Introduction
Anesthetics may damage the brain during the
synaptogeneic phase of neurodevelopment due to their property at
the N-methul-D-aspartic acid and γ-aminoburyric acid A receptors
(1–3). The peak vulnerability window to the
neurotoxicity is during synaptogenesis phase, also called brain
growth spurt period (4,5). Such damage may affect the development
of the neural network, and the effect may persist to adult age for
the behavior and recognition ability (6,7). These
findings raised the concerns about the neurotoxicity of general
anesthetics, particularly the drugs used in pediatric anesthesia,
where the human fetal brain may be damaged during the procedure
(8).
Neuron apoptosis is the primary reason for the
neurotoxicity, and the mechanisms of anesthesia-induced apoptosis
have been well studied (5,9). Activation of proapoptotic proteins such
as Bax, may lead to mitochondrial membrane breakage and activation
of caspases, which execute cell apoptosis (10). On the other hand, generation of free
radicals such as reactive oxygen species (ROS) and reactive
nitrogen species (RNS) leads to lipid peroxidation, which could
cause brain damage. Such free radicals and damages may also elicit
inflammatory responses, which further amplify the apoptotic
response (11). By contrast,
antioxidant enzymes such as superoxide dismutase (SOD), can
scavenge excessive free radicals and alleviate their detrimental
effect (12).
Astragaloside IV (AS IV) is a saponin purified from
a traditional Chinese herbal medicine component Astragalus
membraneaceus (Fisch.) Bunge (13,14). AS
IV has exhibited antioxidant activity and anti-apoptosis function
in various types of cells and tissues (15–19). For
example, it ameliorates renal fibrosis by inhibiting
mitogen-activated protein kinase (MAPK) activation and attenuating
unilateral ureteral obstruction and transforming growth
factor-β-induced renal tubular cell apoptosis (19,20). By
contrast, AS IV is able to protect neuron cells from
1-methyl-4-phenylpyridnium ion-induced neurotoxicity by preventing
ROS production and inhibition of the Bax-mediated proapoptotic
pathway (21). AS IV also reduced
amyloid-β-1-42-induced neurotoxicity by inhibiting the
mitochrondrial permeability transition pore opening (22). However, its role in
anesthesia-induced neuroapoptosis and associated neurotoxicity has
not been investigated.
To determine whether AS IV is able to protect
developing neurons from anesthesia-induced apoptosis, we
investigated the effect of AS IV pre-treatment on
isoflurane-induced neuron apoptosis in hippocampus tissues of new
born rats. The levels of oxidative stress-related enzyme activities
in hippocampus tissues and serum were quantified, and serum levels
of proinflammatory cytokines were determined by ELISA. Protein
expression levels of nuclear factor (NF)-κB, caspase-3, B-cell
lymphoma 2 (Bcl-2), phosphorylated (p)-c-Jun N-terminal kinase
(JNK), extracellular signal-regulated kinase (ERK) and protein
kinase B (Akt) were detected by western blot assay. The results
suggested that AS IV had a protective effect in preventing
isoflurane-induced neurodegeneration, and indicated a potential
mechanism underlying such a protective effect. Overall, the present
study provides novel experimental evidence regarding the role of AS
IV as a potential means for reducing anesthesia-associated
neurotoxicity.
Materials and methods
Animals and reagents
A total of 20 male Sprague-Dawley rats at five days
postpartum (P5) with body weight of 18–25 g were obtained from the
animal experiment facility of the Shanghai Institute of Brain
Functional Genomics Institute (Eastern China Normal University,
Shanghai, China). All animal experiments were approved by the
Institutional Animal Care and Use Committee. AS IV was purchased
from Sigma-Aldrich (St. Louis, MO, USA), and dissolved in 100%
ethanol, then diluted in 0.9% saline. Isoflurane was purchased from
Abbott Laboratories (Lake Bluff, IL, USA). Terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) apoptosis detection kit was from Roche Diagnostics
(Rotkreuz, Switzerland). Antibodies against Akt (cat. no. 9272),
phospho-Akt (p-Akt; cat. no. 4060), JNK (cat. no. 9252), p-JNK
(cat. no. 4668), ERK (cat. no. 4695), p-ERK (cat. no. 4370), NF-κB
p65 (cat. no. 8242), histone H3 (cat. no. 4499) and glyceraldehyde
3-phosphate dehydrogenase (GAPDH; cat. no. 5174) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
against caspase-3 (cat. no. ab2171), glycogen synthase kinase
(GSK)-3β (cat. no. ab32391), p-GSK-3β (cat. no. ab75814) and Klotho
(cat. no. ab181373) were purchased from Abcam, Inc. Anti-BCL2 was
from Santa Cruz Biotechnology, Inc. (La Jolla, CA, USA). BCA
protein quantification kit was from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). RIPA buffer and the IMS cell image analysis
software was from Shanghai JRDUN Biotechnology, Co., Ltd.,
(Shanghai, China).
Experiment groups and anesthesia
procedure
The rats (P5) were randomly divided into five groups
(n=4/group), as follows: Inhaled isoflurane group; isoflurane + AS
IV pre-treatment (10, 40 or 100 mg/kg) groups; and sham group
without anesthesia. The rats were administered AS IV pre-treatment
for three days at 0, 10, 40 or 100 mg/kg/day, then exposed to
anesthesia. The sham group was pre-treated with ethanol in 0.9%
saline vehicle. The general anesthetics procedure was performed on
a platform over a water bath at 37–38°C. The isoflurane was flowed
by oxygen at a concentration of 1.3%. The isoflurane concentration,
oxygen concentration and CO2 concentration inside the
incubator were regulated using a monitoring machine (Datex-Ohmeda,
Inc., Madison, WI, USA). The rats were monitored for
SpO2, skin color and breathing frequency. The body
orientation was frequently changed to maintain SpO2 at
>90% during the 3-h anesthesia. Sham group rats were placed in
the incubator with air only.
