases of NO production through the
elevation of iNOS activities induced by ligature placements.
Consistent with previous studies of EPD (7,8), marked
inflammatory cell infiltrations and edematous changes were detected
in the gingival tissues between the first and second molars, where
the ligature was placed. In addition, absorption of the alveolar
bones due to osteoclast cell activation was detected during the
histopathological observations in this study. Increases in
histological scores based on inflammatory cell infiltration and
alveolar bone damage (2,23), infiltration of inflammatory cells
(including neutrophils), reductions of collagen-occupied regions
associated with edematous changes, reductions of bone volumes,
increases of osteoclast cell numbers and osteoid surface/bone
surface ratios were detected by the histomorphometrical analysis in
the present study, and these findings are quite similar to those of
previous studies (7,8). However, these histopathological changes
associated with periodontitis and alveolar bone loss were
significantly and dose-dependently inhibited by treatment with each
of the three different dosages of PR extracts, and by
indomethacin.
In summary, the results indicate that aqueous PR
extracts contain blue indigo (0.043%) and purple indirubin
(0.009%), and effectively ameliorates ligature placement-induced
periodontitis and associated alveolar bone loss by a combination of
antibacterial, antioxidative and anti-inflammatory activities. PR
exhibits promise as a potent protective agent for various
periodontal diseases in the future.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(grant no. NRF-2012R1A1A2043886).
References
|
1
|
Chambrone LA and Chambrone L: Tooth loss
in well-maintained patients with chronic periodontitis during
long-term supportive therapy in Brazil. J Clin Periodontol.
33:759–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Botelho MA, Rao VS, Carvalho CB,
Bezerra-Filho JG, Fonseca SG, Vale ML, Montenegro D, Cunha F,
Ribeiro RA and Brito GA: Lippia sidoides and Myracrodruon
urundeuva gel prevents alveolar bone resorption in experimental
periodontitis in rats. J Ethnopharmacol. 113:471–478. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samejima Y, Ebisu S and Okada H: Effect of
infection with Eikenella corrodens on the progression of
ligature-induced periodontitis in rats. J Periodontal Res.
25:308–315. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menezes AM, Rocha FA, Chaves HV, Carvalho
CB, Ribeiro RA and Brito GA: Effect of sodium alendronate on
alveolar bone resorption in experimental periodontitis in rats. J
Periodontol. 76:1901–1909. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Listgarten MA: Nature of periodontal
diseases: Pathogenic mechanisms. J Periodontal Res. 22:172–178.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fentoğlu Ö, Kırzıoğlu FY, Bulut MT, Kumbul
Doğuç D, Kulaç E, Önder C and Günhan M: Evaluation of lipid
peroxidation and oxidative DNA damage in patients with
periodontitis and hyperlipidemia. J Periodontol. 86:682–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YS, Kang SJ, Kim JW, Cho HR, Moon SB,
Kim KY, Lee HS, Han CH, Ku SK and Lee YJ: Effects of Polycan, a
β-glucan, on experimental periodontitis and alveolar bone loss in
Sprague-Dawley rats. J Periodontal Res. 47:800–810. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ku SK, Cho HR, Sung YS, Kang SJ and Lee
YJ: Effects of calcium gluconate on experimental periodontitis and
alveolar bone loss in rats. Basic Clin Pharmacol Toxicol.
108:241–250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toker H, Ozdemir H, Eren K, Ozer H and
Sahin G: N-acetylcysteine, a thiol antioxidant, decreases alveolar
bone loss in experimental periodontitis in rats. J Periodontol.
80:672–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JH, Seo BI, Cho SY, Park KR, Choi SH,
Han CK, Song CH, Park SJ and Ku SK: Single oral dose toxicity study
of prebrewed armeniacae semen in rats. Toxicol Res. 29:91–98. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noh JR, Kim YH, Gang GT, Hwang JH, Kim SK,
Ryu SY, Kim YS, Lee HS and Lee CH: Hepatoprotective effect of
Platycodon grandiflorum against chronic ethanol-induced
oxidative stress in C57BL/6 mice. Ann Nutr Metab. 58:224–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woo YM, Kim AJ, Kim JY and Lee CH:
Tyrosinase inhibitory compounds isolated from Persicaria
tinctoria flower. J Appl Biol Chem. 54:47–50. 2011. View Article : Google Scholar
|
|
13
|
Kim SJ, Ko JH, Park SH, Kim MS and Kim KS:
Preparation method of indigo standard solution and variation of
indigo contents in blue dye extract from breeding lines of
Persicaria tinctoria H. Gross. Korean J Medicinal Crop Sci.
21:213–219. 2013.(In Korean). View Article : Google Scholar
|
|
14
|
Xiao Z, Hao Y, Liu B and Qian L: Indirubin
and meisoindigo in the treatment of chronic myelogenous leukemia in
China. Leuk Lymphoma. 43:1763–1768. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mok CK, Kang SS, Chan RW, Yue PY, Mak NK,
Poon LL, Wong RN, Peiris JS and Chan MC: Anti-inflammatory and
antiviral effects of indirubin derivatives in influenza A (H5N1)
virus infected primary human peripheral blood-derived macrophages
and alveolar epithelial cells. Antiviral Res. 106:95–104. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoessel R, Leclerc S, Endicott JA, Nobel
ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, et
al: Indirubin, the active constituent of a Chinese antileukaemia
medicine, inhibits cyclin-dependent kinases. Nat Cell Biol.
