Introduction
Infliximab is a genetically constructed
immunoglobulin (Ig)G1 murine-human chimeric monoclonal antibody
that binds the soluble subunit and the membrane-bound precursor of
tumor necrosis factor-α (TNF-α). Consequently, it helps to decrease
the biological activity of TNF-α (1,2).
Neutralization of TNF-α has been suggested as a therapeutic
strategy for patients with inflammatory bowel disease (IBD),
rheumatoid arthritis (RA), spondyloarthropathy (SpA) and various
other chronic inflammatory conditions (3–5). IBD,
RA, and SpA are common chronic inflammatory conditions,
characterized by episodes of remission and relapse, which have a
major impact on the patients' physical, emotional and social
well-being. TNF-α cytokine, which is expressed by activated
macrophages, has been implicated in the pathogenesis of IBD, RA and
SpA (6–8). Infliximab has been widely used to treat
patients who failed to respond to other anti-inflammatory agents
since FDA approval was attained in 1998. The advent of infliximab
has dramatically improved the quality of life and prognosis of
these patients.
Multicenter randomized clinical trials (RCTs) have
been conducted to evaluate the efficacy and safety of infliximab
throughout the past decade. These RCTs demonstrated that infliximab
is an effective therapy for the treatment of various inflammatory
diseases (9–12). However, the safety of infliximab
remains a major concern to physicians and patients, including its
short- and long-term side effects, which have limited its clinical
use. Although the side effects are uncommon, they may be serious
and potentially life-threatening. The most common side effects
include infection, infusion reactions, tumor and lupus-like
syndrome. Miscellaneous types of infections, such as bacterial,
mycobacteria, invasive fungal, viral and parasitic infections have
been observed since the approval of infliximab (13).
In animal models, TNF-α has been demonstrated to
have a central role in the host response against tuberculosis,
including granuloma formation and the containment of disease
(14,15). Notably, antibodies against TNF-α
induced reactivation of tuberculosis in a mouse model of latent
infection (16). The role of TNF-α
in the human immune response to tuberculosis remains unclear, and
the effect on the tuberculosis infection of infliximab requires
clarification. Previous large retrospective reviews of
infliximab-induced tuberculosis have shown that the frequency of
tuberculosis following infliximab therapy was elevated, as compared
with other opportunistic infections (17). In addition, the rate of reported
cases of tuberculosis treated with infliximab is higher than the
background rate of tuberculosis in patients with rheumatoid
arthritis (18–20). However, the findings of previous RCTs
that examined the association between infliximab and tuberculosis
infection risk were inconsistent. The aim of present study was to
analyze the findings of RCTs that investigated infliximab, with an
emphasis on tuberculosis infection risk.
Materials and methods
Search strategy and study
selection
To perform this review, we conducted a structured
search in PubMed (ncbi.nlm.nih.gov/pubmed), MEDLINE (http://webofknowledge.com/medline) and EMBASE
(http://www.embase.com) databases up to May 2014
using the following search terms: (‘inflammatory bowel disease’ or
‘Crohn's disease’ or ‘ulcerative colitis’ or ‘rheumatoid arthritis’
or ‘spondyloarthropathy’) and (‘remicade’ or ‘infliximab’ or
‘monoclonal antibody cA2’) and (‘tuberculosis’ or ‘mycobacterial
infections’). References from the articles that met the eligibility
criteria were also examined and evaluated, and were selected for
this review if they also met the criteria. Only articles published
in English were included. Titles and abstracts of articles
identified by the initial search were first evaluated by
investigators for appropriateness to the study question, and full
papers of potential eligible studies were subsequently obtained and
reviewed in detail. The present meta-analysis was designed,
analyzed and reported according to the PRISMA statement (21).
Criteria for the inclusion of an article in the
present meta-analysis were as follows: (i) Randomized controlled
trials; (ii) >95% of the patients are aged >18 years; and
(iii) compared infliximab with or without concomitant
immunomodulators therapy with placebo. Furthermore, for inclusion,
studies were required to be independent from other studies in order
to avoid giving double weight to estimates derived from the same
trial, and to have sufficient information to allow adequate
estimation of the relative risk (RR)/odds ratio (OR) and 95%
confidence intervals (CIs).
Studies were excluded if: (i) They were a review,
lecture, comment or research that cannot be extracted with
statistical data; or (ii) they included patients that were
pregnant, hypersensitive to infliximab, exhibited systemic disease,
or were given biological treatment previously.
Outcome assessment
The primary outcome was the occurrence of
tuberculosis infection with infliximab, compared with placebo. The
secondary outcome was mortality due to tuberculosis infection.
Data extraction
Two investigators individually evaluated all
relevant articles identified by the literature search using
pre-defined eligibility forms. Any discrepancies were resolved by
discussion. The following information was obtained from each study:
First author, year of publication, geographical region, disease
type, sample size during the study, dosage of infliximab, duration
of therapy, combination therapy (if any), number of individuals who
experienced tuberculosis infection, tuberculosis manifestation, and
prognosis of tuberculosis. Data were extracted as
intention-to-treat analyses, wherever trial reporting facilitated
this.
Quality evaluation
Jadad scoring was applied to assess the
methodological quality of included trials, which judges the
descriptions of randomization, double blinding, and subject
withdrawal in the included trials (22). The quality scale ranges from 0 to 5
points with a low-quality report scoring ≤2 and a high-quality
report scoring ≥3 (23).
Statistical analysis
Statistical heterogeneity between the studies was
assessed using χ2 test and I2, which assumes
the presence of heterogeneity at P<0.10 and/or I2
>50%. A fixed effects model was used when the heterogeneity test
demonstrated a P-value of >0.10 and a I2 of <50%;
otherwise, a random-effects model was used. Subgroup analyses were
performed according to disease type, sample size, study quality,
duration of therapy, whether patients in both infliximab and
placebo arms were exposed to immunosuppressants and whether
patients enrolled in infliximab and placebo arms were screened for
tuberculosis. We compared individual ORs between these analyses
using the Cochran Q statistic tool.
