Introduction
Heart failure (HF) is a syndrome in which the
quantity of blood being pumped by the heart is insufficient to meet
the requirements of the body. A failing heart usually results in
progressive functional decline, irrespective of the cause of its
failure. HF has high prevalence, mortality and morbidity rates, as
well as significant healthcare costs, and so is an important health
problem worldwide. Atherothrombotic illnesses, which often lead to
HF, are predicted to increase in prevalence and become the primary
cause of mortality worldwide by 2020, thus contributing to an
increase in the prevalence of HF (1).
Elevated sympathetic nervous system activity in
patients with HF is associated with a poor survival rate (2). Although HF improves the contractility
of the heart, sustained activation of β-adrenergic receptors
(β-ARs), particularly of the β1 subtype, causes
contractile dysfunction, arrhythmias of the ventricle, cell loss,
cardiac chamber remodeling and congestive heart failure (2). The resultant cardiac histology is
characterized by hypertrophy of cardiomyocytes, in addition to
perivascular and concomitant interstitial fibrosis (2). Certain β-adrenergic blockers, when
given as long-term treatment, have shown the ability to attenuate
ventricular remodeling (3).
Bisoprolol is a selective and specific blocker of β1-ARs
in cardiac myocytes, and selectively reduces the heart rate without
changing any other cardiac parameters, such as conduction.
Furthermore, bisoprolol has no direct effect on other hemodynamic
parameters (3). Previous studies
have demonstrated that bisoprolol has several pharmacological
functions, including anti-anginal, anti-arrhythmic and
anti-ischemic functions, and it is clinically used for the
treatment of cerebrovascular and cardiovascular illnesses (4).
The up-titration of β-blockers is often suboptimal
in clinical practice (5). It is
commonly observed that patients not only do not take the target
dose as stated in the guidelines but also cease to take the pills
due to a fear of the side effects. From a doctor's perspective, the
administration of a medium dose of a β blocker to patients with
cardiovascular disease, particularly those who are due to undergo
coronary bypass graft or cardiac pacemaker surgeries, may be safer
than up-titration. However, there is little information available
with regard to whether the role of bisoprolol in attenuating
ventricular remodeling is dependent on the achievement of a target
dose, and whether this should be the preferred option. The aim of
the present study was to clarify the underlying benefits of
bisoprolol in the attenuation of pressure overload-induced cardiac
hypertrophy and fibrosis among different doses of the drug in
mice.
Materials and methods
Animal models
All animal procedures were performed in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health (NIH Publication No. 85-23,
revised 1996) and approved by the Institutional Animal Care and Use
Committee at Renmin Hospital of Wuhan University (Wuhan, China).
All surgeries and subsequent analyses were performed in a blinded
manner. Experiments were conducted using 6-8-week-old male C57BL/6J
mice (11400700047102; Beijing Vital River Laboratory Animal
Technology Co., Ltd., Beijing, China) weighing between 20–25 g
(n=62) were used in these experiments. The mice were kept in a
quiet, clean and dim feeding specific pathogen free system with a
12-hour light:dark cycle at a constant temperature of 22°C, and
they had free access to clean water from an inverted plastic
bottle. The mice also had access to rodent chow and to fresh air
that was ventilated using a small VFA-23-BV animal ventilator (Kent
Scientific, Connecticut, USA). Aortic banding (AB) was performed as
previously described (6). The mice
were randomly assigned into the following six groups: Bisoprolol
(10 mg/kg/day)+ sham (n=12), saline + sham (n=12), AB + saline
(n=14), AB + low-dose bisoprolol (2.5 mg/kg/day; n=16), AB +
middle-dose bisoprolol (5 mg/kg/day; n=16) and AB + high-dose
bisoprolol (10 mg/kg/day; n=16). Bisoprolol and inert saline were
administered orally via gastric gavage for 8 weeks starting on day
1 following surgery.
The mice were fasted during the night prior to
surgery. A topical depilatory agent was applied to the neck and
chest area to remove fur at and around the area of the incision.
One dose of penicillin (10 mg/kg, 0.1 ml) was administered by an
intraperitoneal (i.p.) injection prior to the beginning of surgery.
Next, the mice were placed supine and the body temperature was
maintained at 37°C using a heating pad. The mice were then
anesthetized with sodium pentobarbital (80 mg/kg, i.p.;
Sigma-Aldrich, St. Louis, MO, USA) and xylazine (10 mg/kg).
Following endotracheal intubation, mechanical ventilation was
initiated. The skin was cleaned with Germex and Betadine (both from
Winguide Huangpu Pharmaceutical Co., Ltd., Shanghai, China). A
horizontal skin incision of 1 cm in length was made at the level of
2–3 intercostal spaces, once the animal reached a surgical plane of
anesthesia (lack of reflex or response to toe-pinching). A 6-0 silk
suture was snared and pulled back around the aorta to produce a
65–70% constriction following removal of the needle. A bent
27-gauge needle (for 23.5–25.5 g) was then placed next to the
aortic arch and the suture was tightly tied around the needle and
aorta between the left carotid artery and the brachiocephalic
trunk. Doppler analysis was performed to ensure that a
physiological constriction of the aorta was induced. Following
ligation, the needle was quickly removed allowing the suture to
constrict the aorta. The incision was closed in layers and the mice
were allowed to recover on a warming pad until they were fully
awake. The adequacy of anesthesia was monitored during the surgical
procedure using the pedal withdrawal reflex, slow constant
breathing and the lack of a response to surgical manipulation. The
sham animals underwent the same procedure without AB.
Immediately after the surgery, mice received one
dose of buprenorphine (0.1 mg/kg, subcutaneously) for the first 24
h, and were allowed to have food and water when they were fully
awake. On day 1 after the surgery, the administration of bisoprolol
was initiated.
Drugs and reagents
Bisoprolol was purchased from Merck KGaA (Frankfurt,
Germany). The following antibodies were used: Anti-glyceraldehyde
3-phosphate dehydrogenase (2118; anti-GAPDH; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-atrial natriuretic
peptide (sc20158; ANP), anti-brain natriuretic peptide (18817;
BNP), anti-β-myosin heavy chain (sc53090; MHC) (all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and anti-connective tissue
growth factor (23936; CTGF), anti-transforming growth factor
(TGF)-β1 (3709) and anti-collagen 1a (ab90395) (all from
Abcam, Cambridge, UK).
Echocardiographic imaging
After 4 and 8 weeks, mice that had been subjected to
chronic pressure overload generated by AB or to sham surgery, the
latter serving as a control group, were subjected to
echocardiographic imaging. Transthoracic M-mode and Doppler
echocardiographic examination was performed using a MyLab 30 CV
ultrasound instrument (Esaote SpA, Genoa, Italy) with a 10 MHz
linear array ultrasound transducer, as previously described, in
order to assess the internal diameter and wall thickness of the
left ventricle (LV) (7,8). Mice were placed on a heating pad and a
nose cone with 0.75–1% isoflurane in 100% oxygen was applied. The
temperature was maintained at 36.5–37.5°C and ultrasound gel was
spread on the chest of the anesthetized mouse. The ultrasound probe
was placed in contact with the ultrasound gel and scanning was
performed over a period of 30 min. The heart rate, temperature and
blood pressure were constantly monitored during the scan, and
M-mode images were obtained for measurements of the LV wall
thickness, LV end-diastolic diameter (LVEDD), LV end-systolic
diameter (LVESD), left ventricular septum diastolic (IVSD), left
ventricular posterior wall diameter (LVPWD), fractional shortening
(FS) and ejection fraction (EF). The data were stored on a hard
drive and a 230 MB optical disk for image processing.
