Introduction
Esophageal carcinoma (EC) is a global health problem
ranked eighth in terms of incidence, and sixth in terms of
mortality (1,2). The majority of primary tumors in
patients are curable by surgical resection, however, due to a lack
of distinct early symptoms, patients are often diagnosed at
advanced stages, and more than half of patients present with
metastases (3). The remaining
patients without advanced stage disease receive surgery,
chemotherapy and radiotherapy for treatment, however, the majority
eventually succumb to metastases. Therefore, in order to advance
the current radiotherapy and chemotherapy, there is an increasing
interest in developing an effective agent to inhibit tumor cell
prolification and restrain the metastasic capability of EC
cells.
In recent years, interest in the use of traditional
medicines for the prevention and treatment of tumors has increased,
and various therapies have been employed as monotherapy or in
combination with conventional medicine (4,5).
Glycoalkaloids (GAs) are natural toxic compounds present in a
number of vegetables and plants (6).
Previous findings showed that glycoalkaloids exert a strong
inhibitory effect on tumor growth in animals as a result of their
cytotoxic effects on tumor cells (7,8).
α-solanine, a bioactive component and one of the major steroidal
glycoalkaloids in potatoes, is predominantly detected in the tuber
crop potato and the nightshade plant. It was previously
demonstrated that α-solanine causes growth inhibition and apoptosis
induction in multiple cancer cells (9,10). In
addition, certain studies have indicated that α-solanine possesses
anti-metastasis activity in various cancers (11–17).
Thus, to determine the potential contribution of
α-solanine to EC therapy, and the underlying molecular mechanisms
regarding the association between α-solanine and esophageal
tumorigenesis, the aim of the present study was to examine the
effect of α-solanine on the EC9706 and Eca109 cell lines.
Materials and methods
Cell lines and reagents
α-solanine was purchased from Sigma-Aldrich (St.
Louis, MO, USA), and dissolved in dimethylsulfoxide for storage at
−20°C. Human esophageal squamous carcinoma cell lines, EC9706 and
Eca109, were purchased from Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China). The cells
were routinely cultured in DMEM supplemented with 10%
heat-inactivated fetal bovine serum (FBS; both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin in a humidified cell incubator with an
atmosphere of 5% CO2 at 37°C.
In vitro cell proliferation assay
EC9706 and Eca109 cell lines in the logarithmic
phase of growth were seeded into 96-well plates at a density of
1×104 cells/well. Subsequent to the starving of cells
with serum-free medium containing 0.1% BSA for 24 h, once cell
adhesion was complete, the cells were exposed to a range of
concentrations of α-solanine (10, 20, 40 and 60 µg/ml) for 24, 48
and 72 h. The cells were then treated with a Cell Counting kit-8
(CCK-8) solution (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) and incubated for a further 2 h. Cell proliferation was
determined by measuring the absorbance at 450 nm using a plate
reader (Model 680; cat. no. 168-1000; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Triplicate parallel experiments were performed
for each concentration. The rate of inhibition was calculated using
the equation: Rate of growth inhibition (%) = (ODcontrol
- ODtreated)/ODcontrol × 100%, where OD was
the optical density.
Colony-forming survival assay
The overall survival of the cells treated with
α-solanine was assessed by the rate of colony formation. EC9706 and
Eca109 cells were seeded into 6-well plates for 24 h. Following
this, the cells were washed with DMEM, trypsinized and counted.
Base Agar Matrix Layer (1.5 ml; GenMed Scientifics, Inc, Shanghai,
China) was dispensed into each well of a 12-well plate. The plate
was maintained at 18–25°C until solid. Subsequently, 1.5 ml growth
agar layer consisting cells were added into each well and the plate
was kept at room temperature until the growth layer congealed. A
further 500 µl culture media containing various concentrations of
α-solanine (0, 20, 40 and 60 µg/ml) was added to the surface of the
growth layer. The cells were then incubated at 37°C with 5%
CO2 until colony formation was visible, which usually
occurred between 10 and 14 days. The colonies with >50 cells
were considered to be surviving colonies. The plating efficiency
was calculated by dividing the average number of colonies per well
by the amount of cells plated. Survival fractions were calculated
by normalization to the plating efficiency of appropriate control
groups.
Cell migration and invasion
assays
Transwell filters were coated with Matrigel (3.9
g/l, 40 µl) on the upper surface of the polycarbonic membrane (6.5
mm in diameter, 8 µm pore size). The Matrigel, after solidifying at
37°C for 30 min, served as the extracellular matrix for tumor cell
invasion analysis. Subsequent to treatment with different
concentrations (20, 40 and 60 µg/ml) of α-solanine for 24 h, 200 µl
serum-free cell suspension medium containing EC9706 and Eca109
cells was loaded onto the top chamber of the transwell. Medium (500
µl) containing 10% FBS (used as a chemoattractant) was added to the
bottom chamber. The cells were allowed to migrate for 12 h at 37°C
in a humidified incubator with 5% CO2. After 12 h, the
upper surface of the membrane was wiped with a cotton tip to
mechanically remove non-invasive cells, and the invasive cells
attached to the lower surface of the membrane were fixed with
methanol and stained with crystal violet for 20 min. The membranes
were extracted and mounted onto coverslips with the cells on the
upper surface. The number of cells invading the Matrigel were
counted in three randomly selected visual fields from the central
and peripheral portions of the filter with an inverted microscope
at magnification, ×100. Each assessment was performed in
triplicate.
