Introduction
Cystic hydatid disease (CHD) is a result of
infection with Echinococcus (E.) granulosus at the larval
stage. CHD affects sheep, cattle and humans, and occurs throughout
the world (1–2). In endemic regions, approximately
50/100,000 individuals have cystic echinococcosis, and prevalence
is between 5 and 10% in regions of Argentina, Peru, East Africa,
Central Asia and China (3). At
present, the major methods for the treatment of CHD include early
prevention, drug administration, which typically involves the
administration of benzimidazoles such as albendazole and
mebendazole, and surgery (4–6). The disadvantage of these methods is the
high cost, which is particularly challenging in remote areas and
undeveloped countries. Therefore, the development of a treatment
method that is relatively cheap and highly efficient at the same
time is of critical importance.
Vaccination of livestock may provide an additional
approach for the management of CHD. Various studies have reported
vaccines for the protection of certain animals, such as sheep,
goats and cattle, against hydatid disease caused by the cysts of
E. granulosus (7–12).
In a previous study of our group, E.
granulosus myophilin (Eg.myophilin) was isolated from a strain
of the parasite present in China (13). Furthermore, the immune response and
the induced immunoprotection of recombinant Eg.myophilin
(rEg.myophilin) were investigated using an experimental model of
hydatidosis in mice challenged with E. granulosus
protoscoleces (14). It is also
known that cytokines and serum antibodies serve an important role
in CHD. Cytokine response indicates Th1/Th2 polarization, which is
related to the cystic localization, the clinical stage and
evolution. Hydatid infection can induce Th1 and Th2 cytokines. Th1
cytokines are associated with protective immunity in
echinococcosis, while Th2 cytokines have been suggested to induce
susceptibility to the disease. If cytokine response skews Th1/Th2
ratios towards a preferentially immunopathology-associated Th2
polarization, the immune response will benefit parasite growth and
development. In addition, serum antibody response is associated
with (or is a marker for) cystic development, growth and disease
progression (1,15). The present study aimed to investigate
the immunoprotection of rEg.myophilin against the establishment of
a challenge oral infection using E. granulosus eggs in
sheep. In addition, the study investigates the underlying
protection mechanisms in order to assess the value of rEg.myophilin
as a potential molecular vaccine.
Materials and methods
Ethics statement
The present study was performed in strict accordance
with the recommendations reported in the Guidelines for Animal
Experimentation of Ningxia Medical University (Yinchuan, China).
The experimental protocol was approved by the Ethics Committee of
Ningxia Medical University.
Recombinant antigens
The recombinant myophilin antigens to protect sheep
against hydatid CHD caused by the E. granulosus was
expressed and purified by Ni2+ affinity chromatography
as described previously (14)
Briefly, protein expression was induced at 37°C by cultivation of
the transformed E. coli BL21 overnight in the presence of
0.4 mM isopropyl-β-D-thiogalactoside (Promega Corporation, Madison,
WI, USA). The recombinant six His-tagged rEg.myophilin was purified
from the extract of transformed E. coli BL21 (DE3) by
Ni2+ chelate affinity chromatography (Merck Millipore,
Darmstadt, Germany) according to the manufacturer's protocol.
Purified six His-tagged protein was analyzed using 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (Promega
Corporation).
Parasite eggs
Adult tapeworms of the E. granulosus species
were obtained from five dogs (gender, female; age, 18 months;
obtained from Lanzhou Veterinary Research Institute, Lanzhou,
China) using arecoline hydrobromide (Shanghai Seebio Biotech, Inc.,
Shanghai, China) and isolated from the mature proglottids under an
XSP-37XB inverse microscope (Qianke, Shanghai, China). The
procedure was performed as previously described by Lamberti et
al (16). Briefly, dogs were
given an oral dose (4 mg/kg) of arecoline hydrobromide. Purgation
followed for ~30 min. The purged samples were collected, mixed in
5% formal saline then passed through a sieve. The tapeworms were
examined and collected. After the proglottids of tapeworms were
opened with a scalpel and the eggs were carefully removed with
tweezers. The eggs were then packed in capsules, with 1,500 eggs
per capsule, which were used for challenging oral E.
granulosus infection.