Hemodynamic analysis
The in vivo cardiac performance was measured
by both load-dependent and load-independent parameters derived from
pressure-volume (P-V) loops. The invasive hemodynamic measurements
were performed by the same operator who was blinded to the
experimental groups following the echocardiographic examination in
each mouse. The mice were anesthetized with 1.5% isoflurane using
cardiac catheterization. A SPR-839 microtip catheter transducer
(Millar Instruments, Houston, TX, USA) was inserted into the right
carotid artery and moved into the LV. Following stabilization for a
period of 15 min, the pressure signals and heart rate were
continuously recorded with an ARIA pressure-volume conductance
system (MVPS-400, Millar, Inc., TX, USA) coupled with a
Powerlab/4SP A/D converter (ATC1000; World Precision Instruments
Inc., Hilton, Australia) and then stored and displayed on a
personal computer as previously described (7,8).
The mice were sacrificed by cervical dislocation 8
weeks post-operatively after anesthetizing with 1.5% isoflurane
(Lunan Pharmaceutical Group Co., Ltd., Shandong, China) or sodium
pentobarbital (80 mg/kg; i.p; Sigma-Aldrich). The hearts, lungs and
tibiae of the mice were dissected and weighed or measured to
compare the heart weight (HW)/body weight (BW) in mg/g, HW/tibial
length (TL) in mg/mm, and lung weight (LW)/BW in mg/g ratios
amongst the different groups.
Histological and morphometric
analysis
All morphometric and histological measurements were
obtained from the hearts arrested in diastole (intracardiac 40 mM
KCl), rinsed with saline solution and placed in 10% formalin. The
sections were deparaffinized in xylene and rehydrated in ethanol.
The hearts were transversely sectioned close to the apex in order
to visualize the left and right ventricles. Numerous sections (4–5
µm thick) were prepared and stained with hematoxylin and eosin
(H&E) for histopathology or with picrosirius red (PSR) for
interstitial and perivascular collagen volume fraction
quantification. The stained sections were visualized by light
microscopy at magnification, ×400, and cross-sectional images of
the cardiac myocytes were digitized using an Eclipse 80i digital
microscope (Nikon Corporation, Tokyo, Japan). An quantitative
digital image analysis system (Image-Pro Plus 6.0, Media
Cybernetics, Inc., Rockville, MD, USA) was used to measure single
myocytes, with ~100–200 myocytes in the LV being outlined in each
group. The fraction of collagen was calculated as a ratio of the
sum of the total area of interstitial or perivascular fibrosis to
the sum of the total connective tissue area plus the myocyte area
in the entire visual field of a section. For myocyte
cross-sectional area, the sections were stained for membranes with
fluorescein (FITC)-conjugated wheat germ agglutinin (WGA;
Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
for nuclei with 4′,6-diamidino-2-phenylindole according to standard
protocols (9).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was used to detect the RNA expression levels
of fibrotic and hypertrophic markers. The total RNA was extracted
from flash-frozen, pulverized mouse cardiac tissue using the TRIzol
(Roche Diagnostics, Basel, Switzerland) extraction protocol. A
SmartSpec plus spectrophotometer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to estimate the yield and purity using
the A260/A280 and A230/260 ratios. The RNA (2 µg of each sample)
was reverse transcribed into cDNA using oligo (dT) primers and
RTase (04897030001; Roche Diagnostics GmbH, Mannheim, Germany) and
the Transcriptor First Strand cDNA Synthesis kit (04896866001;
Roche Diagnostics). PCR amplifications were quantified using SYBR
Premix Ex Taq II (Tli RNaseH Plus; RR820A; Takara Biotechnology
Co., Ltd., Dalian, China). Reactions were performed in a total
volume of 20 µl, starting with an initial denaturation step at 95°C
for 5 sec, followed by 40 cycles of denaturation at 95°C for 30
sec, annealing at 60°C for 34 sec and an extension step at 72°C for
3 min, followed by a 7-min terminal extension at 72°C. GAPDH gene
expression was used as a reference in order to normalize the
results using the ΔΔCq method (10).
The following primers were used: GAPDH forward:
5′-TCATCAACGGGAAGCCCATC-3′ and reverse: 5′-CTCGTGGTTCACACCCATCA-3′;
ANP forward: 5′-ACCTGCTAGACCACCTGGAG-3′ and reverse:
5′-CCTTGGCTGTTATCTTCGGTACCGG-3′; BNP forward:
5′-GAGGTCACTCCTATCCTCTGG-3′ and reverse:
5′-GCCATTTCCTCCGACTTTTCTC-3′; β-MHC forward:
5′-CCGAGTCCCAGGTCAACAA-3′ and reverse: 5′-CTTCACGGGCACCCTTGGA-3′;
CTGF forward: 5′-TGTGTGATGAGCCCAAGGAC-3′ and reverse:
5′-AGTTGGCTCGCATCATAGTTG-3′; collagen 1a forward:
5′-TGGTACATCAGCCCGAAC-3′ and reverse: 5′-GTCAGCTGGATAGCGACA-3′; and
TGF-β1 forward: 5′-ATCCTGTCCAAACTAAGGCTCG-3′ and
reverse: 5′-ACCTCTTTAGCAAGTAGTCCGC-3′.
Western blot analysis
For western blot analysis, cardiac tissues were
lysed in radioimmunoprecipitation assay lysis buffer. The amount of
protein from each sample was calculated using a bicinchoninic acid
assay kit (23227; Thermo Fisher Scientific, Inc.). Furthermore, the
concentrations of the proteins were normalized prior to running any
western blot experiments. Cardiac extracts were subjected to 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis,
transferred to polyvinylidene difluoride (PVDF) membrane (Amersham;
GE Healthcare Life Sciences, Chalfont, UK), and blocked in 7% milk.
The membrane was washed with Tris-buffered saline Tween-20 for 15
min and then incubated overnight at 4°C with the indicated primary
antibodies. These were the following: GAPDH (1:1,000), ANP (1:200),
BNP (1:200), β-MHC (1:200), CTGF (1:200), Collagen1α (1:200) and
TGF-β1 (1:1,000). Following incubation with the secondary
immunoglobulin G antibodies (1:1,000; alkaline phosphatase) for 1 h
at room temperature in 3% milk. These were the following
antibodies: rabbit antibody (IRDye800CW, 926-32211, LI-COR
Biosciences, NE, USA) for GAPDH, ANP, CTGF and TGF-β1; mouse
antibody (IRDye800CW, 926-32210, LI-COR Biosciences, NE, USA) for
β-MHC and collagen1α; goat antibody for BNP (IRDye800CW, 926-32232,
LI-COR Biosciences, NE, USA). Quantification of western blots was
performed using an Odyssey infrared imaging system (Li-Cor
Biosciences, Lincoln, NE, USA). The GAPDH protein was used to
normalize specific protein expression levels for the total cardiac
lysate proteins on the same PVDF membranes.