Cell migration was determined by performing
wound-healing assays. EC9706 and Eca109 cells (1×105)
were seeded into 24-well plates with wound healing inserts (Cell
Biolabs, Inc., San Diego, CA, USA). Once cells had reached 90%
confluence, the inserts were removed with sterile forceps to create
a wound field of ~500 µm. Following removal of the cellular debris
with PBS, the cells were exposed to various concentrations of
α-solanine (0, 20, 40 or 60 µg/ml) for 24 h. Cell migration was
viewed using an inverted microscope. The wound area was scaled
using Image Pro Plus 6.0 software (National Institutes of Health,
Bethesda, MD, USA). The wound closure percentage was calculated
using the equation: Wound closure% = [1 - (wound area after 24 h /
wound area after 0 h) × 100%.
Cell apoptosis assay
Staining with annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) was performed using an Annexin
V-FITC/PI Apoptosis Detection kit (Vazyme Biotech Co., Ltd,
Nanjing, China) to detect and quantify the number of apoptotic
cells. EC9706 cells in the logarithmic phase of growth were seeded
into 6-well plates with 2×105 cells in each well. The
cells that were exposed to different concentrations of α-solanine
were collected and counted 48 h after incubation at room
temperature for 10 min in the dark. The cell pellets were
resuspended in 195 µl of binding buffer and stained with 5 µl each
of annexin V-FITC and PI staining solution for 10 min at room
temperature in the dark. Flow cytometry (BD FACSCanto II; BD
Biosciences, Franklin Lakes, NJ, USA) was performed with the
FACScan system using CellQuest software, version 7.5. The cell
apoptotic rate was calculated as: (Number of apoptotic cells in
each group / total number of cells in each group) × 100%.
Western blot analysis
The method of cell harvesting was described earlier
in the cell apoptosis assay section. Total protein was extracted
from each group of cells using radioimmunoprecipitation assay
buffer containing phenylmethylsulfonyl fluoride. A Bicinchoninic
Acid Protein Assay kit (Beyotime Institute of Biotechnology,
Haimen, China) was used to determine the total protein
concentration. Protein samples (30 µg) were resolved on 10%
SDS-PAGE gels and transferred onto polyvinylidene difluoride
membranes. After blocking the membranes with 3% bovine serum
albumin (Beyotime Institute of Biotechnology) for 1 h, the
membranes were incubated overnight at 4°C with mouse anti-matrix
metalloproteinase (MMP)-2 (cat. no. sc-13594), MMP-9 (cat. no.
sc-21733), E-cadherin (cat. no. sc-8426), B-cell lymphoma (Bcl)-2
(cat. no. sc-509), Bax (cat. no. sc-23959) and GAPDH (cat. no.
sc-365062) monoclonal antibodies (1:1,000; Santa Cruz Biotechnology
Inc., Dallas, TX, USA). Subsequently, the membranes were incubated
with horseradish peroxidase-conjugated goat anti-mouse secondary
antibody (1:1,000; cat. no. sc-2969; Santa Cruz Biotechnology). The
western blots were scanned and the protein intensities were
analyzed using the Typhoon™ FLA 7000 laser scanner with Typhoon™
FLA 7000 Control Software (GE Healthcare Life Sciences, Chalfont,
UK). Experiments were performed in triplicate.
Caspase-3/7 activity assay
Cells from each α-solanine-treated group were
collected as described in the cell apoptosis assay section earlier.
Caspase 3/7 activity was measured using a Caspase-Glo 3/7 assay
(Promega Corporation, Madison, WI, USA). The plates were incubated
at room temperature for 1 h, and 100 µl Caspase-Glo 3/7 reagent was
added after the incubation period. Luminescence intensity was then
detected using a microplate reader (Infinite 200 PRO; Tecan Trading
AG, Männedorf, Switzerland).