Animals and immunization
Sheep of mixed gender (n=18; Lanzhou Veterinary
Research Institute) were used in the present study. Weaning of the
sheep was performed at 3–4 months of age and vaccination was
performed at 5–6 months. All sheep were raised in the same farm of
Lanzhou Veterinary Research Institute with a natural environment,
and were numerically identified using ear-tags. The animals were
divided into three random groups (n=6 in each) as follows: i)
rEg.myophilin group, in which sheep were immunized twice with 50 µg
rEg.myophilin in 1 ml phosphate-buffered saline (PBS) emulsified in
Freund's Adjuvant solution (Sigma-Aldrich, St. Louis, MO, USA),
with the first immunization initially conduced in Freund's
Adjuvant, Complete (week 0) and then in Freund's Incomplete
Adjuvant after 4 weeks; ii) control group, in which sheep were
injected with corresponding adjuvant plus PBS; and iii) negative
control group (healthy, untreated). Animals were injected
intramuscularly in the neck region. The rEg.myophilin and control
groups were challenged orally with 3,000 parasite eggs at 8 weeks
after the first vaccination.
Serum collection and protective
immunity
Blood samples were obtained from the animals through
venipuncture of the jugular vein using disposable syringes at
different time intervals after the first immunization (0, 1, 2, 4,
8, 12, 20, 28, 36 and 44 weeks), with infection performed at week
8. The blood samples were left to clot for 1 h at 37°C, and serum
was removed following centrifugation at 2,200 × g for 5 min at 4°C,
and then stored at −84°C.
At 44 weeks after challenge infection, the sheep
were sacrificed by captive bolt stunning followed by
exsanguination. The liver and lungs of each animal were removed and
sectioned (3 mm specimens for liver and 5 mm specimens for lungs),
and each slice was inspected and palpated to identify the cyst.
Subsequently, the percentage of protection in sheep was determined
according to the method reported by Dempster et al (17), as follows: Protective immunity in
vaccinated sheep (%) = (1 - mean number of cysts in test group /
average number of cysts in control group) × 100%.
Antibody measurement by ELISA
Serum antibody responses were quantified by ELISA
using the a polyclonal primary antibody purchased from Bio-Rad
Laboratories, Inc. (1:1,000; Hercules, CA, USA) at 0, 1, 2, 4, 8,
12, 20, 28, 36 and 44 weeks after the first immunization: Mouse
anti-bovine (cat no. MCA627); with infection performed at week 8.
Briefly, 96-well microtitre plates (Sino-American Biotechnology
Co., Luoyang, China) were incubated overnight at 4°C with
rEg.myophilin (10 µg per 100 µl per well) in 0.1 M carbonate buffer
(pH 9.6). Next, PBS plus 0.05% Tween-20 (PBST) was used to dilute
the serum samples by 1:1,000. Bound antibody was detected using the
following secondary antibodies diluted in PBST: Horseradish
peroxidase (HRP)-conjugated rabbit anti-sheep immunoglobulin (Ig)G
(heavy/light chains; 1:10,000; cat no. 51842504), mouse anti-bovine
IgG1 (1:20,000; cat no. MCA627), mouse anti-bovine IgG2 (1:20,000;
cat no. MCA6269) and HRP-conjugated rabbit anti-sheep IgA (1:100;
cat no. AHP949P). Goat Anti-Mouse IgG conjugated to HRP (cat no.
ZB-2305; Zhongshan Jinqiao, Inc. Beijing, China) was used to detect
antibodies following IgG1 and IgG2. Antibody titers were read at
450 nm using an ELISA Model 680 Microplate Reader (Bio-Rad
Laboratories, Inc.). The enzyme substrate used for detection was
3,3′5,5′-Tetramethylbenzidine (cat no. CW0050S; Kangwei Biotech
Company, Beijing, China). All samples were tested in duplicate.