Statistical analysis
Graphs were generated with GraphPad Prism 6
(GraphPad Software Inc., La Jolla, CA, USA), and the data in the
figures are expressed as the mean ± standard error of the mean. The
significance of differences between experimental and control groups
was evaluated using one-way analysis of variance with Student
Newman-Keuls test. Comparison of survival was performed using
Kaplan-Meier analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Long-term administration of bisoprolol
reveals substantial benefits
Initially, the potential existence of a
time-response association for various doses of bisoprolol in the
enhancement of cardiac functions was investigated.
Echocardiographic parameters, including LVEDD, LVESD, IVSD, LVPWD,
FS and EF, which have commonly been used in the clinic for
estimating cardiac functions, were measured at 4 and 8 weeks after
sham or AB surgery. There was no clear phenotypic difference among
all of the groups at baseline, as assessed by echocardiography
(Table I), or at 4 weeks (Fig. 1). The latter observation may be
attributed to the compensatory role of the heart. The long-term
administration of bisoprolol to mice until 8 weeks after surgery
(Table II) revealed statistically
significant differences among the groups, in contrast with
administration for 4 weeks (Fig. 1).
Altogether, these data indicate that the role of bisoprolol in the
attenuation of cardiac function is dependent upon the time period
for which it is used, with long-term administration of bisoprolol
demonstrating a greater benefit than short-term use.
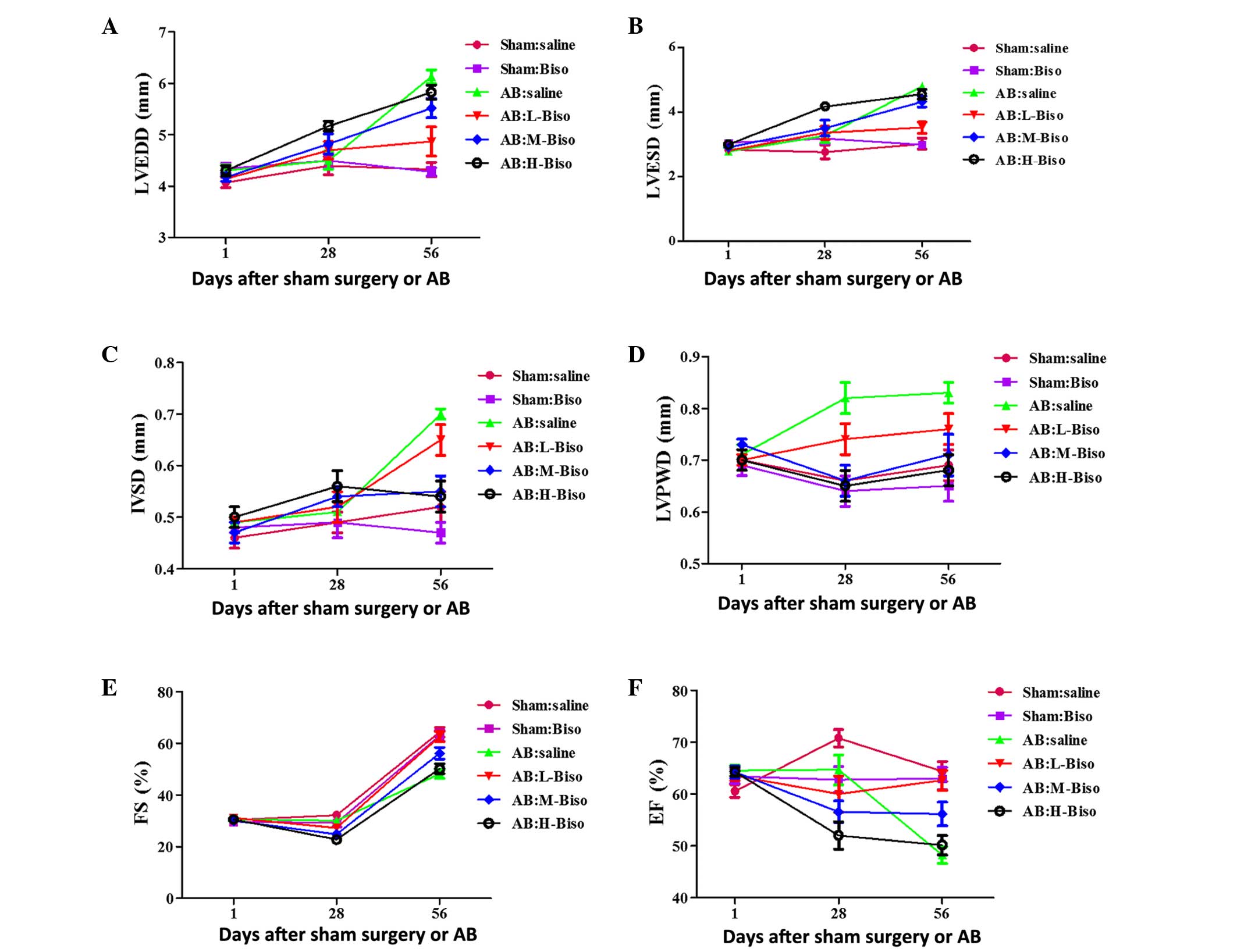 | Figure 1.Echocardiographic parameters at
different time-points. (A) Left ventricular end-diastolic diameter
(LVEDD), (B) left ventricular end-systolic diameter (LVESD), (C)
left ventricular septum diastolic (IVSD), (D) left ventricular
posterior wall diameter (LVPWD), (E) fractional shortening (FS) and
(F) ejection fraction (EF). Data are presented as mean ± standard
error of the mean (n=12–16 in each group) and were analyzed with
2-way repeated-measures analysis of variance. Bonferroni correction
was applied for multiple comparisons. (A-F) P<0.01 for H-Biso
and M-Biso vs. the other four groups at 8 weeks (56 days), but no
significant difference between the M-Biso and H-Biso groups. AB,
aortic banding; Biso, bisoprolol; L, low-dose; M, middle-dose; H,
high-dose. |
 | Table I.Echocardiograhic parameters of
C57BL/6 mice at baseline. |
Table I.
Echocardiograhic parameters of
C57BL/6 mice at baseline.
|
| Sham | AB |
|---|
|
|
|
|
|---|
| Parameter | Saline | Biso | Saline | L-Biso | M-Biso | H-Bsio |
|---|
| Number | 12 | 12 | 14 | 16 | 16 | 16 |
| BW (g) |
24.97±0.24 |
24.87±0.17 |
24.91±0.18 |
25.16±0.13 |
25.50±0.20 |
25.10±0.24 |
| HR (bpm) |
495.50±4.80 |
502.45±4.24 |
508.00±5.43 |
508.94±6.20 |
505.44±6.94 |
506.81±5.56 |
| LVESD (mm) |
2.84±0.09 |
3.05±0.11 |
2.78±0.12 |
2.81±0.06 |
2.91±0.07 |
2.99±0.07 |
| LVEDD (mm) |
4.07±0.10 |
4.34±0.11 |
4.30±0.11 |
4.14±0.06 |
4.17±0.08 |
4.30±0.10 |
| IVSD (mm) |
0.46±0.02 |
0.48±0.01 |
0.49±0.01 |
0.49±0.01 |
0.47±0.02 |
0.50±0.02 |
| LVPWD (mm) |
0.70±0.02 |
0.69±0.02 |
0.71±0.02 |
0.70±0.01 |
0.73±0.01 |
0.70±0.02 |
| FS (%) |
30.40±0.83 |
29.73±0.91 |
30.62±0.80 |
31.13±0.76 |
30.00±0.78 |
30.56±0.86 |
| EF (%) |
64.60±1.26 |
63.45±1.38 |
64.54±1.04 |
63.50±0.93 |
64.25±1.21 |
64.31±0.83 |
 | Table II.Echocardiographic parameters of
C57BL/6 mice at 8 weeks after sham or AB surgery. |
Table II.