Funnel plot graph, and Begg and Egger tests were
performed to evaluate publication bias. All analyses were conducted
using the Revman 5.0 (Nordic Cochrane Centre, Copenhagen, Denmark)
and Stata software (11.0; StataCorp LP, College Station, TX, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Literature search and characteristics
of the included studies
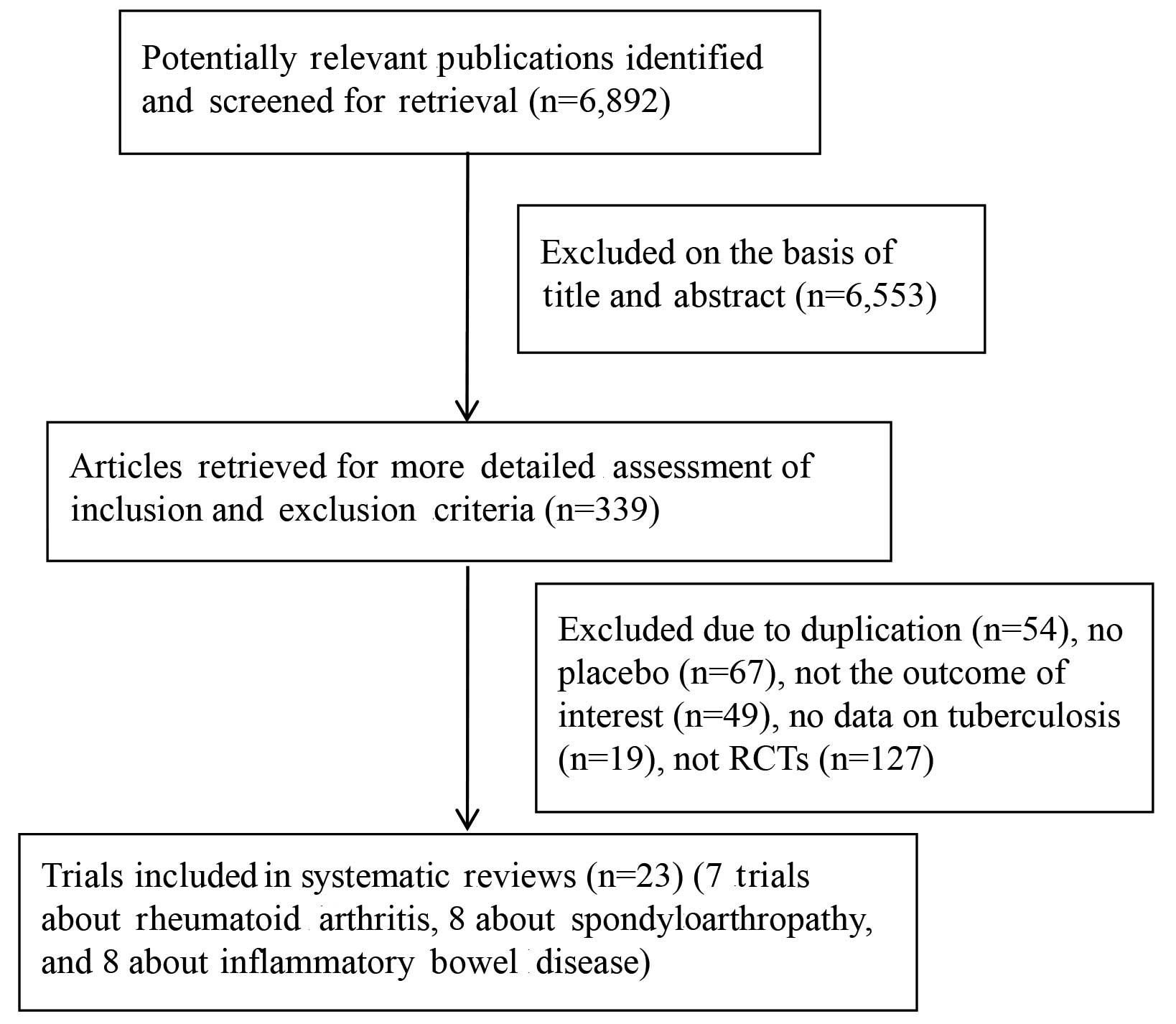
The present search strategy identified 6,892
articles, 6,553 of which were excluded after the title and abstract
were reviewed. For the remaining 339 articles, 316 articles were
excluded due to duplication (n=54), not being an RCTs (n=127), a
lack of placebo (n=67), no outcome of interest (n=49), and no data
on tuberculosis (n=19). Finally, 23 articles were included in the
present meta-analysis, reporting on 24 respective RCTs. Of these, 8
studies studied infliximab-associated tuberculosis incidence in IBD
(24–32), one of which reported two separate
trials (29), 7 trials studied
infliximab-associated tuberculosis incidence rates in RA (33–39), and
8 trials studied infliximab-associated tuberculosis incidence in
SpA (40–47). A flow diagram of the selection
process for the inclusion of studies in the present meta-analysis
is shown in Fig. 1.
Duration of follow-up ranged between 12 and 54
weeks. The characteristics of the 24 trials are presented in
Tables I and II, including the year the studies were
conducted, number of patients treated, dose of infliximab
administered, incidence of tuberculosis infection, tuberculosis
manifestation, prognosis of tuberculosis and tuberculosis screening
test prior to therapy.
 | Table I.RCTs evaluating the incidence of
infliximab-associated tuberculosis in patients with RA, SpA and
IBD. |
Table I.
RCTs evaluating the incidence of
infliximab-associated tuberculosis in patients with RA, SpA and
IBD.
| A, Rheumatoid
arthritis |
|---|
|
|---|
| Author, year | Country | Study design | Disease | Treatment (number
of patients) | Duration (wks) | Ref |
|---|
| Maini et al,
1998 | Netherlands,
Germany, Austria, UK | MC, DB, PC, phase
3 | Active RA | Grp 1: Placebo plus
MTX (n=14) | 26 | (33) |
|
|
| RCT; MTX
allowed |
| Grp 2: Placebo plus
1 mg/kg infliximab q4wks (n=14) |
|
|
|
|
|
|
| Grp 3: MTX plus 1
mg/kg infliximab q4wks (n=15) |
|
|
|
|
|
|
| Grp 4: Placebo plus
3 mg/kg infliximab q4wks (n=15) |
|
|
|
|
|
|
| Grp 5: MTX plus 3
mg/kg infliximab q4wks (n=14) |
|
|
|
|
|
|
| Grp 6: Placebo plus
10 mg/kg infliximab q4wks (n=14) |
|
|
|
|
|
|
| Grp 7: MTX plus 10
mg/kg infliximab q4wks (n=15) |
|
|
| Lipsky et
al, 2000 | USA, Netherlands,
Germany, Austria, UK | MC, DB, PC, phase
3 | Active RA | Grp 1: MTX plus
placebo at wks 0, 2, 6 and q4kws (n=88) | 54/104 | (34) |
|
|
| RCT (ATTRACT);
corticosteroids and NSAIDs allowed |
| Grp 2: MTX plus
infliximab 3 mg/kg at wks 0, 2, 6 and every 8 wk (n=86) |
|
|
|
|
|
|
| Grp 3: MTX plus
infliximab 3 mg/kg at wks 0, 2, 6 and every 4 wk (n=86) |
|
|
|
|
|
|
| Grp 4: MTX plus
infliximab 10 mg/kg at wks 0, 2, 6 and every 8 wk (n=87) |
|
|
|
|
|
|
| Grp 5: MTX plus
infliximab 10 mg/kg at wks 0, 2, 6 and every 4 wk (n=81) |
|
|
| St Clair et
al, 2004 | North America, and
Europe | MC, PC, phase 3
RCT | Early RA | All patients: MTX
7.5 mg/week, which increased to 15 mg/week by wk 4 and 20 mg/week
by wk 8 (n=1,004) | 54 | (35) |
|
|
| (ASPIRE);
corticosteroids and NSAIDs allowed | (≤3 years) |
|
|
|
|
|
|
|
| Grp 1: Placebo at
wks 0, 2, 6 and q8kws through wk 46 (n=282) |
|
|
|
|
|
|
| Grp 2: Infliximab 3
mg/kg at wks 0, 2, 6 and q8kws through wk 46 (n=359) |
|
|
|
|
|
|
| Grp 3: Infliximab 6
mg/kg at wks 0, 2, 6 and q8kws through wk 46 (n=363) |
|
|
| Quinn et al,
2005 | UK | DB, PC RCT; MTX
allowed | Early poor
prognosis RA | Grp 1: Placebo plus
MTX (n=10) | 48 | (36) |
|
|
|
|
| Grp 2: 3 mg/kg
infliximab at wks 0, 2, 6 and q8wks plus MTX (n=10) |
|
|
| Westhovens et
al, 2006 | Belgium, US,
Netherlands | MC, DB, PC, phase
3 | Active RA | Grp 1: Placebo at
wks 0, 2, 6 and 14, and infliximab 3 mg/kg at wks 22, 26, and 30,
and q8wks through wk 46 (n=363) | 54 | (37) |
|
|
| RCT (START); AZA,
6-MP |
|
|
|
|
|
|
| MTX, DMARDs and
corticosteroids allowed |
| Grp 2: Infliximab 3
mg/kg at wks 0, 2, 6, 14 and at least 3mg/kg q8wks through wk 46
(n=360) |
|
|
|
|
|
|
| Grp 3: Infliximab
10 mg/kg at 0, 2, 6, 14 and q8wks through wk 46 (n=361) |
|
|
| Abe et al,
2006 | Japan | MC, DB, PC RCT;
corticosteroids and NSAIDs allowed | RA | Grp 1: Placebo plus
MTX (n=47) | 36 | (38) |
|
|
|
|
| Grp 2: 3 mg/kg
infliximab at wks 0, 2, 6 plus MTX (n=49) |
|
|
|
|
|
|
| Grp 3: 10 mg/kg
infliximab at wks 0, 2 and 6 wk plus MTX (n=51) Most of Grp 1, 2
and 3: 3 mg/kg infliximab q8wks plus MTX thereafter |
|
|
| Schiff et
al, 2008 | Brazil, America,
Argentina, France, Mexico | DB, PC phase 3 | RA | Grp 1: Placebo plus
MTX (n=110) | 52 | (39) |
|
|
| RCT (ATTRACT) |
| Grp 2: 3 mg/kg
infliximab plus MTX at wks 0, 2, 6, 14, every 8 wk (n=165) |
|
|
|
| B,
Spondyloarthropathy |
|
| Author, year | Country | Study design | Disease | Treatment (number
of patients) | Duration (wks) | Ref |
|
| Braun et al,
2002 | Germany | MC, DB, PC, phase
2 | Severe AS | Grp 1: Placebo at
wks 0, 2, 6 (n=35) | 12 | (40) |
|
|
| RCT; NSAIDs
allowed |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6 (n=35) |
|
|
| Van der Heijde
et al, 2005 | Europe, US,
Canada | MC, DB, PC, phase
3 | Active AS | Grp 1: Placebo at