Statistical analysis
SPSS statistical software (version 16.0; SPSS, Inc.,
Chicago, IL, USA was used for statistical analysis. One-way
analysis of variance (ANOVA) was used to analyze the significance
between groups. Multiple comparisons were made using Fisher's Least
Significant Difference test when the probability indicated by ANOVA
was statistically significant. Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
α-solanine affects cell viability and
inhibits colony formation
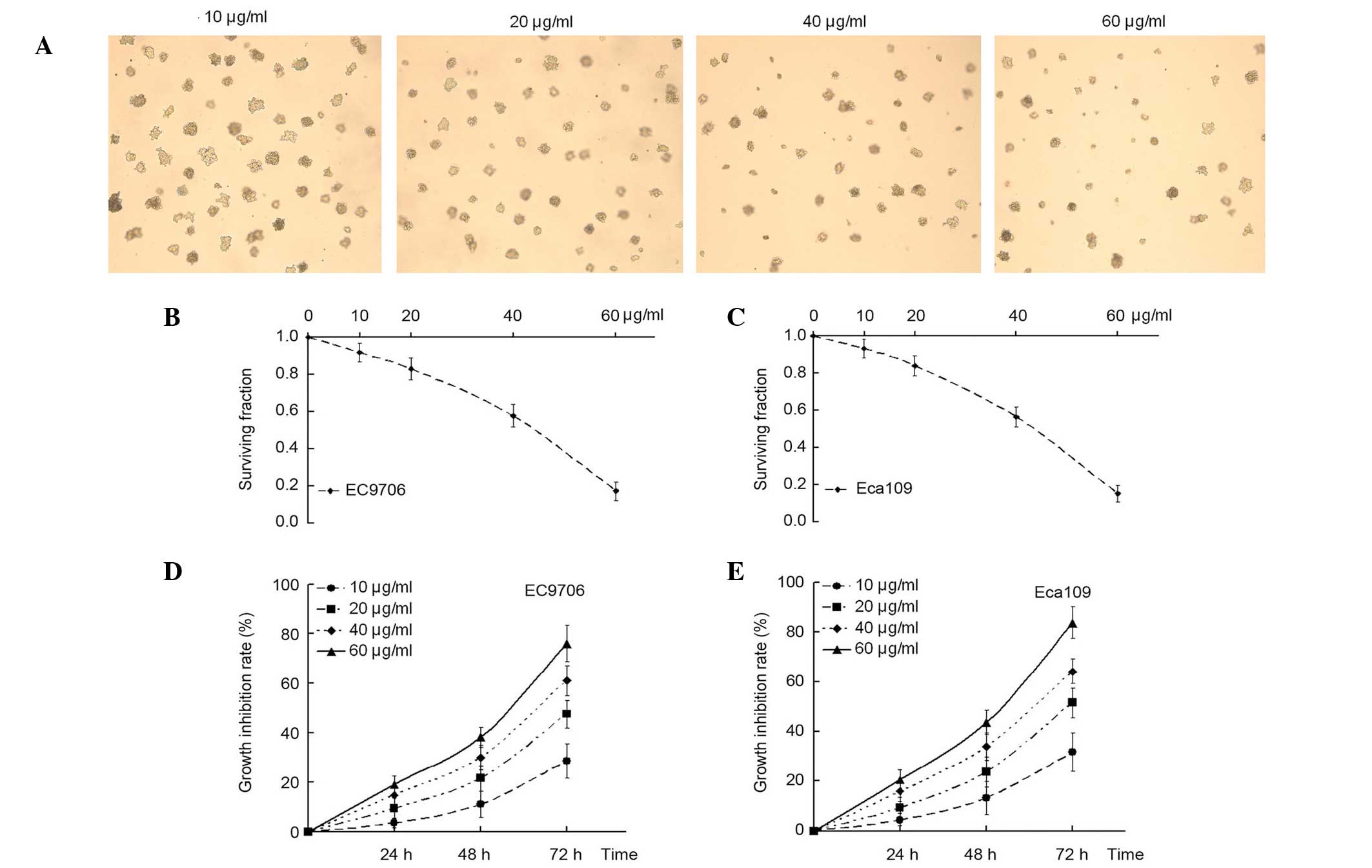
To determine the effects of α-solanine on tumor
cells, a clonogenic survival analysis was performed. It was
observed that the treatment of EC9706 and Eca109 EC cells with
various concentrations of α-solanine (10, 20, 40 and 60 µg/ml)
exerted an inhibitory effect on clonogenic survival. Furthermore,
the inhibitory effect was more pronounced when the concentration of
α-solanine was increased (Fig.
1A-C).
A CCK-8 assay was performed to measure the effect of
α-solanine on the growth and viability of EC9706 and Eca109 cells
in vitro. Compared with the control, (without α-solanine),
cell proliferation was markedly inhibited at different
concentrations in the α-solanine group in a dose- and
time-dependent manner (Fig. 1D and
E). These results suggested that α-solanine may function as a
tumor suppressor in EC cells in vitro.