Echocardiographic parameters of
C57BL/6 mice at 8 weeks after sham or AB surgery.
|
| Sham | AB |
|---|
|
|
|
|
|---|
| Parameter | Saline | Biso | Saline | L-Biso | M-Biso | H-Bsio |
|---|
| Number | 5 | 6 | 4 | 6 | 6 | 6 |
| BW (g) |
31.10±0.68 |
27.11±0.47 |
26.56±0.68a |
27.53±0.64 |
28.28±0.59 |
26.77±1.15 |
| HR (bpm) |
550.80±21.31 |
537.67±21.80 |
482±21.00 |
548.83±17.51 |
544.17±21.72 |
533.00±24.89 |
| LVESD (mm) |
3.02±0.17 |
2.98±0.11 |
4.80±0.07a |
3.52±0.17b |
4.32±0.17 |
4.55±0.15 |
| LVEDD (mm) |
4.32±0.14 |
4.28±0.08 |
6.13±0.13a |
4.87±0.28b |
5.52±0.19 |
5.83±0.14 |
| IVSD (mm) |
0.52±0.03 |
0.47±0.02 |
0.70±0.01a |
0.65±0.03b |
0.55±0.03 |
0.54±0.03 |
| LVPWD (mm) |
0.69±0.03 |
0.65±0.03 |
0.83±0.02a |
0.76±0.03 |
0.71±0.04 |
0.68±0.03 |
| FS (%) |
30.60±0.60 |
30.17±1.11 |
22.50±0.96a |
30.00±0.77 |
26.00±1.15c |
22.00±1.03d |
| EF (%) |
64.40±1.89 |
63.00±2.14 |
48.25±1.60a |
62.67±1.94 |
56.17±2.27c |
50.17±1.89d |
Diversities between middle- and
high-dose bisoprolol are not remarkable in the attenuation of
cardiac hypertrophy induced by pressure overload
To investigate whether there is some diversity among
the different doses of bisoprolol in attenuating the cardiac
hypertrophy induced by pressure overload, the AB model was used to
induce hypertrophy. The degree to which hypertrophy was affected by
different doses of bisoprolol was assessed by echocardiographic,
histomorphological and molecular analysis. After 8 weeks,
echocardiographic and P-V loop analyses were performed in order to
observe the chamber diameter, wall thickness and function of the
left ventricle. Results demonstrated that there were no significant
changes in the two groups mice that underwent sham surgery, whereas
there were marked diversities among the four groups that underwent
AB surgery. However, there was no statistically significant
difference between the middle- and high-dose bisoprolol groups
(Fig. 2A). In the AB groups, mice
that received intragastric administration of saline or low-dose
bisoprolol exhibited deteriorated cardiac hypertrophy and
dysfunction compared with the mice that were treated with middle-
or high-dose bisoprolol, as measured by echocardiographic
parameters such as LVEDD, LVESD, LVPWD, FS and EF% after 8 weeks of
AB (Fig. 2B). A more detailed
examination of cardiac function was performed using invasive
pressure-volume analysis. At 8 weeks after AB, distinct phenotypes
were observed among the groups in terms of the progression of
cardiac dysfunction measured by end-systolic and end-diastolic
pressure, dp/dt max and dp/dt min (Fig.
2C). The mice intragastrically treated with saline following AB
demonstrated an increase in heart size and dilatation of
ventricular chambers as compared with the sham group and other AB
groups (Fig. 3A). Furthermore, not
only were the whole hearts much smaller after bisoprolol treatment
compared with those of saline-treated AB mice but also the HW/BW,
LW/BW, and HW/TL ratios were significantly decreased. However, the
diversities between middle- and high-dose were not remarkable at 8
weeks after surgery (Fig. 3B).
Observation of the hearts, and the H&E and WGA-FITC staining
results were consistent with the echocardiographic results
(Fig. 3A).
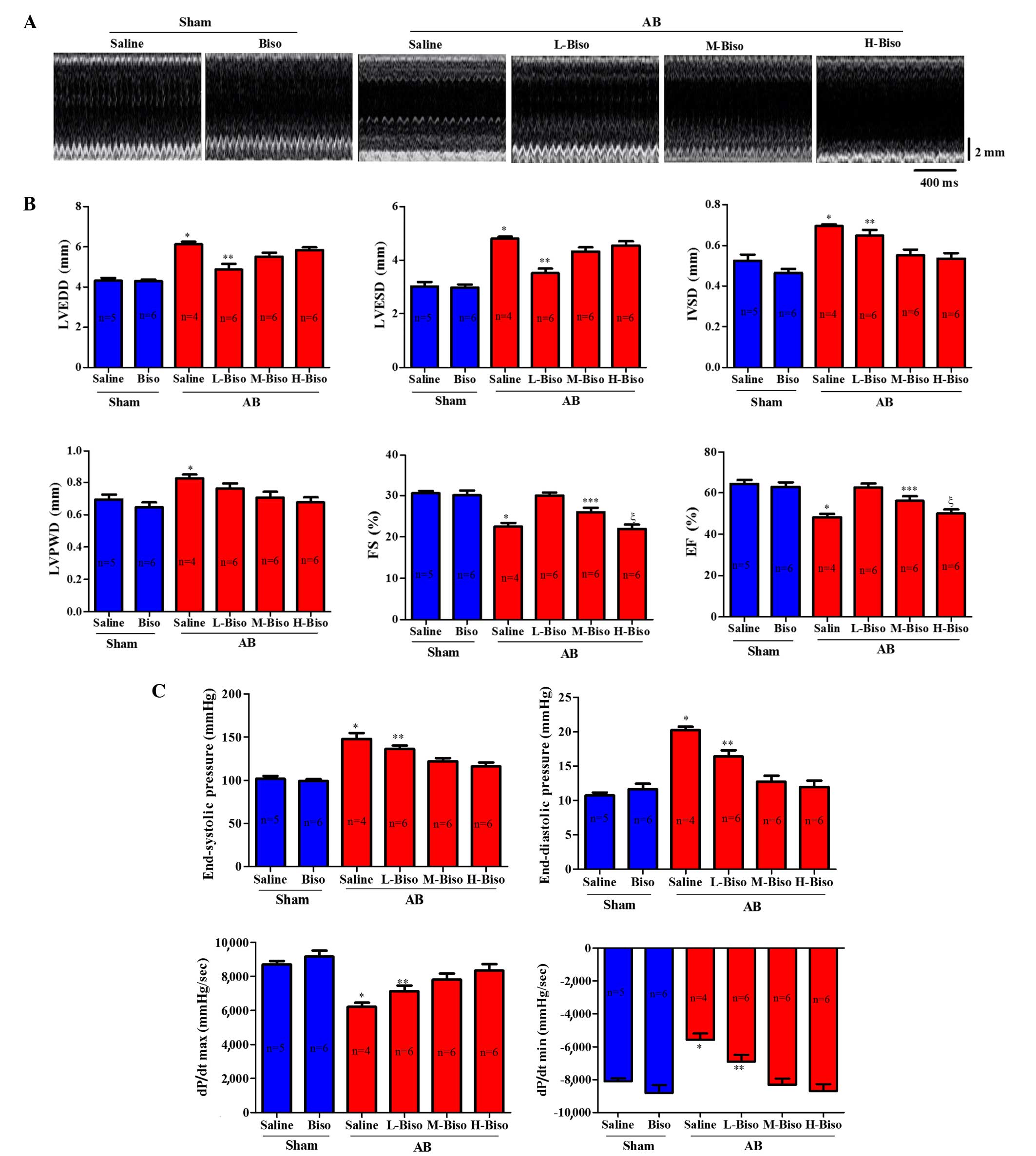 | Figure 2.Bisoprolol increases adverse pressure
overload-induced ventricular remodeling. (A) Representative serial
M-mode echocardiography in conscious mice from the six experimental
groups at 8 weeks after sham or AB surgery. (B) Quantitative
analysis of echocardiographic parameters. (C) Summary of
hemodynamic data on systolic function and diastolic function. All
values are the mean ± standard error of the mean (n=4–6 per group).