Cytokine measurements by ELISA
The optical density value of cytokines was
determined by ELISA in accordance with the manufacturer's
instructions. Briefly, serum samples were added into microplate
ELISA kits pre-coated with rEg.myophilin (cat nos. CSB-E15963Sh,
CSB-E11217Sh, CSB-E12817Sh and CSB-E14018Sh for IL-4, IL-2, IL-10
and IFN-γ kits, respectively; Wuhan Huamei Biotech Co., Ltd.,
Wuhan, China) and maintained at 37°C for 2 h. Subsequent to washing
with PBST, 50 ng (100 µl) biotin-conjugated antibodies were added
per well and cultured for 2 h at 37°C. Following additional
washing, horseradish peroxidase-labeled streptavidin was added for
1.5 h at 37°C. Next, the plates were washed again and substrate was
added for 0.5 h at 37°C. Finally, the reaction was stopped by
adding 100 µl 2 M sulphuric acid, and the optical density was
measured at 450 nm using an ELISA reader (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data comparisons were performed using SPSS
statistical software (version 19.0; IBM SPSS, Armonk, NY, USA) and
were tested for significance using one-way analysis of variance
(ANOVA). Data are presented as mean ± standard deviation. A P-value
of <0.05 was considered to show a statistically significant
difference.
Results
Antibody levels in sheep, measured by
ELISA
The animals were challenge-infected 8 weeks after
the first immunization. Sera from animals treated with adjuvant
plus PBS and rEg.myophilin were prepared and tested using the ELISA
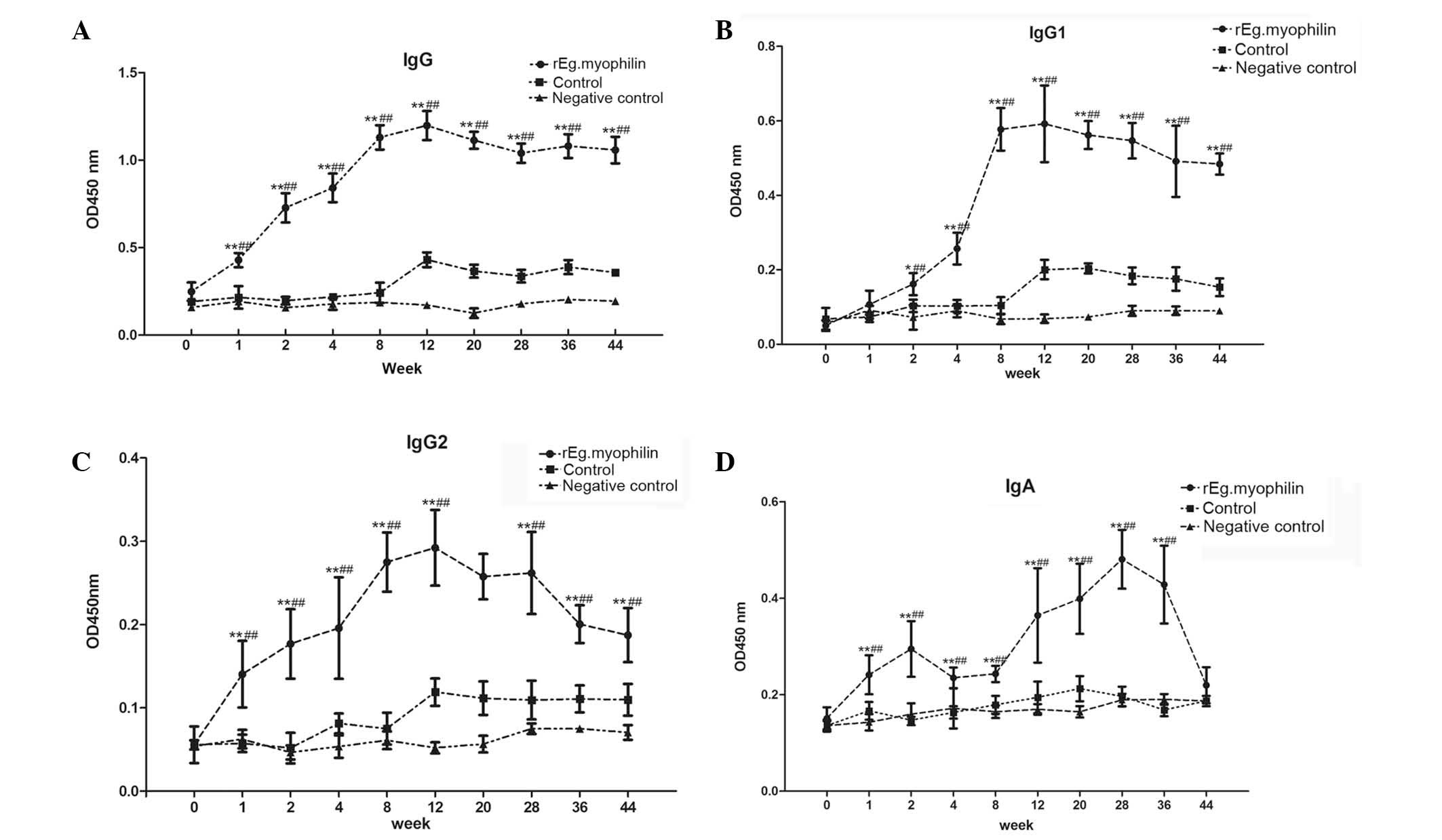
method. As shown in Fig. 1A, the
total IgG rapidly increased following immunization, when compared