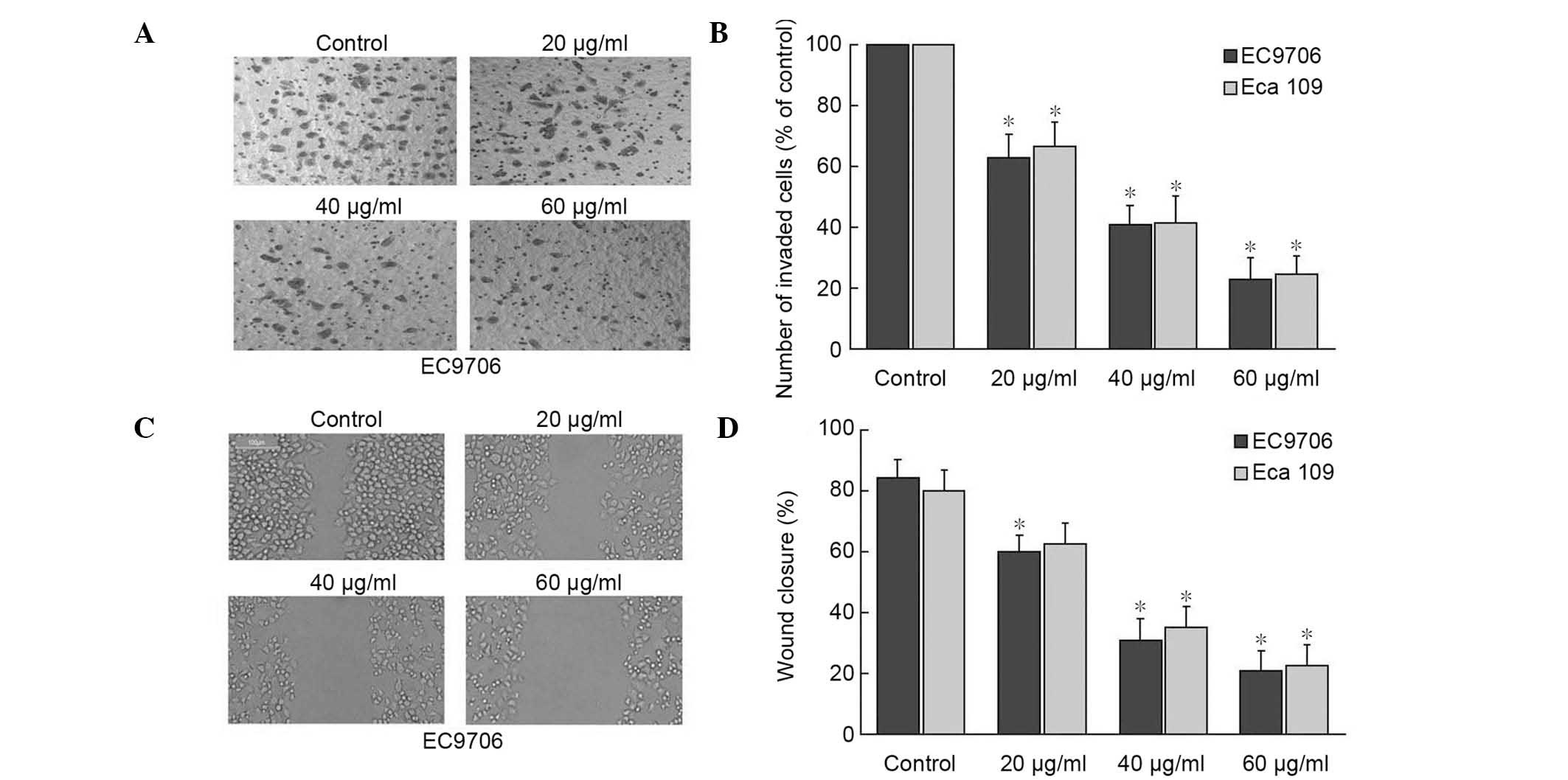
α-solanine decreases the invasion and
migration ability of EC9706 and Eca109 cells
A transwell assay was conducted in addition to a
wound-healing assay to evaluate the effect of α-solanine on the
invasion and migratory activity of EC9706 and Eca109 cells. The
cells were treated with 0, 20, 40 and 60 µg/ml α-solanine, and then
placed in the transwell chambers. Fig.
2A and B shows the average number of migrating cells
penetrating the transwell membrane following treatment with
different concentrations of α-solanine. The results indicated that
the average number of migrating cells penetrating the transwell
membrane was significantly lower for each concentration of
α-solanine, compared with the control group (P<0.05). The effect
of α-solanine on the migration of cells indicated
dose-dependency.
Fig. 2C and D reveal
the migratory ability in the treatment group. The control group had
reached a higher cell density at 24 h post-wounding compared with
each of the α-solanine-treated groups in the two cell lines. These
data indicate that α-solanine suppressed the migration and invasion
of EC cells in a dose-dependent manner.