Sample preparation, ELISA and
biochemistry analysis
Rats were injected with phenobarbital (100 mg/kg)
and sacrificed by CO2 asphyxiation at 12 h
post-anesthesia, and placed on ice. Then the hippocampus tissues
from rat brains were isolated. One part was fixed by 10% formalin
for 12 h at 4°C, then transferred into 50% ethanol for 4–5 h. The
tissues were processed for histology slides at 5-µm and subjected
to hematoxylin and eosin (H&E) staining. Another part was
dissolved in RIPA lysis buffer, and protein was isolated for
western blot assay or to undergo biochemical analysis of enzymatic
activities. Tumor necrosis factor (TNF)-α, interleukin (IL)-6 and
IL-1β levels were measured in blood and brain tissue collected at
12 h after isofluorane exposure using ELISA kits from R&D
Systems, Inc. (Minneapolis, MN, USA). Blood samples were
centrifuged at 3,000 × g for 10 min, and the serum was separated
and stored at −80°C until assayed. For detecting TNF-α, IL-6 and
IL-1β in brain tissue, frozen tissue samples were weighed and
homogenized, then centrifuged at 12,000 × g for 10 min. The
supernatants were stored at −80°C prior to analysis. The nitric
oxide (NO) and malondialdehyde (MDA) levels and enzyme activities
of nitric oxide synthase (iNOS) and superoxide dismutase (SOD) were
quantified using analytical kits from the Nanjing Jiancheng
Biological Engineering Institute (Nanjing, China), following
manufacturers' instruction.
Western blot analysis
Brain tissues were cut into small pieces and
homogenized in RIPA buffer containing protease and phosphatase
inhibitors. The samples were centrifuged at 21,952 × g for 15 min
at 4°C. The supernatant was quantified for protein concentration
using the BCA method and stored at −80°C until use. The nuclear and
cytosolic fractions were separated using the nuclear and
cytoplasmic extraction buffers for detecting NF-κB p65 protein.
Equal quantities of protein from each sample (80 µg) were loaded
per lane and separated on 10% SDS-polyacrylamide gels. The resolved
proteins were electrophoretically transferred to nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA), then blocked in 5%
nonfat milk for 1 h, followed by overnight primary antibody
incubation at 4°C. The primary antibodies used were anti-NF-κB p65
(1:1,000), anti-H3 (1:800), anti-Akt (1:1,000), anti-ERK (1:1,000),
anti-JNK (1:1,000), anti-Bcl-2 (1:100), anti-GSK-3β (1:500),
anti-Klotho (1:800) and anti-GAPDH (1:1,500). Subsequently the
membranes were washed three times with Tris-buffered saline with
0.1% Tween-20, then incubated for 1 h with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:4,000;
cat. no. ab6721; Abcam, Inc.) at room temperature. The membranes
were then incubated with enhanced chemiluminescence detection
reagents and exposed to X-ray films. The optical density of the
bands was measured using the ChemiDoc™ Imaging System (Bio-Rad
laboratories Inc., Hercules, CA, USA).
Apoptosis measurement
The TUNEL assay was performed using a commercially
available kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Six CA1 areas from each slide were
selected and analyzed for the positive cell number using the IMS
cell image analysis system. TUNEL positive cells are apoptotic
cells with dispersed distribution with condensed chromosomes, and
manifested with brown particles in nucleus. The number of apoptotic
cells in each field was counted for samples from different
groups.
Statistic analysis
All the results were presented as the mean ±
standard deviation. SPSS software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA) was used for analysis. Differences among groups
were compared using one-way analysis of variance, followed by
Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Isoflurane-induced neuron apoptosis in
hippocampus CA1 region
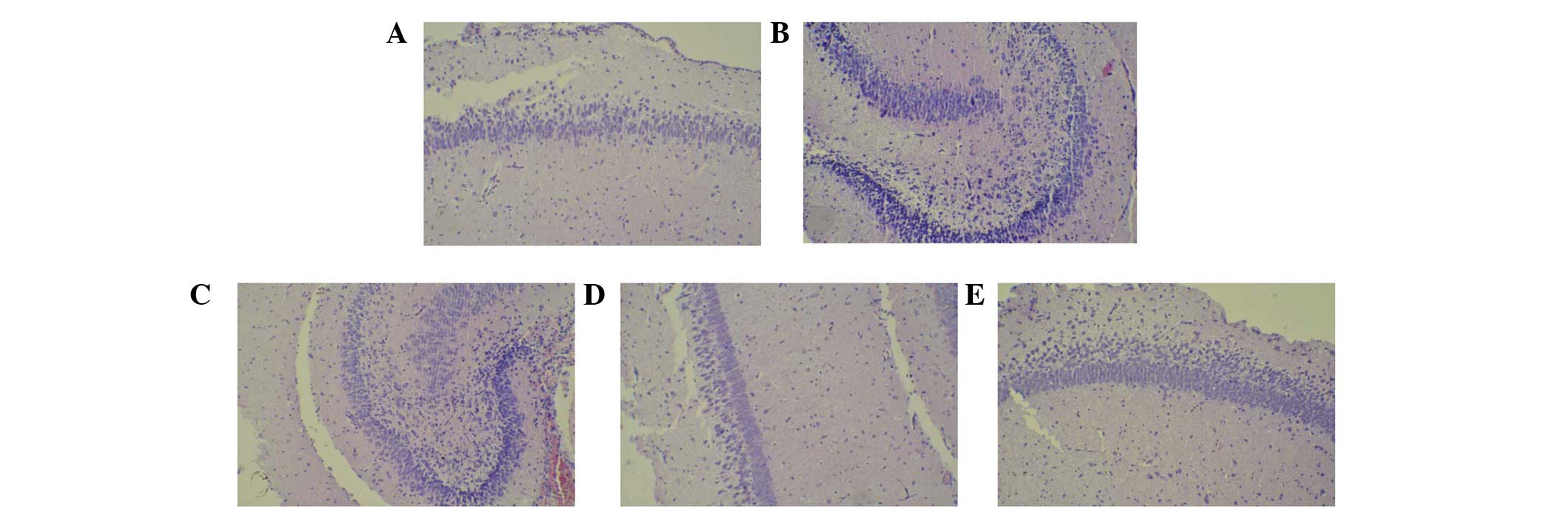
We observed the tissue damage in hippocampus area in
isofluorane-exposed rats, while there were few dead cells in
hippocampus CA1 neuron in sham group rats (Fig. 1A and B); however, the number of dead
cells showing shrunken cytoplasm and degenerated nuclei increased
in tissues from the isoflurane-treated group. These results
suggested that isoflurane exposure induced neuron cell death. By
contrast, AS IV treatment (Fig.