with the control and negative control groups, reaching a maximum
level at week 12. The antibody levels were maintained until week 44
(Fig. 1A). In addition, the levels
of IgG1 and IgG2 tended to increase following rEg.myophilin
immunization, particularly between weeks 4 and 12 after the
beginning of immunization. After week 12, the level of IgG1 was
maintained high (Fig. 1B), whereas
the level of IgG2 declined slowly (Fig.
1C). Notably, the levels of IgA increased subsequent to the
first immunization; however, a declining trend was observed in week
2, followed by further increase between weeks 8 and 28, after which
the levels declined quickly (Fig.
1D).
Analysis of cytokine levels in sheep
immunized with rEg.myophilin, measured by ELISA
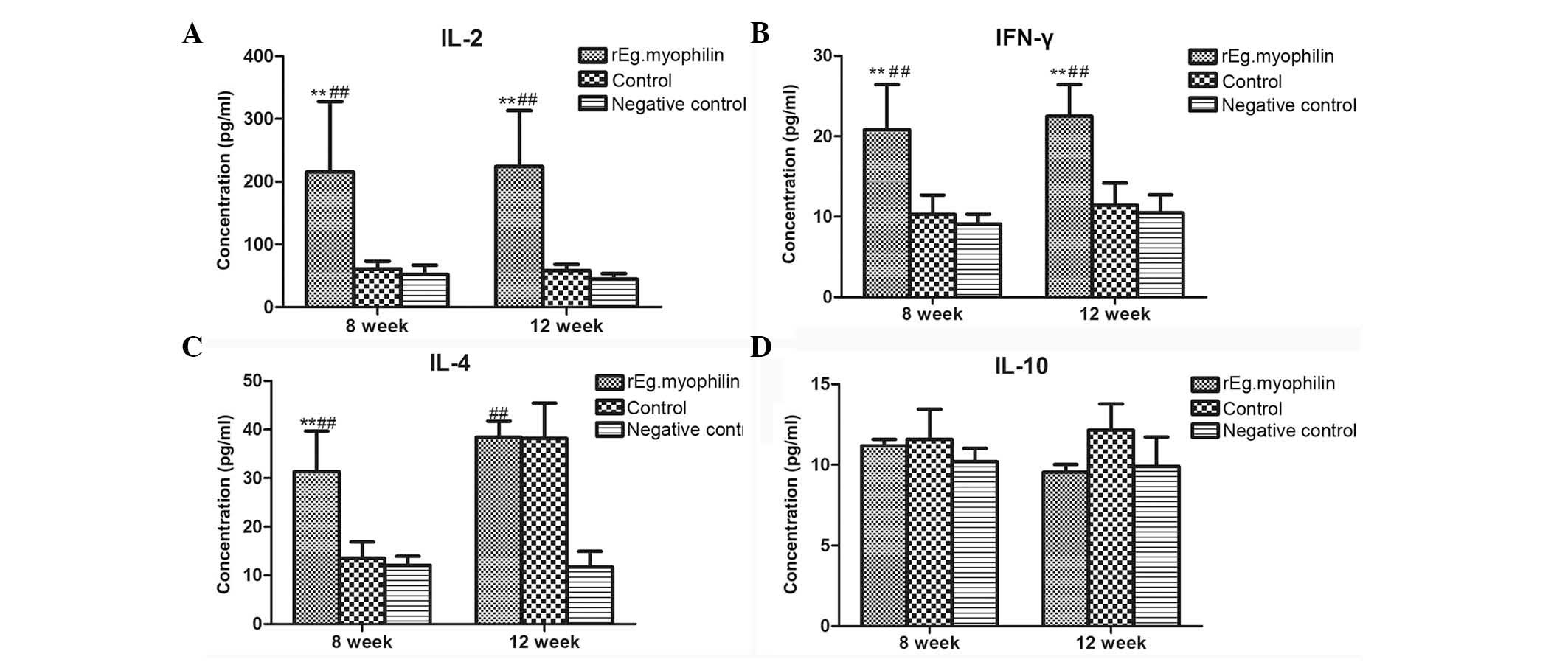
The response of animals to rEg.myophilin vaccination
was determined by measuring the levels of cytokines, including
interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-4 and IL-10. These
levels were measured at week 8 (corresponding to the time point
prior to infection) and week 12 (corresponding to 4 weeks after
infection), as shown in Fig. 2. The
levels of IL-2 (Fig. 2A) and IFN-γ
(Fig. 2B) in the immunized group
were found to be significantly higher when compared with those in
the control group prior to and following infection. Furthermore,
the levels of IL-4 were significantly higher compared with those in
the control group in week 8, while no statistically significantly
difference was observed in week 12 (Fig.
2C). By contrast, the levels of IL-10 were not found to be
significantly different from those of the control group at weeks 8
and 12 (Fig. 2D). The aforementioned