*P<0.05 vs. all other groups, **P<0.05 vs. M-Biso AB and
H-Biso AB, ***P<0.05 vs. H-Biso AB, ξP<0.05 vs.
L-Biso AB and M-Biso AB. AB, aortic banding; Biso, bisoprolol; L,
low-dose; M, middle-dose; H, high-dose; LVEDD, left ventricular
end-diastolic diameter; LVESD, left-ventricular end-systolic
diameter; IVSD, left ventricular septum diastolic; LVPWD, left
ventricular posterior wall diameter; FS, fractional shortening; EF,
ejection fraction; dp/dt, left ventricular contractility. |
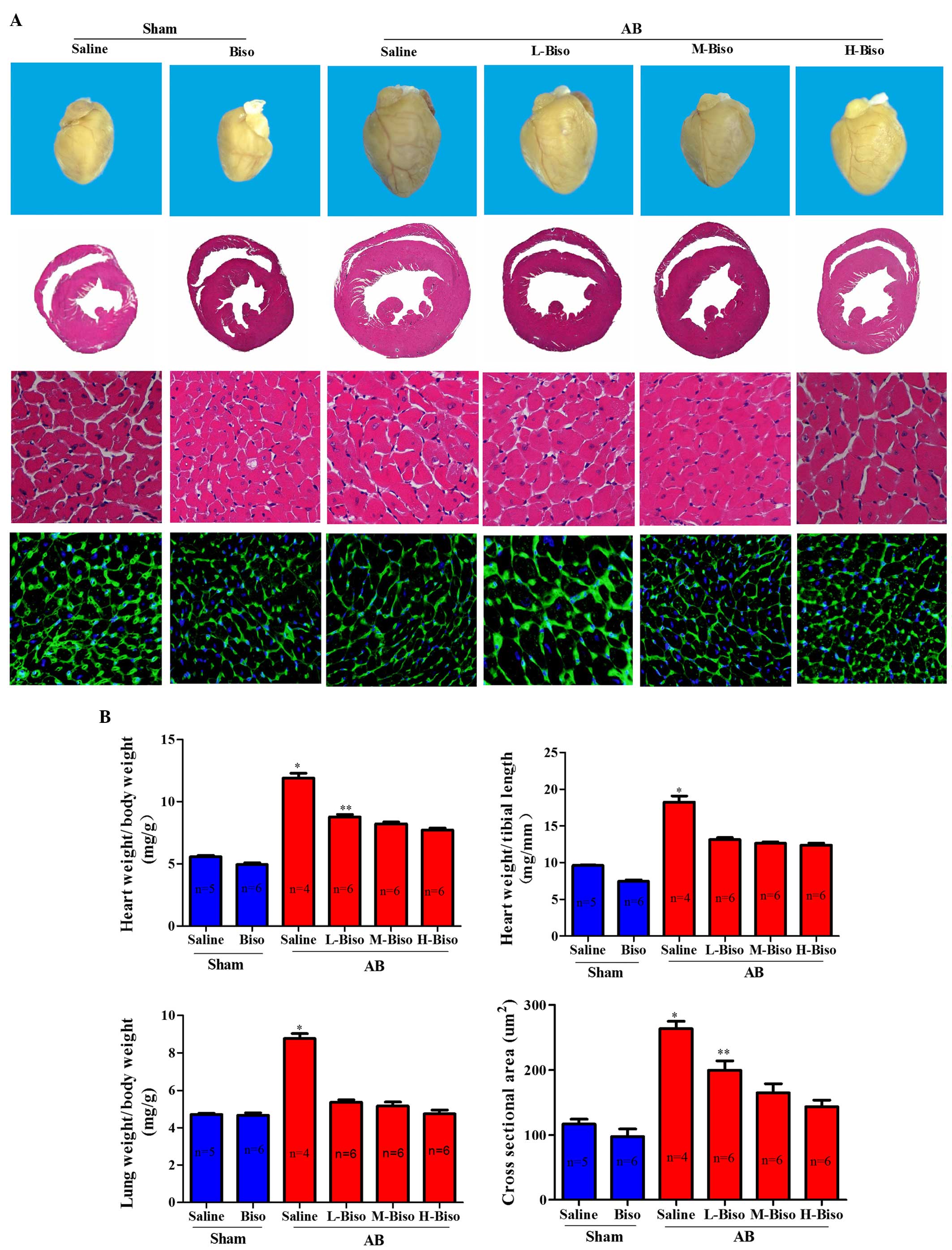 | Figure 3.Bisoprolol attenuated cardiac
hypertrophy induced by pressure overload. (A) Histological changes.
Upper images, gross observation of hearts; middle images,
representative images of hematoxylin and eosin staining of whole
hearts (magnification, ×1) and sections (magnification, ×40); lower
images, wheat germ agglutinin-fluorescein isothiocyanate staining
(magnification, ×40) at 8 weeks post-aortic banding (AB) surgery.
(B) Graphical results of HW/BW ratio, LW/BW ratio, HW/TL ratio, and
myocyte cross-sectional areas (n=100 cells per group) at 8 weeks
post-AB surgery (n=6). *P<0.05 vs. the other five groups,
**P<0.05 vs. M-Biso AB and H-Biso AB groups. Biso, bisoprolol;
L, low-dose; M, middle-dose; H, high-dose; HW, heart weight; BW,
body weight; TL, tibial length. (C) Representative western blots of
atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP)
and β-major histocompatibility complex (MHC) at 8 weeks post-sham
and aortic banding (AB) surgery. (D) Quantitative polymerase chain
reaction analysis of ANP, BNP and β-MHC at 8 weeks post-sham and AB
surgery (n=3 per group). Values represent the mean ± standard error
of the mean. *P<0.05 vs. all other groups. **P<0.05 vs.