1C-E) reduced isoflurane-induced neuron cell death. This
indicated that AS IV exerts neuroprotective effects on
isoflurane-induced neuron cell damage in the CA1 region of
hippocampus in rats. To confirm whether the dead cells identified
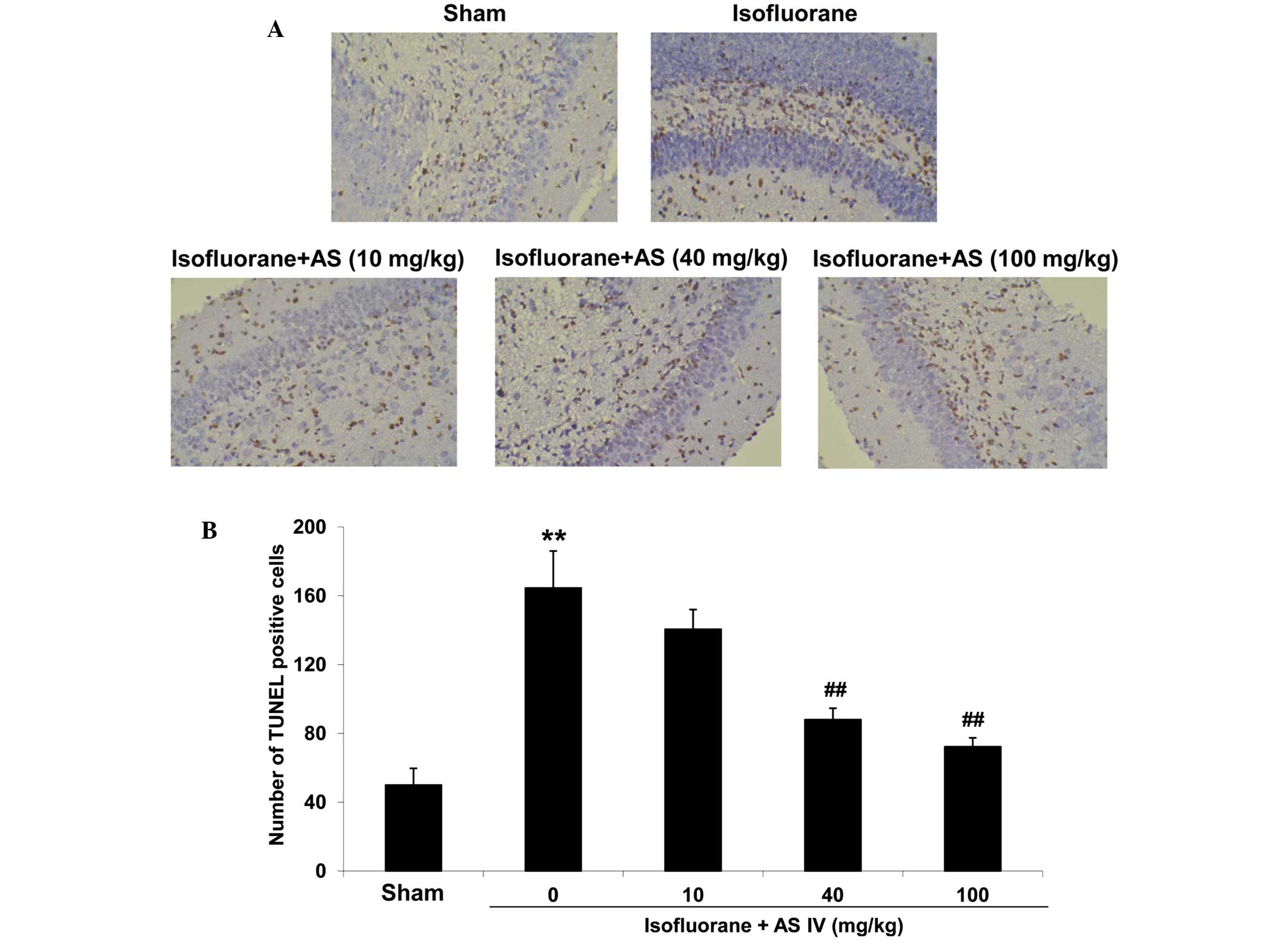
in the H&E stained tissues were apoptotic cells, we further
examined cell apoptosis in these tissues by TUNEL assay (Fig. 2A). In the sham group, the neuron in
hippocampus CA1 zone was rarely stained positive in the TUNEL
assay. Isoflurane exposure significantly increased the apoptotic
neuron numbers (P<0.05 vs. sham group, Fig. 2B). This indicates that the
neurodegenerative damage caused by isoflurane was primarily a
result of hippocampus neuron apoptosis. By contrast, AS IV
treatment (40 and 100 mg/kg) significantly reduced
isoflurane-induced neuron cell apoptosis (P<0.01).