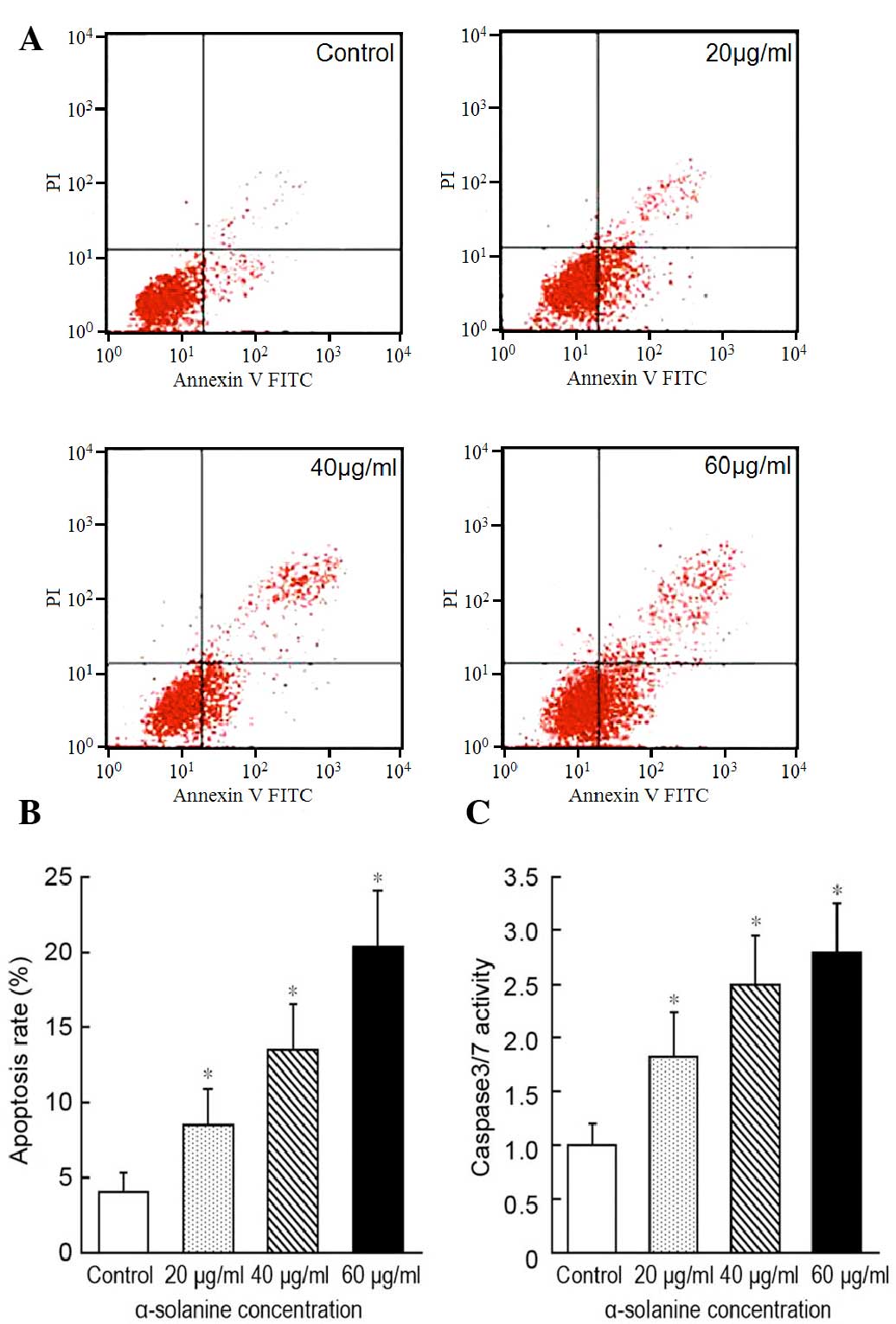
α-solanine induces apoptosis in EC9706
cells
To investigate the ability of α-solanine to induce
cell apoptosis, we used flow cytometry to measure the effect of
different concentrations of α-solanine on apoptosis in EC9706
cells. The cells found to be annexin V-positive or annexin V- and
PI-positive were defined as apoptotic cells (Fig. 3A). Compared to the control, the
apoptotic rate of EC9706 cells was increased following treatment
with each concentration of α-solanine compared with the control
group (P<0.05). Furthermore, the apoptotic rate was
significantly enhanced with increasing concentrations of α-solanine
(P<0.05; Fig. 3B).