1:60–67. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kataoka M, Hirata K, Kunikata T, Ushio S,
Iwaki K, Ohashi K, Ikeda M and Kurimoto M: Antibacterial action of
tryptanthrin and kaempferol, isolated from the indigo plant
(Polygonum tinctorium Lour.), against Helicobacter
pylori-infected Mongolian gerbils. J Gastroenterol. 36:5–9.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang HG, Heo BG, Park YS, Namiesnik J,
Barasch D, Katrich E, Vearasilp K, Trakhtenberg S and Gorinstein S:
Chemical composition, antioxidant and anticancer effects of the
seeds and leaves of indigo (Polygonum tinctorium Ait.)
plant. Appl Biochem Biotechnol. 167:1986–2004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin YK, Leu YL, Huang TH, Wu YH, Chung PJ,
Su Pang JH and Hwang TL: Anti-inflammatory effects of the extract
of indigo naturalis in human neutrophils. J Ethnopharmacol.
125:51–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin YK, Chen HW, Yang SH, Leu YL, Huang YH
and Yen HC: Protective effect of indigo naturalis extract against
oxidative stress in cultured human keratinocytes. J Ethnopharmacol.
139:893–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crawford JM, Taubman MA and Smith DJ: The
natural history of periodontal bone loss in germfree and
gnotobiotic rats infected with periodontopathic microorganisms. J
Periodontal Res. 13:316–325. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Safieh-Garabedian B, Poole S, Allchorne A,
Winter J and Woolf CJ: Contribution of interleukin-1 beta to the
inflammation-induced increase in nerve growth factor levels and
inflammatory hyperalgesia. Br J Pharmacol. 115:1265–1275. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azoubel MC, Menezes AM, Bezerra D, Oria
RB, Ribeiro RA and Brito GA: Comparison of etoricoxib and
indomethacin for the treatment of experimental periodontitis in
rats. Braz J Med Biol Res. 40:117–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ximénez-Fyvie LA, Haffajee AD and
Socransky SS: Microbial composition of supra- and subgingival
plaque in subjects with adult periodontitis. J Clin Periodontol.
27:722–732. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zimmerman BJ, Grisham MB and Granger DN:
Role of oxidants in ischemia/reperfusion-induced granulocyte
infiltration. Am J Physiol. 258:G185–G190. 1990.PubMed/NCBI
|
|
26
|
Sullivan GW, Sarembock IJ and Linden J:
The role of inflammation in vascular diseases. J Leukoc Biol.
67:591–602. 2000.PubMed/NCBI
|
|
27
|
Işeri SO, Sener G, Yüksel M, Contuk G,
Cetinel S, Gedik N and Yegen BC: Ghrelin against
alendronate-induced gastric damage in rats. J Endocrinol.
187:399–406. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holanda Pinto SA, Pinto LM, Cunha GM,
Chaves MH, Santos FA and Rao VS: Anti-inflammatory effect of alpha,
beta-Amyrin, a pentacyclic triterpene from Protium
heptaphyllum in rat model of acute periodontitis.
Inflammopharmacology. 16:48–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lima V, Vidal FD, Rocha FA, Brito GA and
Ribeiro RA: Effects of tumor necrosis factor-alpha inhibitors
pentoxifylline and thalidomide on alveolar bone loss in short-term
experimental periodontal disease in rats. J Periodontol.
75:162–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samira S, Ferrand C, Peled A, Nagler A,
Tovbin Y, Ben-Hur H, Taylor N, Globerson A and Lapidot T: Tumor
necrosis factor promotes human T-cell development in nonobese
diabetic/severe combined immunodeficient mice. Stem Cells.
22:1085–1100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Assuma R, Oates T, Cochran D, Amar S and
Graves DT: IL-1 and TNF antagonists inhibit the inflammatory
response and bone loss in experimental periodontitis. J Immunol.
160:403–409. 1998.PubMed/NCBI
|
|
32
|
Cuzzocrea S, Zingarelli B, Hake P, Salzman
AL and Szabó C: Antiinflammatory effects of mercaptoethylguanidine,
a combined inhibitor of nitric oxide synthase and peroxynitrite
scavenger, in carrageenan-induced models of inflammation. Free
Radic Biol Med. 24:450–459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Paola R, Marzocco S, Mazzon E, Dattola
F, Rotondo F, Britti D, De Majo M, Genovese T and Cuzzocrea S:
Effect of aminoguanidine in ligature-induced periodontitis in rats.
J Dent Res. 83:343–348. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Southan GJ and Szabó C: Selective
pharmacological inhibition of distinct nitric oxide synthase
isoforms. Biochem Pharmacol. 51:383–394. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lohinai Z, Benedek P, Fehér E, Györfi A,
Rosivall L, Fazekas A, Salzman AL and Szabó C: Protective effects
of mercaptoethylguanidine, a selective inhibitor of inducible
nitric oxide synthase, in ligature-induced periodontitis in the
rat. Br J Pharmacol. 123:353–360. 1998. View Article : Google Scholar : PubMed/NCBI
|