SOD, iNOS, MDA and NO measurement in
hippocampus tissue and rat serum
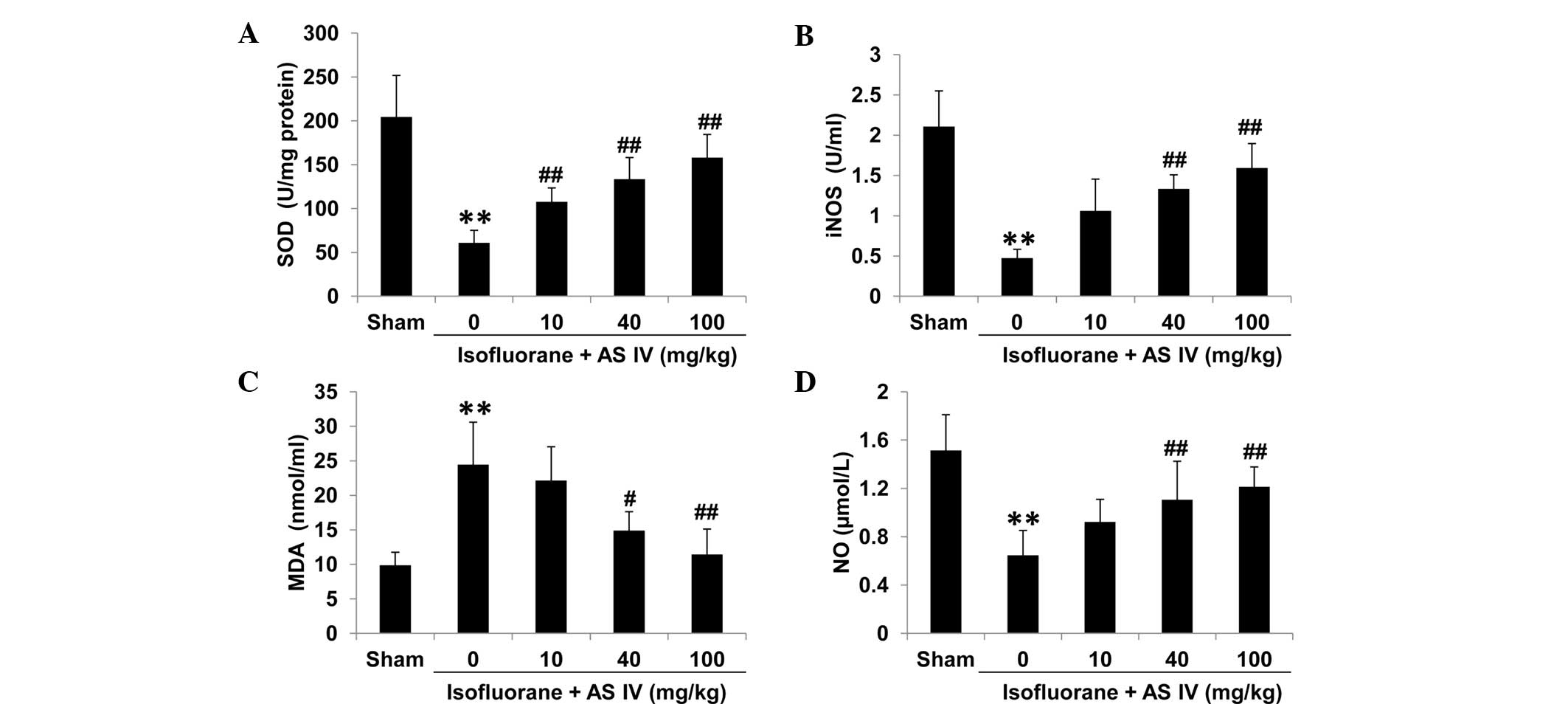
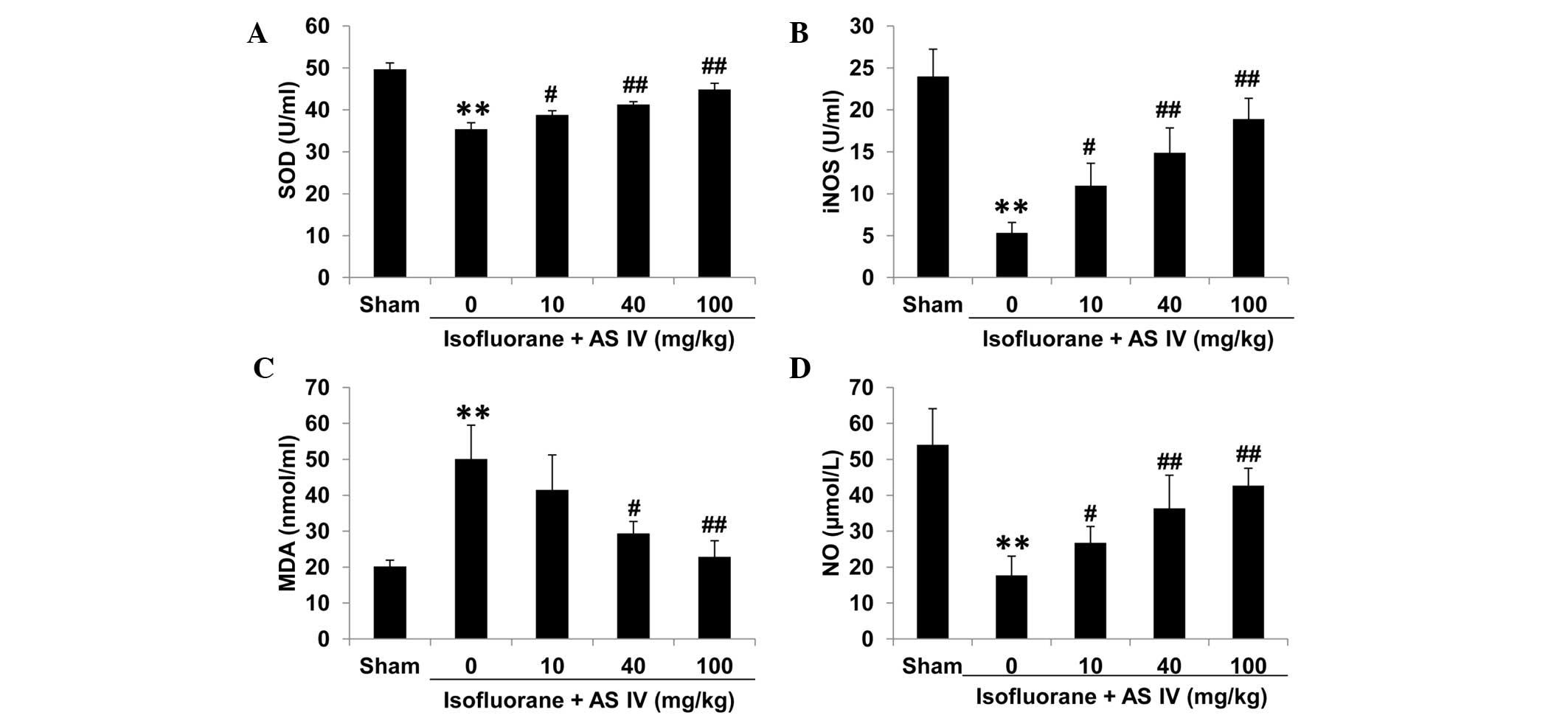
To test the levels of isoflurane-induced oxidative
stress in different groups, we isolated rat serum and hippocampus
tissues and measured the enzyme activities of a number of oxidative
stress-associated antioxidant enzymes, including SOD, iNOS, the
lipid peroxide marker MDA and NO. In the sham group, MDA levels
were relatively low in the hippocampus CA1 zone and rat serum of
the sham group (Figs. 3 and 4). Isoflurane exposure significantly
inhibited the enzyme activities of total iNOS, SOD and NO
(P<0.01 vs. sham), but increased MDA levels, which may
contribute to the neuron apoptosis. By contrast, AS IV treatment