To further assess the effect of α-solanine on tumor
cell apoptosis, a caspase-3/7 activity assay was performed. As
shown in Fig. 3C, the caspase-3/7
activity was higher in the α-solanine group compared with the
control group (P<0.05). These results demonstrate that
α-solanine is able to induce the apoptosis of EC cells.
a-solanine suppresses the expression
of metastasis-associated molecules in EC9706 cells
Subsequent to establishing that α-solanine was able
to decrease the invasion and migration ability of EC cells, the
underlying molecular mechanism was investigated. To determine
whether α-solanine exerted its inhibitory function on tumor cells
by regulating the protein expression levels of matrix MMP-2, MMP-9
and E-cadherin, a western blot analysis was performed to determine
the expression levels in α-solanine-treated EC9706 cells. The MMPs
are crucial molecules for extracellular matrix (ECM) degradation,
which induces cell invasion. The results indicated that α-solanine
suppressed the expression of MMP-2 and MMP-9 in a dose-dependent
manner (Fig. 4). Furthermore,
E-cadherin expression levels were significantly higher in each of
the α-solanine-treated groups compared with those in the control
group (Fig. 4). Thus, α-solanine is
able to upregulate the expression levels of E-cadherin, which may
increase cell adhesion, thereby indicating that α-solanine may be
able to inhibit metastasis by affecting the proteolytic activation
and adhesive capacity of cells.
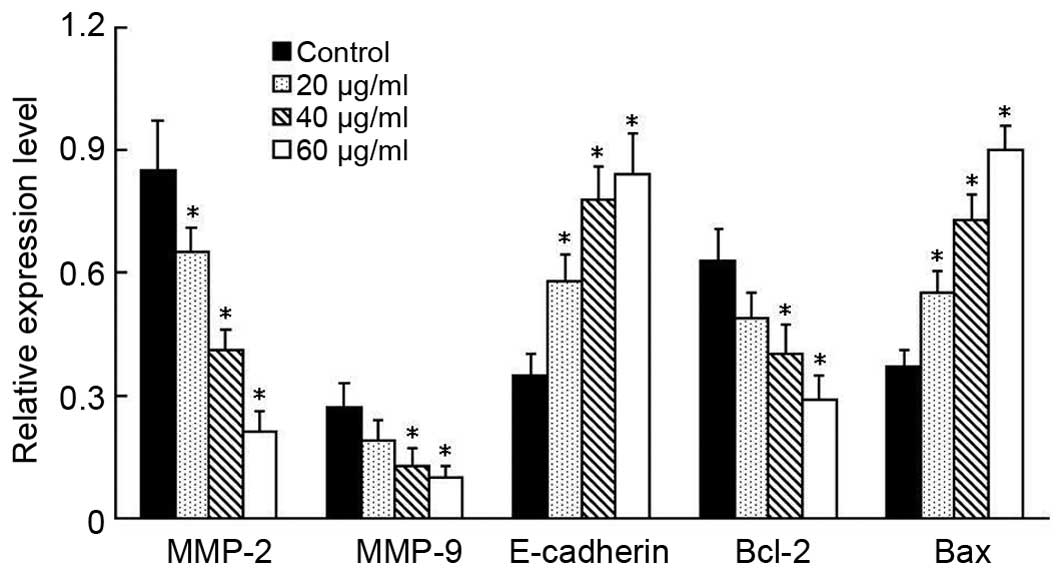 | Figure 4.α-solanine induces apoptosis and
decreases cancer cell invasion and migratory ability on the
alteration of the gene expression, as determined by western blot
analysis. EC9706 cells were seeded into 6-well plates and treated
with the indicated concentration α-solanine. After 48 h, total
protein was extracted for western blot analysis. The relative
expression levels of MMP-2, MMP-9, E-cadherin, Bcl-2 and Bax from
each independent experiment are shown. Greater alterations in the
protein expression levels were observed as the concentration of
α-solanine increased, indicating that α-solanine could promote
apoptosis and decrease the invasion and migration ability of EC9706
cells by regulating the expression levels of MMP-2, MMP-9,
E-cadherin, Bcl-2 and Bax. Data are presented as the mean ±
standard deviation of triplicate experiments. *P<0.05 vs.
control. MMP, matrix metalloproteinase; Bcl-2, B-cell lymphoma. |
α-solanine influences the protein
expression levels of apoptosis-associated genes
The protein expression levels of Bcl-2 and Bax,
which are associated with cell apoptosis, were determined by
western blot analysis. Compared with the control group, the
expression levels of Bcl-2 were decreased in the α-solanine-treated
groups, while the expression levels of Bax were increased
significantly (P<0.05; Fig. 4).
Furthermore, the protein expression levels of Bcl-2 and Bax were
observed to alter in a dose-dependant manner.
Discussion
Glycoalkaloids are produced in the sprouts, roots
and tubers of the potato plant, and are involved in the resistance
of the host-plant to bacteria, fungi, viruses and insects (6). Previous studies have reported that
glycoalkaloids are able to suppress the cell growth of human
cervical, liver, stomach, colon and skin cancers (8,10,18).
α-solanine is a major glycoalkaloids. High concentrations of
α-solanine can cause cytotoxic effects that induce rapid damage to
the plasma membrane, leading to lethal metabolic disorders
(10,19,20). In
addition to the toxic effects of α-solanine in normal physiological
functions, the compound has the potential to be cytotoxic against
cancer cells. Previous studies have demonstrated that α-solanine
exhibits anticarcinogenic potential, such as through inhibiting the
growth of various cancer cells and inducing apoptosis in human
colon cancer cells (8,9,11–14).