results suggest that rEg.myophilin immunization continuously
induces the production of IL-2 and IFN-γ, whilst only temporarily
inducing the production of IL-4, and has no effect on IL-10
level.
Protective immunity in sheep
Sheep in each group were sacrificed 44 weeks after
immunization followed by challenge-infection. The internal organs
of all sheep were carefully examined for the presence of hydatid
cysts. In sheep vaccinated with rEg.myophilin, the number of
hydatid cysts was found to be significantly lower compared with
that in the control group (Table I;
P<0.05). The protective immunity induced by rEg.myophilin was
determined based on the formula described earlier and was found to
be 92%.
 | Table I.Number of hydatid cysts and protective
immunity in vaccinated and control groups of sheep. |
Table I.
Number of hydatid cysts and protective
immunity in vaccinated and control groups of sheep.
| Groups | Eggs for
challenge | No. of cysts | Protective immunity
(%) |
|---|
| Negative control | 0 | 0 | – |
| Control | 3,000 | 25.00±16.59 | – |
| rEg.myophilin | 3,000 |
2.00±1.22a | 92.00 |
Discussion
E. granulosus parasites are known to undergo
numerous development stages in their life cycle, including
oncosphere, protoscoleces, adult worm and egg. The life cycles
involve two mammalian hosts. The adult cestode inhabits the small
intestine of a definitive host, such as a dog or fox, and produces
eggs containing infective oncospheres. Following the oral uptake of
eggs by an intermediate host animal, such as a human or sheep, a
metacestode develops in the internal organs of the host, which is
the larval stage of development (18).