(40 and 100 mg/kg) significantly inhibited isoflurane-induced MDA
production and ameliorated the suppression of iNOS and SOD enzyme
activity and NO production by isoflurane (Figs. 3 and 4).
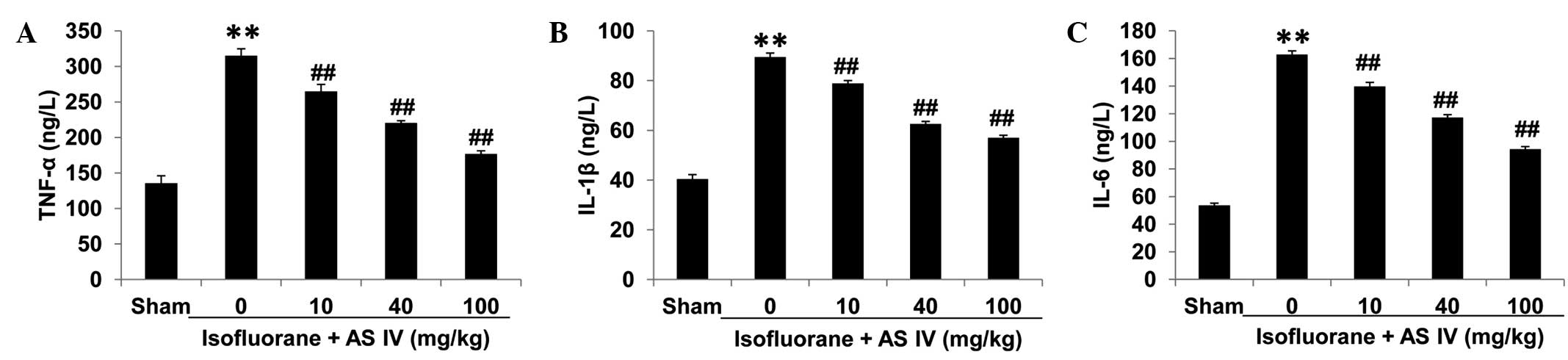
Proinflammatory cytokine measurement
in the serum and culture supernatant
To test the levels of isoflurane-induced
proinflammatory responses in different groups, we isolated rat
serum and measured levels of the major proinflammatory cytokines
TNF-α, IL-6 and IL-1β (Fig. 5). In
the sham group, these cytokine levels were relatively low in rat
serum. Isoflurane exposure significantly increased the levels of
all the three proinflammatory cytokines (P<0.01). By contrast,
AS IV treatment (10, 40 and 100 mg/kg groups) significantly reduced
isoflurane-induced proinflammatory cytokine release (P<0.01).