Therefore, it is reasonable to hypothesize that α-solanine is a
potentially effective therapy against human EC cells tumor
cells.
To assess this hypothesis, initial colony formation
and proliferation assays were conducted in the EC9706 and Eca109 EC
cell lines. It was observed that the cancer cells treated with
different concentrations of α-solanine showed significantly
decreased proliferation and surviving fraction of cells. In
addition, the inhibitory effect of α-solanine was time- and
dose-dependent, with the maximum inhibition detected at 72 h after
treatment. These data demonstrated that α-solanine has potential as
a therapy to treat EC cells in vitro.
Apoptosis has previously been demonstrated to be the
major cause of cell death. It is now widely recognized that
drug-induced apoptosis may be used to measure the sensitivity of
cells to therapies, with an increased rate of apoptosis indicating
that the cells have a higher sensitivity to chemotherapy (21,22). The
current study examined the apoptotic effect induced by α-solanine
at different concentrations. The results indicated that α-solanine
significantly induced apoptosis in EC cells compared with the
control, and the effects of treatment with higher concentrations of
α-solanine were greatest. To further investigate the mechanism
underlying the action of α-solanine on tumor cells apoptosis, the
expression levels of Bcl-2, and Bax were determined, in addition to
the caspase activity (caspase-3/7), which serves an important role
during cell apoptosis (23–25). The data revealed that α-solanine was
able to efficiently decrease Bcl-2 and increase Bax expression
levels, in addition to increasing caspase-3/7 activity, indicating
that α-solanine induced the apoptosis of cancer cells.
Furthermore, to evaluate the effect of α-solanine on
the invasion and migratory activity of EC9706 and Eca109 cells,
transwell and wound-healing assays were performed. The results
revealed that α-solanine was able to prevent the penetration of
cancer cells through the transwell membrane, and inhibited the
migratory ability of EC9706 and Eca109 cells in a dose-dependent
manner. Tumor metastasis is a complex multistep process, in which
cancer cells invade the basement membrane and ECM, and this process
is promoted by various proteolytic enzymes. The MMPs are a family
of zinc-containing endopeptidases with an important role in tumor
cell migration, tissue invasion and metastasis (26,27).
MMP-2 and MMP-9 serve particularly important roles in the process
of metastasis among the MMPs (28,29).
Therefore, the effects of α-solanine on MMP-2 and MMP-9 expression
levels were investigated in the present study. The results
indicated that expression levels of MMP-2 and MMP-9 were
significantly downregulated in the α-solanine-treated groups. In
addition, cell adhesion molecules serve to regulate cell polarity,
differentiation, proliferation and migration through an association
with the actin cytoskeletal network (30). In the present study, it was
ascertained that the expression of E-cadherin was significantly
increased in EC cells treated with α-solanine. These results
suggest that the effect of α-solanine on MMP-2/9 and E-cadherin may
be, at least in part, responsible for its anti-metastatic
potential.
In conclusion, the inhibition of cancer cell
proliferation and metastasis is an important aspect in cancer
prevention and treatment. The results of the current study
demonstrate that α-solanine was able to inhibit the proliferation
and invasion of EC9706 and Eca109 cells in vitro, and
induced apoptosis in these cells. Thus, as it has been indicated
that α-solanine possesses antitumor properties, its use in the
development of chemopreventive and/or chemotherapeutic agents for
the treatment of EC is important. The current results constitute a
novel insight into the anti-tumor mechanism of α-solanine, and
suggest that α-solanine is a potential agent for the prevention and
treatment of EC. Further studies should investigate the effect of
α-solanine on EC cells in vivo.
Acknowledgements
The present study was supported by the Education
Department of Henan Province Science and Technology Research
Projects (grant nos. 12A310015 and 13A310671).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szumiło J: Epidemiology and risk factors
of the esophageal squamous cell carcinoma. Pol Merkur Lekarski.
26:82–85. 2009.(In Polish). PubMed/NCBI
|
|
3
|
Yamashina T, Ishihara R, Nagai K, Matsuura
N, Matsui F, Ito T, Fujii M, Yamamoto S, Hanaoka N, Takeuchi Y, et
al: Long-term outcome and metastatic risk after endoscopic
resection of superficial esophageal squamous cell carcinoma. Am J
Gastroenterol. 108:544–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park B, Jun JH, Jung J, You S and Lee MS:
Herbal medicines for cancer cachexia: Protocol for a systematic
review. BMJ Open. 4:e0050162014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leng JC and Gany F: Traditional Chinese
medicine use among Chinese immigrant cancer patients. J Cancer
Educ. 29:56–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedman M: Potato glycoalkaloids and
metabolites: Roles in the plant and in the diet. J Agric Food Chem.