The research group of the present study has
previously reported that the recombinant protein 14-3-3 (19), ferritin (20) and p29 (21) derived from the E. granulosus
exhibit strong immunogenic properties. In addition, rEg.myophilin
has been reported to show an effectively protective immunity in
mice (14). However, these
experiments present two deficiencies: E. granulosus
protoscoleces were intraperitoneally inoculated (secondary
infection) due to the experimental condition at the time of the
experiments, and thus the natural infection mode was peroral with
E. granulosus eggs. Furthermore, infection commonly affects
sheep, cattle and humans, not mice; thus, secondary infection in
mice may not accurately reflect the E. granulosus immune
mechanism.
In the present study, we attempted to investigate
the immunoprotection properties of rEg.myophilin against CHD
following oral infection of E. granulosus eggs in sheep. The
study examined whether immunization with rEg.myophilin leads to
effective immunity protection compared with the control group,
which may provide a foundation for the use of rEg.myophilin as a
valuable vaccine.
The results of the current experiments showed that
immunization with rEg.myophilin induced a high level of humoral
antibodies, and a mixed IgG1 and IgG2 response. Notably, the
analysis of IgG subtypes revealed a gradual shift from a
predominant IgG2 to a predominant IgG1 isotype at week 4
post-immunization (which corresponds to 4 weeks before infection).
With the extension of infection time, IgG and IgG1 were maintained
at higher levels compared with the control, whereas the levels of
IgG2 declined slowly. The results may suggest that protection
against E. granulosus in the rEg.myophilin group sheep was
correlated with the increased levels of IgG1 and total IgG, but not
with IgG2. In addition, the level of IgA increased subsequent to
the first immunization, while tended to decline at week 2. Next, a
rapid increase was observed following infection, reaching a maximum
level at week 28 (corresponding to 20 weeks after infection), after
which IgA levels quickly declined.
Although echinococcus IgA has been extensively
studied in dogs and several experimental models have been described
(22–24), little is known regarding E.
granulosus infection in sheep. Carol and Nieto (25) demonstrated that nasal immunization of
immunostimulating complexes produced from protoscoleces showed
significant induction of the secretory IgA antibody response, as
detected in the saliva and serum of dogs infected with E.
granulosus. Kouguchi et al (26) observed a sharp increase in serum IgA
responses immediately after challenge infection with E.
multilocularis in dogs (26).
The results of the present study showed that IgA may be involved in
protective immune response. However, the detailed mechanisms
through which this process leads to protective immunity remain
unknown.
Cytokines are commonly produced by T cells in the
regulation of parasitic diseases of humoral immunity. Th1 cells
produce IL-2 and IFN-γ to promote IgG2 production, while Th2 cells
produce IL-4 and IL-10 to promote a humoral response, particularly
by inducing IgG1. Th1 protective response mediates protective
immunity and helps the host to clear hydatid disease. Furthermore,
Th2 response promotes the humoral immune response and is beneficial
to parasitism (27–29). In the present study, four types of
cytokines were selected (IFN-γ, IL-2, IL-10 and IL-4), and their
dynamic changes in sheep were examined at weeks 8 (prior to
infection) and week 12 (4 weeks following infection). The levels of
Th1-type cytokines (IFN-γ and IL-2) were significantly higher
compared with that in the control group; however, Th2-type cytokine
(IL-10) were not significantly changed in sheep prior to and
following infection. The levels of IL-4 were significantly higher
compared with that of the control group after the second
immunization, while no significantly different was detected at 4
weeks after egg challenge.
These results suggested that rEg.myophilin likely
induced Th1-type immune response following immunization and
infection. The changes in cytokine levels appeared to be associated
with changes in the levels of specific antibodies. Furthermore,
after rEg.myophilin immunization, the increase of IgG2 levels was
coordinated with the increase of Th1-type cytokines. However, the
increase in IgG1 levels was not associated with an increase in the
levels of Th2-type cytokines. Furthermore, after rEg.myophilin
immunization, the increase in IgG2 levels was coordinated with the
increase of Th1-type cytokines (IL-2 and IFN-γ). However, the
increase in IgG1 levels was not associated with an increase in the
levels of Th2-type cytokines (IL-4 and IL-10). In addition, the
levels of IgG subtypes showed that IgG2 was predominant following
the second immunization. However, the mechanism through which
rEg.myophilin stimulates such an immune response in sheep remains
unclear.