These results suggested that isoflurane exposure induced systemic
inflammatory responses in neonatal rats, and AS IV alleviated such
inflammation.
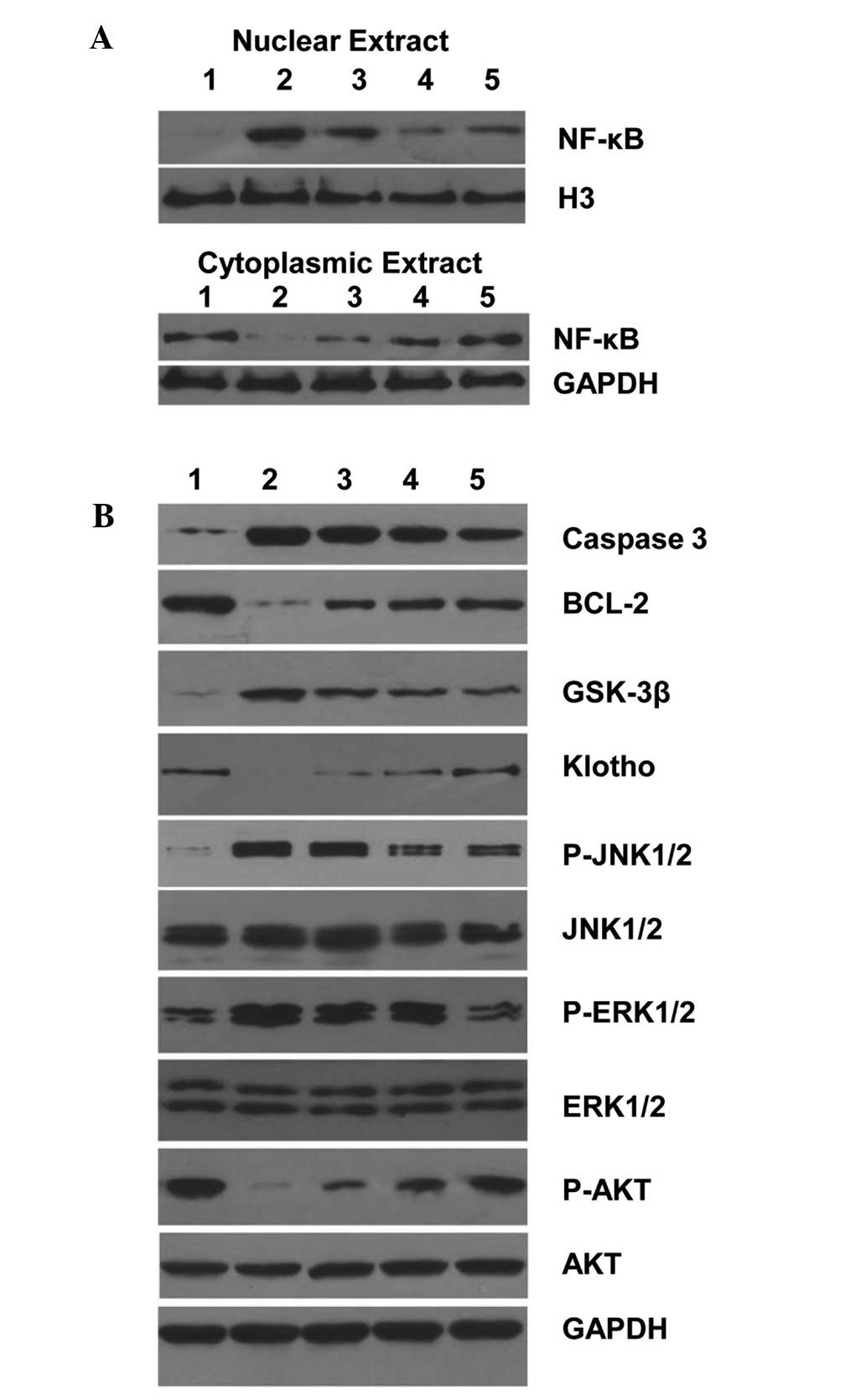
Inflammatory and antiapoptotic
signaling pathway analysis
The proinflammatory cytokine responses and reactive
nitrogen species production are primarily regulated by the NF-κB
pathway. Thus, we measured the NF-κB activity in rat hippocampus
tissues by western blot. In protein samples from the sham group,
NF-κB protein was detected predominantly in the cytoplasm and
levels were low in the nucleus, suggesting low NF-κB activity
(Fig. 6A). Isoflurane exposure
markedly increased nuclear levels of NF-κB protein, indicating
NF-κB activation in hippocampus tissue. By contrast, AS IV
treatment reduced isoflurane-induced NF-κB nuclear localization,
indicating suppressed NF-κB activation (Fig. 6A).
We further measured the expression levels of a
number of apoptosis-associated proteins in different groups. In
protein samples from the sham group, the expression of the
proapoptotic marker protein caspase-3 was relatively low. By
contrast, the antiapoptotic marker protein Bcl-2 was abundantly
expressed. Isoflurane exposure markedly increased caspase-3 protein
levels and decreased Bcl-2 protein levels (Fig. 6B). By contrast, AS IV treatment
inhibited isoflurane-induced caspase-3 upregulation and Bcl-2
downregulation, suggesting that AS IV suppressed isoflurane-induced
hippocampus apoptosis. Similarly, isoflurane exposure induced
GSK-3β and decreased Klotho and phosphorylated Akt protein levels;
however, AS IV pre-treatment inhibited isoflurane-induced changes
in these proteins.