wks 0, 2, 6, 12 and 18 (n=78) | 24 | (41) |
|
|
| RCT (ASSERT);
NSAIDs allowed |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6, 12 and 18 (n=201) |
|
|
| Marzo-Ortega et
al, 2005 | UK | DB, PC, phase 2
RCT; | Active AS | Grp 1: Infliximab 5
mg/kg at wks 0, 2, 6 14, 22 + MTX 7.5 mg/week (n=28) | 30 | (42) |
|
|
| NSAIDS and
corticost-eroids allowed |
| Grp 2: Placebo+MTX
7.5 mg/sem (n=14) |
|
|
| Inman et al,
2010 | Canada | MC, DB, PC, phase
3b | Active AS | Grp 1: Placebo
(n=37) | 52 | (43) |
|
|
| RCT; NSAIDS and
corti-costeroids allowed |
| Grp 2: Infliximab 3
mg/kg at wks 0, 2, 6 and q8wks through wk 52 (n=39) |
|
|
|
|
|
|
| All patients:
Infliximab 5 mg/kg at wks 22 or 38 if BASDAI>3 and a relative
decrease of <50% in BASDAI |
|
| Van den Bosch et
al, 2002 | Belgium | DB, PC, phase 2
RCT; corticosteroids and NSAIDs allowed | Active SpA | Grp 1: Placebo at
wks 0, 2, 6 (n=20) | 12 | (44) |
|
|
|
|
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6 (n=20) |
|
|
| Antoni et
al, 2002 | Europe, US,
Canada | MC, DB, PC, phase 3
RCT | Active SpA | Grp 1: Placebo at
wks 0, 2, 6, and 14 (n=52) then infliximab 5 mg/kg at wks 16, 18,
22, 30,38 and 46 (n=50) | 50 | (45) |
|
|
| (IMPACT); one of
MDARDs allowed |
|
|
|
|
|
|
|
|
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6, and 14 (n=52), placebo at wks 16, 18 and
infliximab 5 mg/kg at wks 22, 30, 38 and 46 (n=49) |
|
|
| Kavanaugh et
al, 2005 | Europe, US,
Canada | MC, DB, PC, phase
3 | Active SpA | Grp 1: Placebo at
wks 0, 2, 6, and q8wks through wk 22 (n=100) | 24 | (46) |
|
|
| RCT (IMPACT II);
MTX and corticosteroids allowed |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6, and q8wks to wk 22 (n=100) |
|
|
| Sieper et
al, 2007 | Austria, Europe,
South Korea | MC, DB, PC Phase
3b | MTS active axial
SpA | Grp 1: Placebo+
naproxen 1000 mg/d at wks 0, 2, 6, 12, 18, 24 wks (n=52) | 24 | (47) |
|
|
| RCT (INFAST) |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6, 12, 18, 24 wks (n=105) |
|
|
|
| C, Inflammatory
bowel disease |
|
| Author, year | Country | Study design | Disease | Treatment (number
of patients) | Duration (wks) | Ref |
|
| Present et
al, 1999 | North America and
Europe | MC, DB, PC phase
3 | Fistulizing CD | Grp 1: Infliximab
10 mg/kg at wks 0, 2, 6 (n=32) | 52 | (25) |
|
|
| RCT |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6 (n=31) |
|
|
|
|
|
|
| Grp 3: Placebo at
wks 0, 2, 6 (n=31) |
|
|
| Sands et al,
2001 | US | MC, DB, PC phase
2, | Active UC | Grp 1: Single dose
of placebo (n =3) | 12 | (26) |
|
|
| RCT | (MT&W score
>10) | Grp 2: Single dose
of infliximab 5 mg/kg (n=3) |
|
|
|
|
|
|
| Grp 3: Single dose
of infliximab 10 mg/kg (n=3) |
|
|
|
|
|
|
| Grp 4: Single dose
of infliximab 20 mg/kg (n=2) |
|
|
| Hanauer et
al, 2002 | North America, | MC, DB, PC, phase
3 | MTS CD | All patients:
Infliximab 5 mg/kg at wk 0 (n=573) | 54 | (27) |
| Europe, Israel | RCT (ACCENT I);
AZA |
| Grp 1: Placebo at
wks 2, 6 and q8wks to wk 46 (n=188) |
|
|
|
| 6-MP, MTX and
cortico-steroids allowed |
| Grp 2: Infliximab 5
mg/kg at wks 2, 6 and q8wks to wk 46 (n=192) |
|
|
|
|
|
|
| Grp 3: Infliximab 5
mg/kg at wks 2 and 6 and 10 mg/kg q8wks to wk 46 (n=193) |
|
|
| Sands et al,
2004 | North America,
Europe, Israel | MC, DB, PC, phase
3 | Fistulizing CD | All patients:
Infliximab 5mg/kg at wks 0, 2, 6 (n=306) | 54 | (28) |
|
|
| RCT (ACCENT
II); |
| Grp 1: Placebo at
wk 14 and q8wks through wk 46 (n=144) |
|
|
|
|
| AZA, 6-MP, MTX and
corticosteroids allowed |
| Grp 2: Infliximab 5
mg/kg at wk14 and q8wks to wk 46 (n=138) |
|
|
| Rutgeerts et
al, 2005 | Belgium, Canada,
Israel, America, France | MC, DB, PC, phase
3 | Active UC | Grp 1: Placebo at
wks 0, 2, 6 and q8wks to wk 46 (n=121) | 54 | (29) |
|
|
| RCT (ACT I); AZA,
6-MP and corticoster-oids allowed |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6 and q8wks to wk 46 (n=121) |
|
|
|
|
|
|
| Grp 3: Infliximab
10 mg/kg at wks 0, 2, 6 and q8wks to wk 46 (n=122) |
|
|
| Rutgeerts et
al, 2005 | Belgium, Canada,
Israel, America, France | MC, DB, PC, phase
3 | Active UC | Grp 1: Placebo at
wks 0, 2, 6 and q8wks through wk 22 (n=123) | 54 | (29) |
|
|
| RCT (ACT II); AZA,
6-MP and corticoster-oids allowed |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6 and q8wks to wk 22 (n=121) |
|
|
|
|
|
|
| Grp 3: Infliximab
10 mg/kg at wks 0, 2, 6 and 10 mg/kg q8wks to wk 22 (n=120) |
|
|
| Lemann et
al, 2006 | France | MC, DB, PC, phase
2 | Luminal
steroid-dependent CD | Grp 1: Placebo
(n=58) | 52 | (30) |
|
|
| RCT; AZA and 6-MP
allowed |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6 (n=57) |
|
|
| Colombel et
al, 2010 | Netherlands,
Belgium, France | MC, DB, phase 3
RCT | MTS CD | Grp 1: AZA 2.5
mg/kg capsules/placebo infusion (n=161) | 50 | (31) |
|
|
| (SONIC); AZA, 6-MP
and corticosteroids allowed |
| Grp 2: Placebo
capsules/infliximab 5 mg/kg infusions (n=63) |
|
|
|
|
|
|
| Grp 3: AZA 2.5
mg/kg capsules/infliximab 5 mg/kg infusions (n=179) Capsules
(daily)/infusions (wks 0, 2, 6, q8wks to wk 22) |
|
|
| Ochsenkuhn et
al, 2004 | Germany | SC, OL, PC phase
2 | Active UC | Grp 1: Prednisolone
1.5 mg/kg qd for 2 wks, followed by a tapering regimen with a
weekly reduction of 5 mg (n=7) | 14 | (32) |
|
|
| RCT; AZA and 6-MP
allowed |
| Grp 2: Infliximab 5
mg/kg at wks 0, 2, 6 (n=6) |
|
|
 | Table II.Characteristics of the included
randomized clinical trials. |
Table II.