M-Biso AB and H-Biso AB. Biso, bisoprolol; L, low-dose; M,
middle-dose; H, high-dose; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
In order to explore whether the different doses of
bisoprolol correspond with changes in the protein levels and mRNA
expression of markers of cardiac hypertrophy, western blot analysis
and qPCR analysis of fetal genes, including ANP, BNP and β-MHC were
performed. The results demonstrated a significant reduction of the
mRNA expression and protein levels of ANP, BNP and β-MHC from those
in the saline-treated AB group when the animals were treated with
bisoprolol (Fig. 3C and D). In
addition, the results indicated that the middle- and high-dose of
bisoprolol inhibited the protein and mRNA expression levels of the
cardiac hypertrophy markers ANP, BNP, and β-MHC the most notably;
however, there were no statistically significant differences
between these two groups (Fig. 3C and
D). Altogether, the aforementioned data clearly indicate that
there is no noteworthy difference between the high-dose, i.e., the
target-dose, and middle-dose bisoprolol in the attenuation of
cardiac hypertrophy induced by pressure overload.
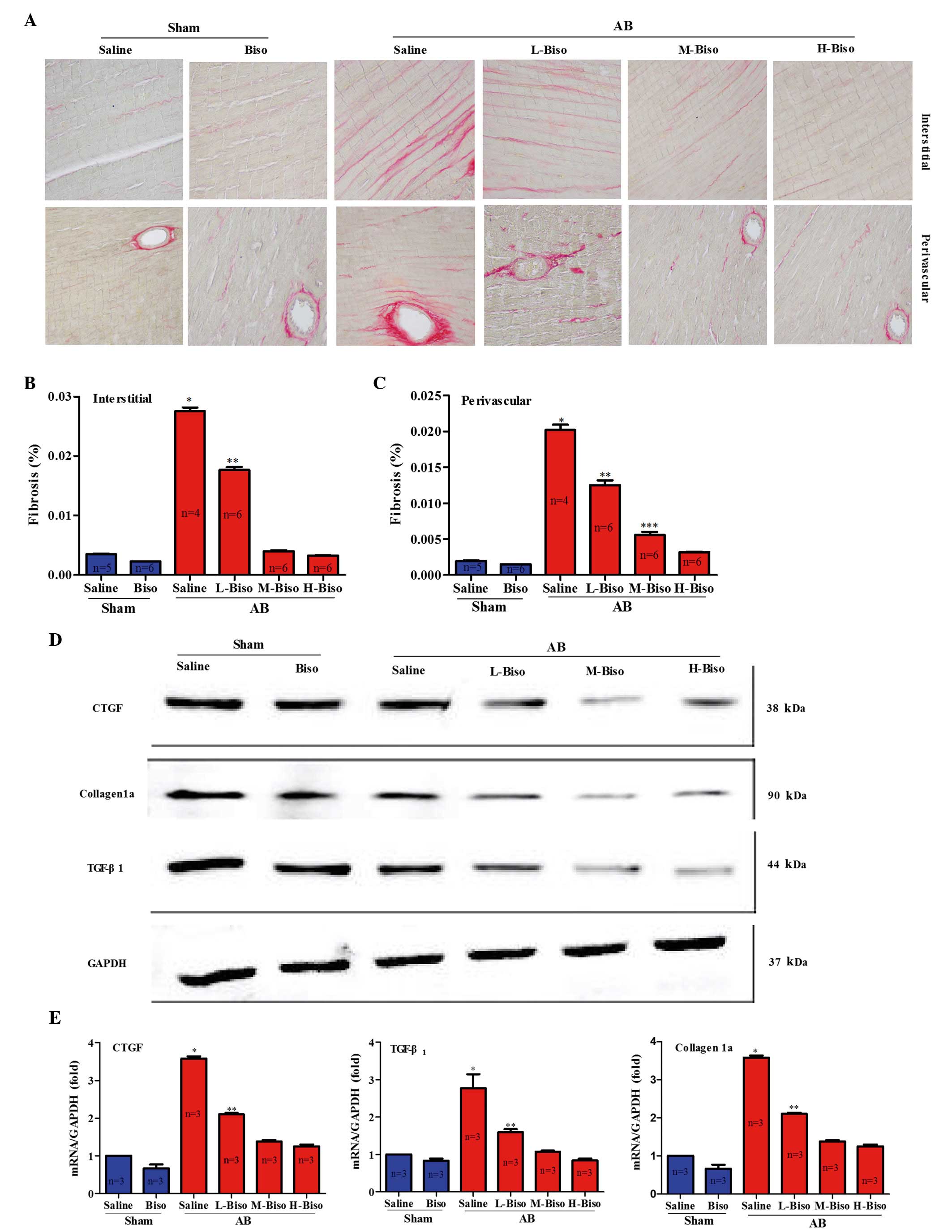
Amelioration of the degree of cardiac
fibrosis is not evidently different between middle- and high-dose
bisoprolol
Pathological cardiac hypertrophy correlates with
increased fibrosis in the myocardium (11). Fibrosis is a typical feature of
pathological cardiac hypertrophy, characterized by the accumulation
of collagen. To investigate the extent by which different doses of
bisoprolol inhibit cardiac fibrosis, paraffin-embedded slides were
stained with PSR at 8 weeks after AB surgery, and the staining was
quantitatively analyzed from interstitial and perivascular regions
of the left ventricles. Also, the extent of fibrosis was
investigated by assessing the protein and mRNA expression levels of
fibrotic genes, including Tgf-β1 (encoding
TGF-β1), Col1a (encoding collagen 1a) and
Ctgf (encoding CTGF) (12,13). The
results revealed that long-term treatment of mice with bisoprolol
following AB surgery reduced interstitial and perivascular cardiac
fibrosis (Fig. 4A). Furthermore, a
marked attenuating effect of middle- and high-doses of bisoprolol
on cardiac fibrosis was identified by quantitative analysis of
collagen volume in the interstitial and perivascular regions;
however, no statistically significant difference was observed
between these two groups (Fig. 4B and
C). Reduced fibrosis in the mice treated with middle- and
high-dose bisoprolol may represent decreased collagen synthesis or
increased collagen degradation in response to tissue damage.
Therefore, the synthesis of collagen was assessed by examining the
protein and mRNA expression levels of fibrotic markers CTGF,
collagen 1a, and TGF-β1, all of which have a function in
the proliferation of cardiac fibroblasts and the biosynthesis of
extracellular matrix (ECM) proteins. The data revealed that the
mRNA and protein expression levels of CTGF, Collagen 1a, and
TGF-β1 were significantly lower in the middle- and
high-dose bisoprolol-treated mice than in the saline and low-dose
bisoprolol-treated mice at 8 weeks after AB surgery. However, there
were no statistically significant differences between the middle-
and high-dose bisoprolol groups (Fig. 4D
and E). These data demonstrate that middle- and high-dose
bisoprolol have comparable effects in the attenuation of cardiac
fibrosis induced by pressure overload.