MAPK signaling pathways are intricately involved in
apoptotic responses (23). We
observed the activation of JNK and ERK by isoflurane exposure;
indicated by increased phosphorylated protein levels. Furthermore,
AS IV treatment inhibited isoflurane-induced MAPK activation
(Fig. 6B).
Discussion
Apoptotic neuron cell death after anesthesia
exposure has an important consequence in neurotoxicity (24). The two major pathways for cell
apoptosis are the death receptor (Fas and TNFR)-mediated intrinsic
pathway and mitochondria pathway (24). The mitochondria pathway can be
activated by extrinsic and intrinsic stimuli, which includes
oxidation and cytotoxic drug treatment. For the immature animal
brain, particularly in specific development stages such as
synaptogenesis period, general anesthesia can induce severe
neurotoxicity, and mitochondria pathway activation is considered
the initial step (5). The key step
is cytochrome c release from mitochondria to the cytoplasm, and the
mechanism is that isoflurane may activate IP3 receptor on
endoplasmic reticulum and disrupt the calcium homeostasis, and
changed the structure and function of the outer membrane-located
Bcl-2 family proteins, mitochrondrial permeability transition, MPT
and the channel (MPT pore) (25).
Clinically, a mixture of laughing gas and isoflurane has been shown
to activate the mitochondria pathway in neurons by downregulating
antiapoptotic protein Bcl-xL, and immature brains are even more
sensitive to isoflurane-induced neuron apoptosis by increasing
proapoptotic protein Bax, decreasing antiapoptotic protein Bcl-2,
and allowing free radical accumulation and cytochrome c release
into cytoplasm, initiating apoptosis (26). A similar neurodegenerative effect was
observed in the present study.
Oxidative stress, which is a result of the
overproduction and accumulation of free radicals, including ROS and
RNS, plays a major role in a variety of neurological pathogenesis
(27). Disruption of the balance of
free radical generation and antioxidant enzyme system may result in
oxidative stress. Previous studies have reported that AS IV is an
effective free radical scavenger and potentiates the intrinsic
antioxidant system in the animal model of cerebral
ischemia/reperfusion (16). In the
present study, the results indicated that AS IV enhanced intrinsic
antioxidant enzyme activity and decreased RNS accumulation in the
hippocampus tissue and serum.
Notably, we also observed a systemic increase of
proinflammatory cytokines (TNF-α, IL-1β and IL-6) in neonatal rats
following isoflurane exposure, suggesting an inflammatory responses
triggered by the anesthesia procedure. AS IV also exhibited
anti-inflammatory activity, in this case by alleviating
anesthesia-induced proinflammatory cytokine production. As a master
regulator of inflammatory responses and cell survival, NF-κB
activation has been documented in multiple neurological pathology
conditions (28). Although NF-κB is
primarily a survival factor in numerous cell types, its role in
neuronal apoptosis may vary depending on the specific contexts and
treatments (29). In the present
study, we observed isoflurane-induced NF-κB activation, which could
be from accumulated oxidative stress. Crucially, AS IV reduced such
NF-κB activation, and ameliorated systemic proinflammatory cytokine
production.
It has been reported that isoflurane exposure
downregulates Bcl-2 and ERK signaling in the developing rat brain
(30). However, we identified
increased level of phosphorylated ERK and JNK in neuron cells after
isoflurane exposure. The JNK pathway is generally considered a
proapoptotic pathway, while ERK signaling is often considered
neuroprotective (23). However,
MAPKs are often activated simultaneously in response to stress or
stimulation (23). Thus, we consider
ERK activation as an early reaction in response to isoflurane
exposure; however, its neuroprotective effect may be masked by
other signaling pathways such as JNK pathway.
AS IV is a potent agent for antioxidant and ROS
clearance. AS IV may inhibit oxidized product formation in cell
membrane and nuclear DNA adducts, reduce the accumulation of ROS
inside the mitochondria and alleviate oxidative stress-induced cell
apoptosis (17,21). AS IV has been speculated to exert a
protective effect in treating degenerative diseases in central
neural system, such as Alzheimer's disease, Parkinson's disease,
ischemia-reperfusion-induced brain damage, and physical damage in
the brain (15,22,31,32). The
results of the present study confirmed that AS IV is protective
against isoflurane-induced neuronal degeneration in the developing
rat brain. The underlying mechanism may be associated with the
inhibition of oxidative stress and inflammation in the hippocampus
region and systemic level, thereby reducing abnormal apoptosis of
developing neuron. Thus, the antiapoptotic and antioxidative
function of AS IV in general anesthesia-induced neuron toxicity may
have potential clinical relevance.
References
|
1
|
Ikonomidou C, Bosch F, Miksa M, Bittigau
P, Vöckler J, Dikranian K, Tenkova TI, Stefovska V, Turski L and
Olney JW: Blockade of NMDA receptors and apoptotic
neurodegeneration in the developing brain. Science. 283:70–74.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikonomidou C, Bittigau P, Ishimaru MJ,
Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F,
Tenkova T, et al: Ethanol-induced apoptotic neurodegeneration and
fetal alcohol syndrome. Science. 287:1056–1060. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
Benshoff ND, Dikranian K, Zorumski CF, Olney JW and Wozniak DF:
Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.PubMed/NCBI
|
|
4
|
Dobbing J and Sands J: Comparative aspects
of the brain growth spurt. Early Hum Dev. 3:79–83. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felderhoff-Mueser U and Ikonomidou C:
Mechanisms of neurodegeneration after paediatric brain injury. Curr
Opin Neurol. 13:141–145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fredriksson A, Pontén E, Gordh T and
Eriksson P: Neonatal exposure to a combination of
N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor
anesthetic agents potentiates apoptotic neurodegeneration and
persistent behavioral deficits. Anesthesiology. 107:427–436. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilder RT, Flick RP, Sprung J, Katusic SK,
Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL and
Warner DO: Early exposure to anesthesia and learning disabilities
in a population-based birth cohort. Anesthesiology. 110:796–804.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Servick K: Biomedical Research.