Characteristics of the included
randomized clinical trials.
| Author, year | Patients treated
with infliximab | Patients that
developed TB with infliximab | Patients treated
with placebo | Patients that
developed TB with placebo | TB
manifestation | Prognosis | Screened | Ref. |
|---|
| Inflammatory bowel
disease |
|
| Present
et al, 1999 | 63 | 0 | 31 | 0 | NS | NS | NS | (25) |
| Sands
et al, 2001 |
8 | 0 |
3 | 0 | NS | NS | NS | (26) |
| Hanauer
et al, 2002 | 385 | 1 | 188 | 0 | NS | Recovered | PPD and CXR | (27) |
| Sands
et al, 2004 |
6 | 0 |
7 | 0 | NS | NS | NS | (28) |
|
Rutgeerts et al,
2005 | 241 | 1 | 123 | 0 | NS | NS | PPD and CXR | (29) |
|
Rutgeerts et al,
2005 | 243 | 0 | 212 | 0 | NS | NS | PPD and CXR | (29) |
| Lemann
et al, 2006 | 57 | 0 | 58 | 0 | NS | NS | PPD and CXR | (30) |
|
Colombel et al,
2010 | 242 | 1 | 161 | 0 | NS | Recovered | PPD and CXR | (31) |
|
Ochsenkuhn et al,
2004 | 138 | 0 | 144 | 0 | NS | NS | NS | (32) |
| Rheumatoid
arthritis |
|
| Maini
et al, 1998 | 87 | 0 | 14 | 0 | NS | NS | No | (33) |
| Lipsky
et al, 2000 | 340 | 1 | 88 | 0 | Disseminated
TB | DNS (resistant
TB) | No | (34) |
| St
Clair et al, 2004 | 722 | 4 (US, 1; Europe,
3) | 282 | 0 | 4 Pulmonary | Recovered | No | (35) |
| Quinn
et al, 2005 | 10 | 0 | 10 | 0 | NS | NS | CXR | (36) |
|
Westovens et al,
2006 | 724 | 8 | 363 | 0 | 2 Pulmonary, 5
extrapulmonary (not disseminated) | 1 DNS | CXR | (37) |
| Abe
et al, 2006 | 100 | 0 | 47 | 0 | NS | NS | CXR | (38) |
| Schiff
et al, 2008 | 165 | 2 | 110 | 0 | 1 Pulmonary, 1
extrapulmonary | Recovered | Yes | (39) |
|
Spondyloarthropathy |
|
| Braun
et al, 2002 | 35 | 1 | 35 | 0 | Disseminated
TB | Recovered | CXR, not PPD | (40) |
| van der
Heijde et al, 2005 | 201 | 0 | 78 | 0 | NS | NS | CXR or PPD | (41) |
|
Marzo-Ortega et al,
2005 | 28 | 0 | 14 | 0 | NS | NS | Yes | (42) |
| Inman
et al, 2010 | 39 | 0 | 37 | 0 | NS | NS | NS | (43) |
| Van den
Bosch et al, 2002 | 20 | 1 | 20 | 0 | Disseminated
TB | Recovered | Yes | (44) |
| Antoni
et al, 2005 | 100 | 0 | 100 | 0 | NS | NS | NS | (45) |
|
Kavanaugh et al,
2007 | 52 | 0 | 52 | 0 | NS | NS | CXR, PPD | (46) |
| Sieper
et al, 2014 | 105 | 1 | 52 | 0 | NS | Recovered | CXR, PPD | (47) |
Rates of tuberculosis infection in
IBD, RA and SpA
Table III presents
the rates of tuberculosis infection within each trial arm in each
inflammatory disease assessed. Rates of tuberculosis infection with
infliximab therapy were 0.70, 0.22 and 0.52%, respectively; whereas
the rates of tuberculosis infection with placebo were consistently
0%, in RA, SpA and IBD. The rates of tuberculosis infection with
infliximab therapy in IBD, RA and SpA were all higher than the
rates with placebo.
 | Table III.Rates of tuberculosis infection
following infliximab therapy versus placebo in patients with RA,
IBD and SpA. |
Table III.
Rates of tuberculosis infection
following infliximab therapy versus placebo in patients with RA,
IBD and SpA.
| Disease | Number of
trials | Total number of
infliximab patients | Number of
infliximab patients infected with tuberculosis (%) | Total number of
placebo patients | Number of placebo
patients infected with tuberculosis (%) |
|---|
| RA | 7 | 2,148 | 15 (0.70) |
914 | 0 (0) |
|
IBD | 8 |
580 | 3
(0.52) |
388 | 0 (0) |
|
SpA | 9 | 1,383 | 3
(0.22) |
927 | 0 (0) |
|
Total | 24 | 4,111 | 21 (0.51) | 2,229 | 0 (0) |
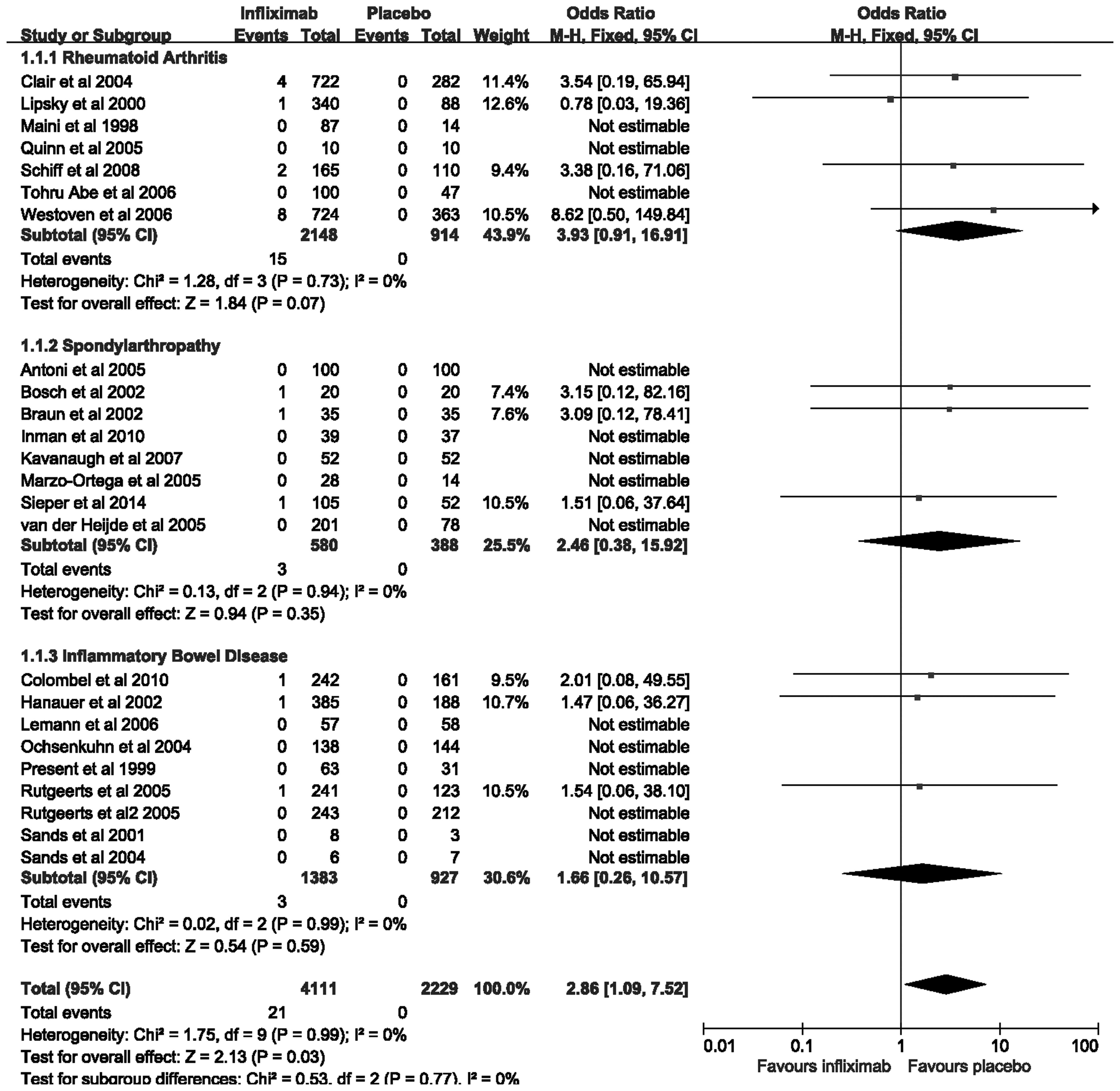
Overall risk of tuberculosis infection
with infliximab therapy vs. placebo in IBD, RA and SpA
The 24 trials examined in the present meta-analysis
contain a total of 6,340 patients with IBD, RA and SpA. Of these,
4,111 (64.8%) patients were randomized to receive infliximab
therapy, and 2,229 (35.2%) patients were administered a placebo. In
total, there were 21 (0.51%) patients assigned to infliximab
therapy who developed tuberculosis infection, as compared with 0
(0%) among patients allocated to placebo therapy. The pooled OR of
developing tuberculosis infection was significantly increased with
infliximab therapy, as compared with the placebo [2.86; 95% CI,
1.09–7.52] (Fig. 2), with no
statistically significant heterogeneity detected between the
studies (I2=0%; P=0.99). However, in each disease
subgroup, the trends were not statistically significant. The OR of
tuberculosis infection with infliximab therapy was 3.93 (95% CI,
0.91–16.91) in RA, 2.46 (95% CI, 0.38–15.92) in SpA, and 1.66 (95%
CI, 0.26–10.57) in IBD. The difference among these subgroups was
not statistically significant (Cochran Q=0.53; P=0.77)
(Fig. 2). The findings demonstrate
that infliximab therapy may increase the risk of developing
tuberculosis compared with that for treatment with placebo.