54:8655–8681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuo CI, Chao CH and Lu MK: Effects of
auxins on the production of steroidal alkaloids in rapidly
proliferating tissue and cell cultures of Solanum lyratum.
Phytochem Anal. 23:400–404. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedman M, Lee KR, Kim HJ, Lee IS and
Kozukue N: Anticarcinogenic effects of glycoalkaloids from potatoes
against human cervical, liver, lymphoma and stomach cancer cells. J
Agric Food Chem. 53:6162–6169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang SA, Paek SH, Kozukue N, Lee KR and
Kim JA: Alpha-chaconine, a potato glycoalkaloid, induces apoptosis
of HT-29 human colon cancer cells through caspase-3 activation and
inhibition of ERK 1/2 phosphorylation. Food Chem Toxicol.
44:839–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee KR, Kozukue N, Han JS, Park JH, Chang
EY, Baek EJ, Chang JS and Friedman M: Glycoalkaloids and
metabolites inhibit the growth of human colon (HT29) and liver
(HepG2) cancer cells. J Agric Food Chem. 52:2832–2839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu MK, Shih YW, Chang Chien TT, Fang LH,
Huang HC and Chen PS: α-Solanine inhibits human melanoma cell
migration and invasion by reducing matrix metalloproteinase-2/9
activities. Biol Pharm Bull. 33:1685–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv C, Kong H, Dong G, Liu L, Tong K, Sun
H, Chen B, Zhang C and Zhou M: Antitumor efficacy of α-solanine
against pancreatic cancer in vitro and in vivo. PLoS One.
9:e878682014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H, Lv C, Yang L, Wang Y, Zhang Q, Yu
S, Kong H, Wang M, Xie J, Zhang C and Zhou M: Solanine induces
mitochondria-mediated apoptosis in human pancreatic cancer cells.
Biomed Res Int. 2014:8059262014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji YB, Gao SY, Ji CF and Zou X: Induction
of apoptosis in HepG2 cells by solanine and Bcl-2 protein. J
Ethnopharmacol. 115:194–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu MK, Chen PH, Shih YW, Chang YT, Huang
ET, Liu CR and Chen PS: Alpha-Chaconine inhibits angiogenesis in
vitro by reducing matrix metalloproteinase-2. Biol Pharm Bull.
33:622–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao SY, Wang QJ and Ji YB: Effect of
solanine on the membrane potential of mitochondria in HepG2 cells
and [Ca2+]i in the cells. World J Gastroenterol. 12:3359–3367.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosenkranz V and Wink M: Induction of
apoptosis by alkaloids. non-protein amino acids and cardiac
glycosides in human promyelotic HL-60 cells. Z Naturforsch C.
62:458–466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cham BE and Meares HM: Glycoalkaloids from
Solanum sodomaeum are effective in the treatment of skin cancers in
man. Cancer Lett. 36:111–118. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamashoji S and Matsuda T: Synergistic
cytotoxicity induced by α-solanine and α-chaconine. Food Chem.
141:669–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Friedman M, Henika PR and Mackey BE:
Effect of feeding solanidine, solasodine and tomatidine to
non-pregnant and pregnant mice. Food Chem Toxicol. 41:61–71. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Konstantinidou AE, Korkolopoulou P and
Patsouris E: Apoptotic markers for tumor recurrence: A minireview.
Apoptosis. 7:461–470. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yano M, Inoue M and Shiozaki H:
Preoperative concurrent chemotherapy and radiation therapy followed
by surgery for esophageal cancer. Ann Thorac Cardiovasc Surg.
8:123–130. 2002.PubMed/NCBI
|
|
23
|
Hsia JY, Chen CY, Hsu CP, Shai SE, Yang
SS, Chuang CY, Wang PY and Chen JT: Expression of
apoptosis-regulating proteins p53, Bcl-2 and Bax in primary
resected esophageal squamous cell carcinoma. Neoplasma. 48:483–488.
2001.PubMed/NCBI
|
|
24
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuribayashi K, Mayes PA and El-Deiry WS:
What are caspases 3 and 7 doing upstream of the mitochondria?
Cancer Biol Ther. 5:763–765. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vargová V, Pytliak M and Mechírová V:
Matrix metalloproteinases. EXS. 103:1–33. 2012.PubMed/NCBI
|
|
27
|
Pytliak M, Vargová V and Mechírová V:
Matrix metalloproteinases and their role in oncogenesis: A review.
Onkologie. 35:49–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hofmann UB, Westphal JR, Van Muijen GN and
Ruiter DJ: Matrix metalloproteinases in human melanoma. J Invest
Dermatol. 115:337–344. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
30
|
Buda A and Pignatelli M: E-cadherin and
the cytoskeletal network in colorectal cancer development and
metastasis. Cell Commun Adhes. 18:133–143. 2011. View Article : Google Scholar : PubMed/NCBI
|