Researchers struggle to gauge risks of childhood anesthesia.
Science. 346:1161–1162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olney JW, Ishimaru MJ, Bittigau P and
Ikonomidou C: Ethanol-induced apoptotic neurodegeneration in the
developing brain. Apoptosis. 5:515–521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Renault TT, Floros KV, Elkholi R, Corrigan
KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla
JJ, Buettner C, et al: Mitochondrial shape governs BAX-induced
membrane permeabilization and apoptosis. Mol Cell. 57:69–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin H and Zhu M: Free radical oxidation of
cardiolipin: Chemical mechanisms, detection and implication in
apoptosis, mitochondrial dysfunction and human diseases. Free Radic
Res. 46:959–974. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jawaid P, ur Rehman M, Yoshihisa Y, Li P,
Zhao Ql, Hassan MA, Miyamoto Y, Shimizu T and Kondo T: Effects of
SOD/catalase mimetic platinum nanoparticles on radiation-induced
apoptosis in human lymphoma U937 cells. Apoptosis. 19:1006–1016.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Wang W, Xue J, Gu Y and Lin S:
Meta-analysis of the clinical value of Astragalus membranaceus in
diabetic nephropathy. J Ethnopharmacol. 133:412–419. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matkovic Z, Zivkovic V, Korica M, Plavec
D, Pecanic S and Tudoric N: Efficacy and safety of Astragalus
membranaceus in the treatment of patients with seasonal allergic
rhinitis. Phytother Res. 24:175–181. 2010.PubMed/NCBI
|
|
15
|
Qu YZ, Li M, Zhao YL, Zhao ZW, Wei XY, Liu
JP, Gao L and Gao GD: Astragaloside IV attenuates cerebral
ischemia-reperfusion-induced increase in permeability of the
blood-brain barrier in rats. Eur J Pharmacol. 606:137–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, Ma RN, Li LH, Qu YZ and Gao GD:
Astragaloside IV reduces cerebral edema post-ischemia/reperfusion
correlating the suppression of MMP-9 and AQP4. Eur J Pharmacol.
715:189–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Y, Du M, Gao Y, Liu H, Wang H, Wu X and
Wang Z: Astragaloside IV attenuates experimental autoimmune
encephalomyelitis of mice by counteracting oxidative stress at
multiple levels. PLoS One. 8:e764952013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gui D, Huang J, Guo Y, Chen J, Chen Y,
Xiao W, Liu X and Wang N: Astragaloside IV ameliorates renal injury
in streptozotocin-induced diabetic rats through inhibiting
NF-κB-mediated inflammatory genes expression. Cytokine. 61:970–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding Y, Yuan S, Liu X, Mao P, Zhao C,
Huang Q, Zhang R, Fang Y, Song Q, Yuan D, et al: Protective effects
of astragaloside IV on db/db mice with diabetic retinopathy. PLoS
One. 9:e1122072014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang ZG, Wu L, Wang JL, Yang JD, Zhang J,
Zhang J, Li LH, Xia Y, Yao LB, Qin HZ and Gao GD: Astragaloside IV
prevents MPP+-induced SH-SY5Y cell death via the
inhibition of Bax-mediated pathways and ROS production. Mol Cell
Biochem. 364:209–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Q, Jia N, Wang W, Jin H, Xu J and Hu
H: Protective effects of astragaloside IV against amyloid beta1-42
neurotoxicity by inhibiting the mitochondrial permeability
transition pore opening. PLoS One. 9:e988662014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miloso M, Scuteri A, Foudah D and Tredici
G: MAPKs as mediators of cell fate determination: An approach to
neurodegenerative diseases. Curr Med Chem. 15:538–548. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Friedlander RM: Apoptosis and caspases in
neurodegenerative diseases. N Engl J Med. 348:1365–1375. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang H, Liang G, Hawkins BJ, Madesh M,
Pierwola A and Wei H: Inhalational anesthetics induce cell damage
by disruption of intracellular calcium homeostasis with different
potencies. Anesthesiology. 109:243–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blaylock M, Engelhardt T and Bissonnette
B: Fundamentals of neuronal apoptosis relevant to pediatric
anesthesia. Paediatr Anaesth. 20:383–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skowrońska M and Albrecht J: Oxidative and
nitrosative stress in ammonia neurotoxicity. Neurochem Int.
62:731–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mincheva-Tasheva S and Soler RM: NF-κB
signaling pathways: Role in nervous system physiology and
pathology. Neuroscientist. 19:175–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanders RD, Sun P, Patel S, Li M, Maze M
and Ma D: Dexmedetomidine provides cortical neuroprotection: Impact
on anaesthetic-induced neuroapoptosis in the rat developing brain.
Acta Anaesthesiol Scand. 54:710–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao A, Guo S, Tu S, Ammar AB, Tang J,
Hong Y, Wu H and Zhang J: Astragaloside IV alleviates early brain
injury following experimental subarachnoid hemorrhage in rats. Int
J Med Sci. 11:1073–1081. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo Y, Qin Z, Hong Z, Zhang X, Ding D, Fu
JH, Zhang WD and Chen J: Astragaloside IV protects against ischemic
brain injury in a murine model of transient focal ischemia.
Neurosci Lett. 363:218–223. 2004. View Article : Google Scholar : PubMed/NCBI
|