Meta-analysis of tuberculosis
infection with infliximab therapy vs. placebo in each subgroup
Subgroup analyses were performed (Table IV). The OR of tuberculosis infection
with infliximab therapy was elevated in trials with ≥50 weeks of
treatment (3.00; 95% CI, 0.97–9.29), as compared with those with a
duration of <50 weeks (2.46; 95% CI, 0.38–15.92); however, there
was no statistically significance (Cochran Q=0.03; P=0.86).
Furthermore, the OR was also increased in the larger sample studies
(2.94; 95% CI, 0.87–9.94), as compared with the smaller sample
studies (2.71; 95% CI, 0.55–13.26); however, again there was no
significant differences detected (Cochran Q=0.01; P=0.93).
There were also no significant differences in the OR of
tuberculosis infection with infliximab therapy accompanied with or
without immunosuppressor (2.96; 95% CI, 0.94–9.29; and 2.59; 95%
CI, 0.42–15.92; respectively, Cochran Q=0.02, P=0.90). When
screening for tuberculosis prior to the examination of therapy
according to the study design, the OR was also increased in trials
that screened for tuberculosis (3.10; 95% CI, 1.04–9.21), as
compared with those without screening (2.09; 95% CI, 0.26–17.13)
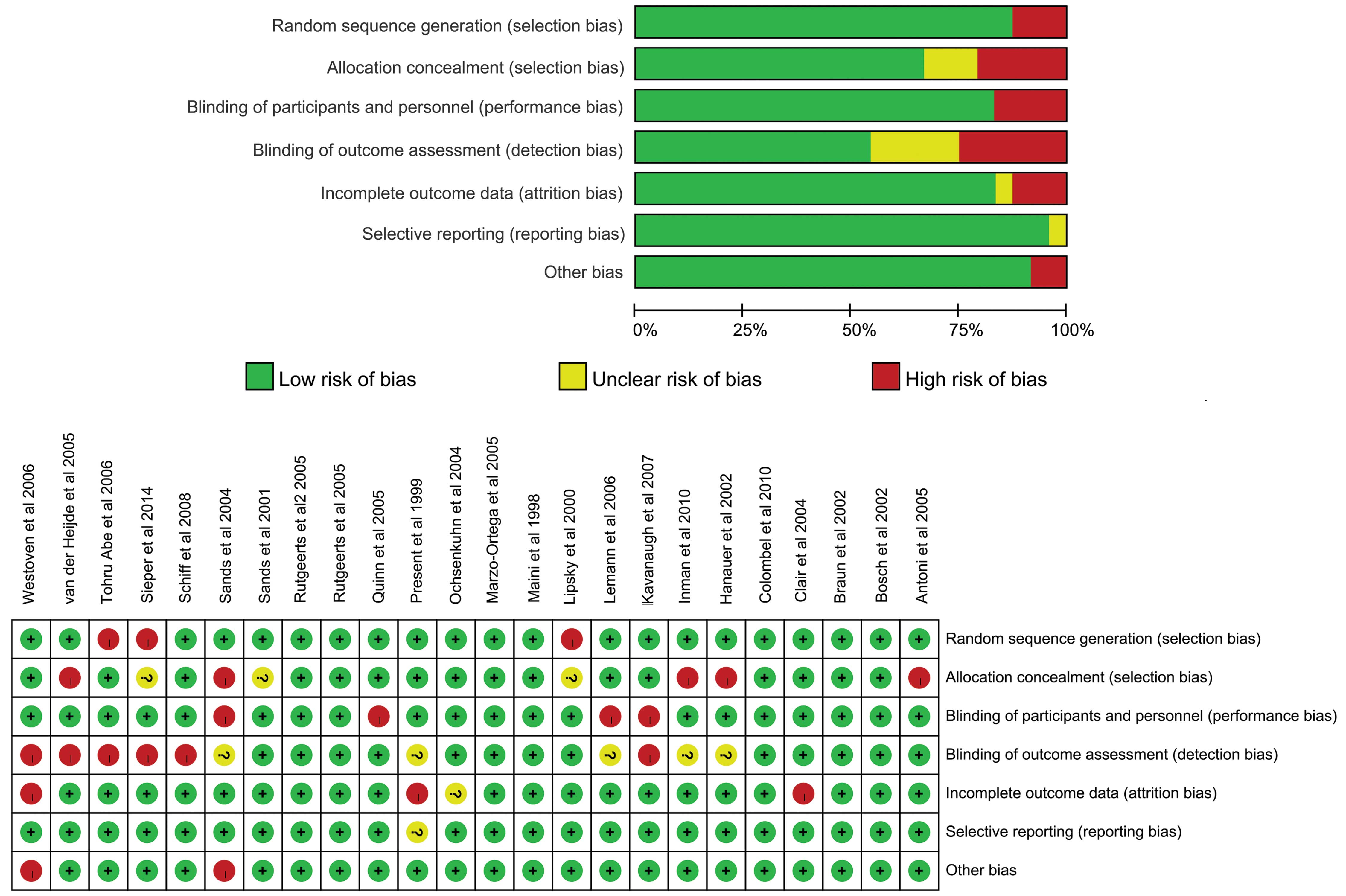
(Cochran Q=0.11, P=0.74). Finally, risk of bias was judged in the
RCTs (Fig. 3), the OR was elevated
in trials with high or unclear risk (3.14; 95% CI, 0.93–10.54), as
compared with those at low risk (2.35; 95% CI, 0.47–11.77);
however, there was also no significant differences detected
(Cochran Q=0.08; P=0.78). The results showed that subgroup
differences did not increase the risk of tuberculosis infection
with infliximab therapy compared with placebo.
 | Table IV.Subgroup analyses of the odds ratio
of TB infection with infliximab therapy vs. placebo in IBD, RA and
SpA. |
Table IV.