In conclusion, the results of the present study
showed that immune reaction can be effectively activated by
treatment with rEg.myophilin. The results also demonstrated that
92% protection can be induced by rEg.myophilin against a challenge
oral infection with E. granulosus eggs in sheep. However,
the associated mechanism detailing how rEg.myophilin induces
protection requires further investigation prior to its development
as a practical vaccine.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81360249). The
authors would like to thank the Center of Scientific Technology of
Ningxia Medical University for providing the instruments.
References
|
1
|
Zhang W and McManus DP: Vaccination of
dogs against Echinococcus granulosus: A means to control
hydatid disease. Trends Parasitol. 24:419–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Battelli G: Echinococcosis: Costs, losses
and social consequences of a neglected zoonosis. Vet Res Commun.
33(Suppl 1): S21–S26. 2009.
|
|
3
|
Craig PS: Echinococcosis Working Group in
China: Epidemiology of human alveolar echinococcosis in China.
Parasitol Int. 55(Suppl): S221–S225. 2005.PubMed/NCBI
|
|
4
|
Eser I, Karabag H, Gunay S, Seker A, Cevik
M, Ali Sak ZH, Yalcin F and Aydin MS: Surgical approach for
patients with unusually located hydatid cyst. Ann Ital Chir.
85:50–55. 2014.PubMed/NCBI
|
|
5
|
Tamarozzi F, Nicoletti GJ, Neumayr A and
Brunetti E: Acceptance of standardized ultrasound classification,
use of albendazole, and long-term follow-up in clinical management
of cystic echinococcosis: A systematic review. Curr Opin Infect
Dis. 27:425–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barnes TS, Deplazes P, Gottstein B,
Jenkins DJ, Mathis A, Siles-Lucas M, Torgerson PR, Ziadinov I and
Heath DD: Challenges for diagnosis and control of cystic hydatid
disease. Acta Trop. 123:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heath DD and Lawrence SB: Antigenic
polypeptides of echinococcus granulosusoncospheres and definition
of protective molecules. Parasite Immunol. 18:347–357. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lightowlers MW, Lawrence SB, Gauci CG,
Young J, Ralston MJ, Maas D and Health DD: Vaccination against
hydatidosis using a defined recombinant antigen. Parasite Immunol.
18:457–462. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dempster RP, Robinson CM and Harrison GB:
Parasite vaccine development: Large-scale recovery of immunogenic
taenia ovisfusion protein GST-45W (B/X) from escherichia coli
inclusion bodies. Parasitol Res. 82:291–296. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manderson D, Dempster R and Chisti Y: A
recombinant vaccine against hydatidosis: Production of the antigen
in escherichia coli. J Ind Micro Biotechnol. 33:173–182. 2006.
View Article : Google Scholar
|
|
11
|
Larrieu E, Herrero E, Mujica G, Labanchi
JL, Araya D, Grizmado C, Calabro A, Talmon G, Ruesta G, Perez A, et
al: Pilot field trial of the EG95 vaccine against ovine cystic
echinococcosis in rio negro, Argentina: Early impact and
preliminary Data. Acta Trop. 127:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heath DD, Robinson C and Lightowlers MW:
Maternal antibody parameters of cattle and calves receiving EG95
vaccine to protect against Echinococcus granulosus. Vaccine.
30:7321–7326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao P, Xiong Y, Du J, Li JY, Wand YN, Li
ZJ and Zhao W: Immune protection of recombinant protein glutathione
s-transferase (rEgGST) of Echinococcus granulosus (Chinese
mainland strain). Zhong Guo Ren Shou Gong Huan Bing Xue Bao.
27:238–240. 2011.(In Chinese).
|
|
14
|
Sun J, Wang Y, Li Z, Ma R, Ji H, Xiong Y,
Wang Y, Li Z and Zhao W: Echinococcus granulosus:
Immunoprotection accompanied by humoral and cytokine response
against secondary hydatidosis in mice immunized with rEg.myophilin.