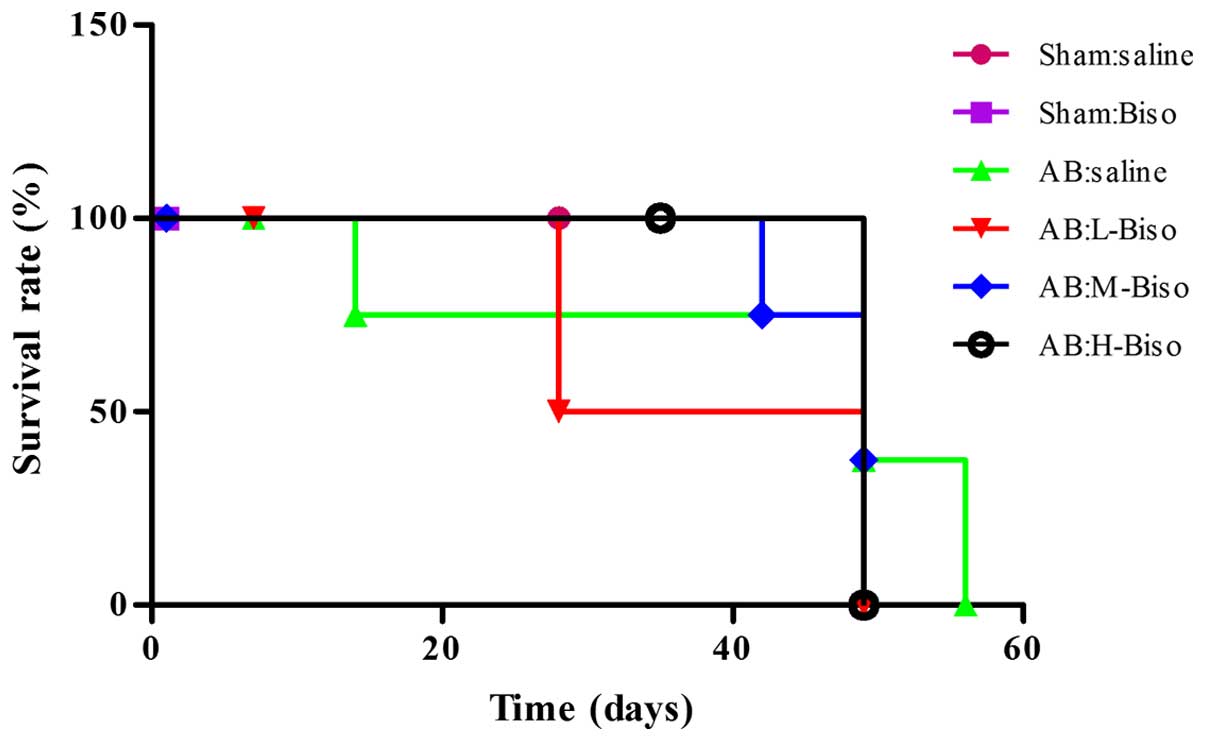
Survival following AB
Treatment with middle- or high-dose bisoprolol
therapy was associated with a significantly improved survival rate
at 8 weeks after AB compared with saline therapy (P<0.05 vs.
saline). However, no statistically significant difference in
survival was observed between the middle- and high-dose bisoprolol
groups (Fig. 5).
Discussion
Hemodynamic overload and ischemic or oxidative
stress promote adverse cardiac remodeling, which is a leading cause
of worsening heart failure (14).
The pathophysiological conditions of heart failure are associated
with adrenergic stimulation and catecholamine release, resulting in
adrenoceptor (AR) activation on different cell types within the
myocardium. Among these, β1-ARs are classically
considered to mediate short-term positive effects on all aspects of
myocardial contractility; however, long-term stimulation produces
adverse effects on myocardial remodeling (15). The aim of the present study was to
clarify the underlying benefits of bisoprolol in the attenuation of
the ventricular remodeling induced by pressure overload with the
use of different doses in mice.
When the heart undergoes pressure or volume
overload, myocardial hypertrophy begins as an adaptive response.
This is often observed in valve disease, arterial hypertension or
following myocardial infarction. When cardiomyocyte hypertrophy
occurs, an increase in cell size and protein content is observed,
as well as a repeated expression of a fetal gene programme that
includes ANP, BNP and β-MHC. Furthermore, the expression of
immediate early genes is also observed (16). When overload is prolonged, the
initially compensatory hypertrophic response may become
maladaptive, which may result in chronic heart failure (17,18).
Previous studies have revealed that the
β1-AR subtype is not only the major mediator of
pathological hypertrophy, but also is associated with a notable
increase in interstitial fibrosis and heart failure (19,20).
This is also supported by the beneficial effect of β1-AR
blockade in clinical heart failure. β-blockers are thought to
function by reducing the sympathetic activity and the workload of
the heart, and by exerting beneficial effects on the ventricular
remodeling process (21,22). According to European and American
guidelines, the use of β-blockers in symptomatic patients with
heart failure has a class 1A recommendation (23,24).
Nonetheless, uptake of therapy in clinical practice remains
suboptimum, with patients who are at greatest risk of mortality
being the least likely to receive evidence-based therapy (25). There have also been concerns with
regard to the treatment efficacy in several groups, notably
patients with atrial fibrillation, coronary artery bypass graft
(26,27) and cardiac pacemaker surgeries
(28), women, and elderly
individuals (29).
Although β-blockers have been widely used in the
treatment of endoscopic sinus surgery (30), hypertension, coronary artery disease,
dilated cardiomyopathy and heart failure (27,31), the
clinical efficacy among different β-blockers does not appear to be
equivalent (32). In the present
study, different doses of a highly selective
β1-AR-blocking antagonist, bisoprolol, not metoprolol
(which induces fibrosis and cardiac dysfunction) (33) was used in order to improve cardiac
function in the AB model. The assessment of echocardiographic
results in the present study demonstrated a greater improvement in
cardiac function after 8 weeks of treatment with bisoprolol
compared with that in mice treated with bisoprolol after AB or sham
surgery at 4 weeks. No statistically significant difference was
observed between the middle- and high-dose bisoprolol treatment
groups (Fig. 1). These outcomes
highlight that the attenuating effect of bisoprolol on ventricular
cardiac hypertrophy induced by pressure overload occurs in a
time-dependent manner. In subsequent experiments, the aim was to
further elucidate this phenomenon in mice treated with different
doses of bisoprolol following AB or sham surgery by investigating
changes of the hearts using echocardiographic imaging (Fig. 2A and B), hemodynamic analysis
(Fig. 2C) and histomorphology
(Fig. 3A and B) after 8 weeks. The
experimental results confirmed that the middle- and high-doses were
important in enhancing cardiac function following AB surgery;
however, no statistically significant difference was observed
between them. The aforementioned outcomes were also confirmed by
exploring the protein and mRNA expression levels of markers of
cardiac hypertrophy, namely ANP, BNP and β-MHC (Fig. 3C and D). Altogether, these data
clearly indicate that there is a time-dependent effect of high- and
middle-dose bisoprolol treatment in the attenuation of cardiac
hypertrophy induced by pressure overload, but no notable difference
between these two doses.
Cardiac fibrosis is an important hallmark of
maladaptive hypertrophy. Increased fibrosis decreases myocardial
compliance, impairs diastolic relaxation and causes cardiac
dysfunction (34,35). In the present study, the
anti-fibrotic properties of bisoprolol in the heart were analyzed.
Initially the anti-fibrotic role of bisoprolol was examined through
PSR staining of sections from interstitial and perivascular regions
of the left ventricles (Fig. 4A-C).
In agreement with the results from pathological images and
quantification, middle- and high-dose bisoprolol-treated mice
demonstrated increased collagen degradation or decreased collagen
synthesis in response to tissue damage. However, no statistically
significant difference was observed between them. TGF-β1
has been reported to be one of the main mediators of cardiac
fibroblast activation (36) and is
considered as a profibrogenic cytokine that contributes to various
types of fibrosis, including cardiac fibrosis associated with heart
failure (37,38). Studies have demonstrated that the
expression of TGF-β1 is increased in patients with
cardiomyopathic conditions and animal models of cardiac fibrosis
(39,40). This was firmly verified in the
present study by analyzing the protein and mRNA expression levels
of the fibrotic markers CTGF, collagen 1a and TGF-β1,
that are known to be important in the biosynthesis of ECM proteins
and the proliferation of cardiac fibroblasts (Fig. 4D and E). These results are also
consistent with a previous study by Fukui et al (41).