Subgroup analyses of the odds ratio
of TB infection with infliximab therapy vs. placebo in IBD, RA and
SpA.
| Variable | Number of
trials | Number of
infliximab patients | Number of placebo
patients | OR of TB
infection | 95% CI |
I2 value (%) |
|---|
| All trials | 24 | 4,111 | 2,229 | 2.86 | 1.09–7.52 | 0 |
| Disease |
|
| RA | 7 | 2,148 |
914 | 3.93 | 0.91–16.91 | 0 |
|
SpA | 8 |
580 |
388 | 2.46 | 0.38–15.92 | 0 |
|
IBD | 9 | 1,383 |
927 | 1.66 | 0.26–10.57 | 0 |
| Duration of
therapy |
|
| ≥50
weeks | 13 | 3,327 | 1,760 | 3.00 | 0.97–9.29 | 0 |
| <50
weeks | 11 |
784 |
469 | 2.46 | 0.38–15.92 | 0 |
| Immunosuppressor
use |
|
|
Yes | 17 | 3,390 | 1,771 | 2.96 | 0.94–9.29 | 0 |
| No | 7 |
721 |
458 | 2.59 | 0.42–15.92 | 0 |
| Screened for
TB |
|
|
Yes | 16 | 2,556 | 1,471 | 3.10 | 1.04–9.21 | 0 |
| No | 5 | 1,149 |
384 | 2.09 | 0.26–17.13 | 0 |
| Sample
sizea |
|
|
Large | 8 | 3,098 | 1,495 | 2.94 | 0.87–9.94 | 0 |
|
Small | 16 | 1,013 |
734 | 2.71 | 0.55–13.26 | 0 |
| Risk of bias |
|
| Low
risk | 7 |
896 |
579 | 2.35 | 0.47–11.77 | 0 |
| High or
unclear | 17 | 3,215 | 1,650 | 3.14 | 0.93–10.54 | 0 |
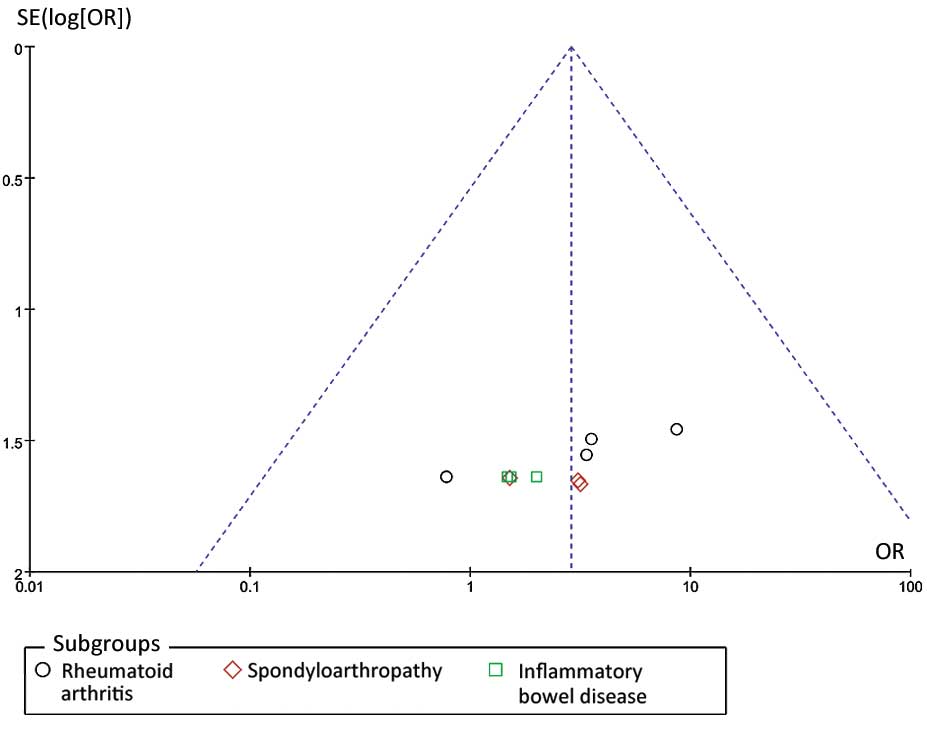
Publication bias
Funnel plot analysis was performed, as demonstrated
in Fig. 4. Funnel plot asymmetry was
detected in the present meta-analysis (Egger test, P=0.002 and
Begger test, P=0.004). These results provide some evidence of
publication bias in the present study.
Discussion
TNF-α is required for granuloma formation and
maintenance, and has an important role in host defense against
diseases caused by intracellular pathogens, such as
Mycobacterium tuberculosis, Histoplasma capsulatum
and Listeria monocytogenes (48–50). The
increased clinical use of TNF-α antagonists has markedly improved
the management of immunomediated diseases; however, it may have led
to an increase in the incidence of infections with intracellular
agents. To the best of our knowledge, the present meta-analysis was
the first to evaluate the potential risk of tuberculosis infection
with infliximab therapy in the management of RA, SpA and IBD, by
collating all obtainable data from 24 individual RCTs. The present
meta-analysis demonstrated that the OR of tuberculosis infection
with infliximab therapy was 2.86-fold greater than when using the
placebo. However, the ORs in all three disease subgroups were not
demonstrated to be statistically significant.
The role of TNF-α in the human immune response to
M. tuberculosis is yet to be fully elucidated. An in
vitro study has proposed that TNF-α has a significant role in
the regulation of granuloma formation, which limits microbial
growth (51). TNF-α, which is a
pleiotropic cytokine produced by infected and activated
macrophages/proinflammatory T cells, enhances macrophage
activation, chemokine production by macrophages, and immune cell
recruitment during tuberculosis infection (51). Anti-TNF-α monoclonal antibody
administration may subsequently increase the risk of the
dissolution of intact granulomas, the production of viable
mycobacteria, and disease reactivation. This may clarify the
increased likelihood of tuberculosis that was observed in patients
receiving infliximab therapy.
In the present meta-analysis, 21 cases of
tuberculosis, including 15 patients with RA, 3 patients with SpA
and 3 patients with IBD, were evaluated. There were some
limitations to the present study, even though the probability in
each disease subgroup was not statistically significant. Firstly,
as a consequence of latent tuberculosis infection (LTBI) screening,
a relatively low number of cases of TB activation were recorded in
clinical trials of infliximab in IBD, RA and SpA. Overall, there
were 0.51% cases of tuberculosis, 0.70% of which were in patients
with RA, and 0.52 and 0.22% cases were in those with IBD and SpA,
respectively. Compared with the tuberculosis incidence
(38–300/100,000) in the general population based on data from the
WHO in 2013, infliximab infusion appears to increase the occurrence
of tuberculosis (52). However, a
major limitation of the present meta-analysis is that there were
only 21 tuberculosis cases included; thus the small sample size may
account for why the ORs for the risk of infliximab-associated
tuberculosis were consistently demonstrated to lack significance in
IBD, RA and SpA. Therefore, the results of the present
meta-analysis should be interpreted with caution. Secondly, besides
a single trial performed in Japan, the remaining studies were
conducted in Europe and the USA, with apparent variations in
tuberculosis risk among the different countries. Due to the low
incidence of tuberculosis in the Western countries, the incidence
of tuberculosis infection in patients treated with inflaximab may
be underestimated. Thirdly, the majority of tuberculosis cases were
in RA trials, suggesting that the underlying disease and previous
immunosuppressive treatment may constitute adjunctive risk factors
for tuberculosis activation. Indeed, patients with SpA and IBD
predominantly have limited background of immunosuppressive
therapies when compared with sufferers of RA. However, not all
trials included in the present study constrained the
immunosuppressive therapies prior to enrollment, which may have
impacted the precision of assessing the risk of infliximab therapy
without or with immunosuppressors. The screening procedure for LTBI
was reported in 14 trials, whereas it was not documented in the
others. Conversely, despite the elevated risk that was demonstrated
in the trials after the introduction of procedures to identify
LTBI, screening procedures for LTBI prior to treatment are still
required due to the limited sample size, relatively low incidence
of tuberculosis infection and publication bias. For that reason,
screening and management for latent tuberculosis is essential prior
to the administration of anti-TNF-α treatments.
It has been demonstrated that infliximab infusion
raises the tuberculosis incidence rate (35,38,39). The
incidence rate of tuberculosis within this critique may be an
underestimation, as the majority of the data that was analyzed
originated from Western countries, whereas tuberculosis infection
has a higher prevalence in developing countries. As is the
situation in China, with the evolution of diagnostic techniques and
medical treatment, RA, SpA and IBD incidence increased due to an
increase in diagnosis. Despite the cost of infliximab, some
patients are able to afford the cost and they are now at an
increased risk for tuberculosis infection with a high latent
infection rate among the population. It may be interesting to
undertake multi-center clinical trials in East Asia in order to
avoid inclusion bias.