Vet Res Commun. 35:193–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Daeki AO, Craig PS and Shambesh MK:
IgG-subclass antibody responses and the natural history of hepatic
cystic echinococcosis in asymptomatic patients. Ann Trop Med
Parasitol. 94:319–328. 2000.PubMed/NCBI
|
|
16
|
Lamberti R, Cavagion L, Gatti A, Calvo C,
Gino L, Puches VV, Alvarez AR, Alvarez E, Cachau MG, Morete M and
Larrieu E: Humoral response and evolution of echinococcus infection
in experimentally infected sheep. Rev Bras Parasitol Vet.
23:237–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dempster R, Berridge M, Harrison G and
Heath D: Echinococcus granulosus: Development of an
intermediate host mouse model for use in vaccination studies. Int J
Parasitol. 21:549–554. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eckert J and Deplazes P: Biological,
epidemiological and clinical aspects of echinococcosis, a zoonosis
of increasing concern. Clin Microbiol Rev. 17:107–135. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Wang Y, Wang Q and Zhao W:
Echinococcus granulosus 14-3-3 protein: A potential vaccine
candidate against challenge with Echinococcus granulosus in
mice. Biomed Environ Sci. 25:352–358. 2012.PubMed/NCBI
|
|
20
|
Wang Y, Li ZJ, Li Z, Bo Y and Wei Z:
Recombinant ferritin protects mice against challenge with
Echinococcus granulosus? Acta Parasitol. 54:335–340. 2009.
View Article : Google Scholar
|
|
21
|
Shi Z, Wang Y, Li Z, Li Z, Bo Y, Ma R and
Zhao W: Cloning, expression and protective immunity in mice of a
gene encoding the diagnostic antigen P-29 of Echinococcus
granulosus. Acta Biochim Biophys Sin (Shanghai). 41:79–85.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gasser RB, Parada L, Acuna A, Burges C,
Laurenson MK, Gulland FM, Reichel MP and Paolillo E: Immunological
assessment of exposure to Echinococcus granulosus in a rural dog
population in Uruguay. Acta Trop. 58:179–185. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deplazes P, Thompson RC, Constantine CC
and Penhale WJ: Primary infection of dogs with Echinococcus
granulosus: Systemic and local (Peyer's patches) immune responses.
Vet Immunol Immunopathol. 40:171–184. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moreno M, Benavidez U, Carol H, Rosenkranz
C, Welle M, Carmona C, Nieto A and Chabalgoity JA: Local and
systemic immune responses to Echinococcus granulosus in
experimentally infected dogs. Vet Parasitol. 119:37–50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carol H and Nieto A: A mucosal IgA
response, but no systemic antibody response, is evoked by
intranasal immunization of dogs with Echinococcus granulosus
surface antigens iscoms. Vet Immunol Immunopathol. 65:29–41. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kouguchi H, Matsumoto J, Nakao R, Yamano
K, Oku Y and Yagi K: Characterization of a surface glycoprotein
from Echinococcus multilocularis and its mucosal vaccine
potential in dogs. PLoS One. 8:e698212013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shainheit MG, Saraceno R, Bazzone LE,
Rutitzky LI and Stadecker MJ: Disruption of interleukin-27
signaling results in impaired gamma interferon production but does
not significantly affect immunopathology in murine schistosome
infection. Infect Immun. 75:3169–3177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nono JK, Lutz MB and Brehm K: EmTIP, a
T-Cell immunomodulatory protein secreted by the tapeworm
echinococcus multilocularis is important for early metacestode
development. PLoS Negl Trop Dis. 8:e26322014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boutennoune H, Qaqish A, Al-Aghbar M,
Abdel-Hafez S and Al-Qaoud K: Induction of T helper 1 response by
immunization of BALB/c mice with the gene encoding the second
subunit of Echinococcus granulosus antigen B (EgAgB8/2).
Parasite. 19:183–188. 2012. View Article : Google Scholar : PubMed/NCBI
|