By comparison, a significant proportion of
extra-pulmonary and disseminated forms of tuberculosis were
recognized, regardless of the preceding latent tuberculosis
screening and treatment (38,39). The
frame from the initial infusion to the incidence of tuberculosis
varies and no obvious dose dependent effect was observed. These
findings suggest that merely screening for TB and treating it prior
to infliximab is not sufficient. Additional follow-up is required
in order to carefully assess the potential of extra-pulmonary
tuberculosis occurring at any dosage, months or years after
infusion. The present meta-analysis established that infliximab
therapy induced tuberculosis. This indicated that the overall
likelihood of TB infection in patients receiving infliximab
infusion is sufficient to justify the overall screening,
prophylaxis treatment, and close observation of this potentially
fatal side effect. Therefore, we consider a PPD test or tuberculin
skin test and chest X-ray to be essential prior to treatment,
particularly in developing countries.
The present research exhibited numerous positive
aspects. Firstly, demanding and conventional methodologies were
applied to conduct the present meta-analysis, including the
reporting of our search strategy, inclusion criteria, and data
extraction process. Moreover, data extraction was conducted by two
independent reviewers. Secondly, all trials enrolled in the
meta-analysis were high quality RCTs. Finally, no significant
heterogeneity was detected between the studies when data were
pooled to estimate the OR of tuberculosis infection with infliximab
therapy vs. placebo.
The present meta-analysis has several limitations,
as a result of the qualities of the published literature that is
readily available for analysis. Firstly, only seven of the RCTs
were at a low risk of bias, and the quantity of subjects
incorporated into the present meta-analysis was limited. Secondly,
with the exception of one RCT, all included trials were from
developed countries with low tuberculosis incidence rates, and
studies from developing countries with a high incidence of
tuberculosis were not available, which resulted in a publication
bias. Finally, other concerns regarding potential biases remain. In
particular, the included trials did not provide any information
regarding host-related risk factors, including ethnicity,
malnutrition, drug abuse, comorbidity, and contact with infected
persons.
In conclusion, the present meta-analysis of 24 RCTs,
comprising details from >6,340 patients with RA, SpA and IBD,
demonstrated that the OR of tuberculosis infection was markedly
increased with infliximab therapy, as compared with placebo
therapy. The overall rates of tuberculosis infection were low
(0.51%).
Acknowledgements
The present study was supported by Zhejiang
Provincial Traditional Chinese Medicine Science Research Foundation
(grant no. 2015ZA010) and Zhejiang Provincial Medical and Health
science Foundation (grant no. 2015KYB020).
References
|
1
|
Knight DM, Trinh H, Le J, Siegel S, Shealy
D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, et al:
Construction and initial characterization of a mouse-human chimeric
anti-TNF antibody. Mol Immunol. 30:1443–1453. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scallon BJ, Moore MA, Trinh H, Knight DM
and Ghrayeb J: Chimeric anti-TNF-alpha monoclonal antibody cA2
binds recombinant transmembrane TNF-alpha and activates immune
effector functions. Cytokine. 7:251–259. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Deventer SJ: Tumour necrosis factor
and Crohn's disease. Gut. 40:443–448. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feldmann M, Brennan FM and Maini RN:
Rheumatoid arthritis. Cell. 85:307–310. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guignard S, Gossec L, Salliot C,
Ruyssen-Witrand A, Luc M, Duclos M and Dougados M: Efficacy of
tumour necrosis factor blockers in reducing uveitis flares in
patients with spondylarthropathy: A retrospective study. Ann Rheum
Dis. 65:1631–1634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murch SH, Braegger CP, Walker-Smith JA and
MacDonald TT: Location of tumour necrosis factor alpha by
immunohistochemistry in chronic inflammatory bowel disease. Gut.
34:1705–1709. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Husby G and Williams RC Jr: Synovial
localization of tumor necrosis factor in patients with rheumatoid
arthritis. J Autoimmun. 1:363–371. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tse SM, Burgos-Vargas R and Laxer RM:
Anti-tumor necrosis factor alpha blockade in the treatment of
juvenile spondylarthropathy. Arthritis Rheum. 52:2103–2108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aaltonen KJ, Virkki LM, Malmivaara A,
Konttinen YT, Nordström DC and Blom M: Systematic review and
meta-analysis of the efficacy and safety of existing TNF blocking
agents in treatment of rheumatoid arthritis. PLoS One.
7:e302752012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiens A, Correr CJ, Venson R, Grochocki
MC, Otuki MF and Pontarolo R: A meta-analysis of the efficacy and
safety of using infliximab for the treatment of rheumatoid
arthritis. Clin Rheumatol. 28:1365–1373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiens A, Venson R, Correr CJ, Otuki MF and
Pontarolo R: Meta-analysis of the efficacy and safety of
adalimumab, etanercept and infliximab for the treatment of
rheumatoid arthritis. Pharmacotherapy. 30:339–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stidham RW, Lee TC, Higgins PD, Deshpande
AR, Sussman DA, Singal AG, Elmunzer BJ, Saini SD, Vijan S and
Waljee AK: Systematic review with network meta-analysis: The
efficacy of anti-tumour necrosis factor-alpha agents for the
treatment of ulcerative colitis. Aliment Pharmacol Ther.
39:660–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scheinfeld N: A comprehensive review and
evaluation of the side effects of the tumor necrosis factor alpha
blockers etanercept, infliximab and adalimumab. J Dermatolog Treat.
15:280–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flynn JL, Goldstein MM, Chan J, Triebold
KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW and Bloom BR:
Tumor necrosis factor-alpha is required in the protective immune
response against Mycobacterium tuberculosis in mice. Immunity.
2:561–572. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kindler V, Sappino AP, Grau GE, Piguet PF
and Vassalli P: The inducing role of tumor necrosis factor in the
development of bactericidal granulomas during BCG infection. Cell.
56:731–740. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohan VP, Scanga CA, Yu K, Scott HM,
Tanaka KE, Tsang E, Tsai MM, Flynn JL and Chan J: Effects of tumor
necrosis factor alpha on host immune response in chronic persistent
tuberculosis: Possible role for limiting pathology. Infect Immun.
69:1847–1855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ford AC and Peyrin-Biroulet L:
Opportunistic infections with anti-tumor necrosis factor-alpha
therapy in inflammatory bowel disease: Meta-analysis of randomized
controlled trials. Am J Gastroenterol. 108:1268–1276. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keane J, Gershon S, Wise RP,
Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN and Braun
MM: Tuberculosis associated with infliximab, a tumor necrosis
factor alpha-neutralizing agent. N Engl J Med. 345:1098–1104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wallis RS, Broder MS, Wong JY, Hanson ME
and Beenhouwer DO: Granulomatous infectious diseases associated
with tumor necrosis factor antagonists. Clin Infect Dis.
38:1261–1265. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Askling J, Fored CM, Brandt L, Baecklund
E, Bertilsson L, Cöster L, Geborek P, Jacobsson LT, Lindblad S,
Lysholm J, et al: Risk and case characteristics of tuberculosis in
rheumatoid arthritis associated with tumor necrosis factor
antagonists in Sweden. Arthritis Rheum. 52:1986–1992. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moher D, Pham B, Jones A, Cook DJ, Jadad
AR, Moher M, Tugwell P and Klassen TP: Does quality of reports of
randomised trials affect estimates of intervention efficacy
reported in meta-analyses? Lancet. 352:609–613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Targan SR, Hanauer SB, van Deventer SJ,
Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF and
Rutgeerts PJ: A short-term study of chimeric monoclonal antibody
cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's
Disease cA2 Study Group. N Engl J Med. 337:1029–1035. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Present DH, Rutgeerts P, Targan S, Hanauer
SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T,
DeWoody KL, et al: Infliximab for the treatment of fistulas in
patients with Crohn's disease. N Engl J Med. 340:1398–1405. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sands BE, Tremaine WJ, Sandborn WJ,
Rutgeerts PJ, Hanauer SB, Mayer L, Targan SR and Podolsky DK:
Infliximab in the treatment of severe, steroid-refractory
ulcerative colitis: A pilot study. Inflamm Bowel Dis. 7:83–88.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanauer SB, Feagan BG, Lichtenstein GR,
Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson
A, Bao W, et al: ACCENT I Study Group: Maintenance infliximab for
Crohn's disease: The ACCENT I randomised trial. Lancet.
359:1541–1549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sands BE, Anderson FH, Bernstein CN, Chey
WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken
JE, et al: Infliximab maintenance therapy for fistulizing Crohn's
disease. N Engl J Med. 350:876–885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rutgeerts P, Sandborn WJ, Feagan BG,
Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer
SB, Lichtenstein GR, et al: Infliximab for induction and
maintenance therapy for ulcerative colitis. N Engl J Med.
353:2462–2476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lemann M, Mary JY, Duclos B, Veyrac M,
Dupas JL, Delchier JC, Laharie D, Moreau J, Cadiot G, Picon L, et
al: Infliximab plus azathioprine for steroid-dependent Crohn's
disease patients: A randomized placebo-controlled trial.
Gastroenterology. 130:1054–1061. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Colombel JF, Sandborn WJ, Reinisch W,
Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G,
Diamond RH, Broussard DL, et al: Infliximab, azathioprine, or
combination therapy for Crohn's disease. N Engl J Med.
362:1383–1395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ochsenkuhn T, Sackmann M and Göke B:
Infliximab for acute, not steroid-refractory ulcerative colitis: A
randomized pilot study. Eur J Gastroenterol Hepatol. 16:1167–1171.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maini RN, Breedveld FC, Kalden JR, Smolen
JS, Davis D, Macfarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN,
et al: Therapeutic efficacy of multiple intravenous infusions of
anti-tumor necrosis factor alpha monoclonal antibody combined with
low-dose weekly methotrexate in rheumatoid arthritis. Arthritis
Rheum. 41:1552–1563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lipsky PE, van der Heijde DM, St Clair EW,
Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P,
Feldmann M, et al: Infliximab and methotrexate in the treatment of
rheumatoid arthritis. Anti-tumor necrosis factor trial in
rheumatoid arthritis with concomitant therapy study group. N Engl J
Med. 343:1594–1602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
St Clair EW, van der Heijde DM, Smolen JS,
Maini RN, Bathon JM, Emery P, Keystone E, Schiff M, Kalden JR, Wang
B, et al: Active-Controlled Study of Patients Receiving Infliximab
for the Treatment of Rheumatoid Arthritis of Early Onset Study
Group: Combination of infliximab and methotrexate therapy for early
rheumatoid arthritis: A randomized, controlled trial. Arthritis
Rheum. 50:3432–3443. 2004. View Article : Google Scholar
|
|
36
|
Quinn MA, Conaghan PG, O'Connor PJ, Karim
Z, Greenstein A, Brown A, Brown C, Fraser A, Jarret S and Emery P:
Very early treatment with infliximab in addition to methotrexate in
early, poor-prognosis rheumatoid arthritis reduces magnetic
resonance imaging evidence of synovitis and damage, with sustained
benefit after infliximab withdrawal: Results from a twelve-month
randomized, double-blind, placebo-controlled trial. Arthritis
Rheum. 52:27–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Westhovens R, Yocum D, Han J, Berman A,
Strusberg I, Geusens P and Rahman MU: START Study Group: The safety
of infliximab, combined with background treatments, among patients
with rheumatoid arthritis and various comorbidities: A large,
randomized, placebo-controlled trial. Arthritis Rheum.
54:1075–1086. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abe T, Takeuchi T, Miyasaka N, Hashimoto
H, Kondo H, Ichikawa Y and Nagaya I: A multicenter, double-blind,
randomized, placebo controlled trial of infliximab combined with
low dose methotrexate in Japanese patients with rheumatoid
arthritis. J Rheumatol. 33:37–44. 2006.PubMed/NCBI
|
|
39
|
Schiff M, Keiserman M, Codding C,
Songcharoen S, Berman A, Nayiager S, Saldate C, Li T, Aranda R,
Becker JC, et al: Efficacy and safety of abatacept or infliximab vs
placebo in ATTEST: A phase III, multi-centre, randomised,
double-blind, placebo-controlled study in patients with rheumatoid
arthritis and an inadequate response to methotrexate. Ann Rheum
Dis. 67:1096–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Braun J, Brandt J, Listing J, Zink A,
Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider
M, et al: Treatment of active ankylosing spondylitis with
infliximab: A randomised controlled multicentre trial. Lancet.
359:1187–1193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van der Heijde D, Dijkmans B, Geusens P,
Sieper J, DeWoody K, Williamson P and Braun J: Ankylosing
Spondylitis Study for the Evaluation of Recombinant Infliximab
Therapy Study Group: Efficacy and safety of infliximab in patients
with ankylosing spondylitis: Results of a randomized,
placebo-controlled trial (ASSERT). Arthritis Rheum. 52:582–591.
2005. View Article : Google Scholar
|
|
42
|
Marzo-Ortega H, McGonagle D, Jarrett S,
Haugeberg G, Hensor E, O'connor P, Tan AL, Conaghan PG, Greenstein
A and Emery P: Infliximab in combination with methotrexate in
active ankylosing spondylitis: A clinical and imaging study. Ann
Rheum Dis. 64:1568–1575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Inman RD and Maksymowych WP: CANDLE Study
Group: Adouble-blind, placebo-controlled trial of low dose
infliximab in ankylosing spondylitis. J Rheumatol. 37:1203–1210.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Van den Bosch F, Kruithof E, Baeten D,
Herssens A, de Keyser F, Mielants H and Veys EM: Randomized
double-blind comparison of chimeric monoclonal antibody to tumor
necrosis factor alpha (infliximab) versus placebo in active
spondylarthropathy. Arthritis Rheum. 46:755–765. 2002. View Article : Google Scholar
|
|
45
|
Antoni C, Krueger GG, de Vlam K, Birbara
C, Beutler A, Guzzo C, Zhou B, Dooley LT and Kavanaugh A: IMPACT 2
Trial Investigators: Infliximab improves signs and symptoms of
psoriatic arthritis: Results of the IMPACT 2 trial. Ann Rheum Dis.
64:1150–1157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kavanaugh A, Krueger GG, Beutler A, Guzzo
C, Zhou B, Dooley LT, Mease PJ, Gladman DD, de Vlam K, Geusens PP,
et al: Infliximab maintains a high degree of clinical response in
patients with active psoriatic arthritis through 1 year of
treatment: Results from the IMPACT 2 trial. Ann Rheum Dis.
66:498–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sieper J, Lenaerts J, Wollenhaupt J,
Rudwaleit M, Mazurov VI, Myasoutova L, Park S, Song Y, Yao R,
Chitkara D, et al: Efficacy and safety of infliximab plus naproxen
vs. naproxen alone in patients with early, active axial
spondyloarthritis: Results from the double-blind,
placebo-controlled INFAST study, Part 1. Ann Rheum Dis. 73:101–107.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Algood HM, Lin PL and Flynn JL: Tumor
necrosis factor and chemokine interactions in the formation and
maintenance of granulomas in tuberculosis. Clin Infect Dis.
41(Suppl 3): S189–S193. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
White DW and Harty JT: Perforin-deficient
CD8+ T cells provide immunity to Listeria monocytogenes
by a mechanism that is independent of CD95 and IFN-gamma but
requires TNF-alpha. J Immunol. 160:898–905. 1998.PubMed/NCBI
|
|
50
|
Zhou P, Miller G and Seder RA: Factors
involved in regulating primary and secondary immunity to infection
with Histoplasma capsulatum: TNF-alpha plays a critical role in
maintaining secondary immunity in the absence of IFN-gamma. J
Immunol. 160:1359–1368. 1998.PubMed/NCBI
|
|
51
|
Roach DR, Bean AG, Demangel C, France MP,
Briscoe H and Britton WJ: TNF regulates chemokine induction
essential for cell recruitment, granuloma formation, and clearance
of mycobacterial infection. J Immunol. 168:4620–4627. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zumla A, George A, Sharma V, Herbert N and
Baroness Masham: of Ilton: WHO's 2013 global report on
tuberculosis: Successes, threats, and opportunities. Lancet.
382:1765–1767. 2013. View Article : Google Scholar : PubMed/NCBI
|