The current study demonstrates that the effects of
bisoprolol in the attenuation of ventricular remodeling induced by
hypertrophic stimuli are time-dependent. Thus, achieving a target
dose of bisoprolol for attenuating ventricular remodeling may not
be a preferred option in some cases (42,43).
Future studies should focus on determining whether the results
obtained in mice are also observed among patients from different
regions, and with different professions and states of disease
(44,45). The translation of this knowledge into
clinical use should be challenging and exciting.
Furthermore, the results of the present study must
be evaluated in light of several study limitations. Firstly, the
clinical efficacy of different β-blockers does not appear to be
equivalent (29). A previous study
suggests that nebivolol, a β1-selective AR blocker
improves LV dysfunction and survival early after myocardial
infarction and possibly beyond the effects provided by conventional
β1-receptor blockade (46). Secondly, the sample size was small.
Finally, the present study did not assess the findings through the
levels of cardiac electrophysiology. Nevertheless, the observations
reported in the present study may have clinical significance for
the pharmacological modulation of catecholamine-mediated myocardial
remodeling in the stressed heart, for example giving the right
person the correct medication and dose, and knowing the correct
duration of use of the medication.
Acknowledgements
The authors thank Xiang Gao and Lina Zhou of the
Central Laboratory, Renmin Hospital of Wuhan University for
technical assistance. This study was supported by the Doctoral
Program Foundation of the Department of Education (grant no.
20130141130010), which is used in the field of preferential
development in China.
References
|
1
|
LloydJones D, Adams RJ, Brown TM,
Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K,
Gillespie C, et al: American Heart Association Statistics Committee
and Stroke Statistics Subcommittee: Executive summary: Heart
disease and stroke statistics - 2010 update: A report from the
American Heart Association. Circulation. 121:948–954. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lohse MJ, Engelhardt S and Eschenhagen T:
What is the role of beta-adrenergic signaling in heart failure?
Circ Res. 93:896–906. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J, Liu Y, Fan X, Li Z and Cheng Y: A
pathway and network review on beta-adrenoceptor signaling and beta
blockers in cardiac remodeling. Heart Fail Rev. 19:799–814. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tzingounis AV, von Zastrow M and Yudowski
GA: {Beta}-blocker drugs mediate calcium signaling in native
central nervous system neurons by {beta}-arrestin-biased agonism.
Proc Natl Acad Sci USA. 107:21028–21033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gheorghiade M, Albert NM, Curtis AB,
Thomas Heywood J, McBride ML, Inge PJ, Mehra MR, O'Connor CM,
Reynolds D, Walsh MN, Yancy CW and Fonarow GC: Medication dosing in
outpatients with heart failure after implementation of a
practice-based performance improvement intervention: Findings from
IMPROVE HF. Congest Heart Fail. 18:9–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen DF, Tang QZ, Yan L, Zhang Y, Zhu LH,
Wang L, Liu C, Bian ZY and Li H: Tetrandrine blocks cardiac
hypertrophy by disrupting reactive oxygen species-dependent ERK1/2
signalling. Br J Pharmacol. 159:970–981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bian Z, Cai J, Shen DF, Chen L, Yan L,
Tang Q and Li H: Cellular repressor of E1A-stimulated genes
attenuates cardiac hypertrophy and fibrosis. J Cell Mol Med.
13:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bian ZY, Huang H, Jiang H, Shen DF, Yan L,
Zhu LH, Wang L, Cao F, Liu C, Tang QZ and Li H: LIM and
cysteine-rich domains 1 regulates cardiac hypertrophy by targeting
calcineurin/nuclear factor of activated T cells signaling.
Hypertension. 55:257–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novoyatleva T, Schymura Y, Janssen W,
Strobl F, Swiercz JM, Patra C, Posern G, Wietelmann A, Zheng TS,
Schermuly RT and Engel FB: Deletion of Fn14 receptor protects from
right heart fibrosis and dysfunction. Basic Res Cardiol.
108:3252013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noguchi A, Nakamura K, Sakata K,
SatoFukuda N, Ishigaki T, Mano J, Takabatake R, Kitta K, Teshima R,
Kondo K, et al: Development and interlaboratory validation of a
simple screening method for genetically modified maize using a
ΔΔCq-based multiplex real-time PCR assay. Anal Chem. 88:4285–4293.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eghbali M and Weber KT: Collagen and the
myocardium: Fibrillar structure, biosynthesis and degradation in
relation to hypertrophy and its regression. Mol Cell Biochem.
96:1–14. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daniels A, van Bilsen M, Goldschmeding R,
van der Vusse GJ and van Nieuwenhoven FA: Connective tissue growth
factor and cardiac fibrosis. Acta Physiol (Oxf). 195:321–338. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
RuizOrtega M, Rodríguez-Vita J,
Sanchez-Lopez E, Carvajal G and Egido J: TGF-beta signaling in
vascular fibrosis. Cardiovasc Res. 74:196–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giordano FJ: Oxygen, oxidative stress,
hypoxia, and heart failure. J Clin Invest. 115:500–508. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belge C, Hammond J, DuboisDeruy E, Manoury
B, Hamelet J, Beauloye C, Markl A, Pouleur AC, Bertrand L, Esfahani
H, et al: Enhanced expression of β3-adrenoceptors in cardiac
myocytes attenuates neurohormone-induced hypertrophic remodeling
through nitric oxide synthase. Circulation. 129:451–462. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heineke J and Molkentin JD: Regulation of
cardiac hypertrophy by intracellular signalling pathways. Nat Rev
Mol Cell Biol. 7:589–600. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hill JA and Olson EN: Cardiac plasticity.
N Engl J Med. 358:1370–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drazner MH: The progression of
hypertensive heart disease. Circulation. 123:327–334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lohse MJ, Engelhardt S and Eschenhagen T:
What is the role of beta-adrenergic signaling in heart failure?
Circ Res. 93:896–906. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Port JD and Bristow MR: Altered
beta-adrenergic receptor gene regulation and signaling in chronic
heart failure. J Mol Cell Cardiol. 33:887–905. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mason RP, Giles TD and Sowers JR: Evolving
mechanisms of action of beta blockers: Focus on nebivolol. J
Cardiovasc Pharmacol. 54:123–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Liu Y, Fan X, Li Z and Cheng Y: A
pathway and network review on beta-adrenoceptor signaling and beta
blockers in cardiac remodeling. Heart Fail Rev. 19:799–814. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McMurray JJ, Adamopoulos S, Anker SD,
Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C,
Gomez-Sanchez MA, et al: ESC Committee for Practice Guidelines: ESC
Guidelines for the diagnosis and treatment of acute and chronic
heart failure 2012: The Task Force for the Diagnosis and Treatment
of Acute and Chronic Heart Failure 2012 of the European Society of
Cardiology. Developed in collaboration with the Heart Failure
Association (HFA) of the ESC. Eur Heart J. 33:1787–1847. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Writing Committee Members, . Yancy CW,
Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC,
Geraci SA, Horwich T, et al: American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines. 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American College of cardiology
Foundation/American Heart Association task force on practice
guidelines. Circulation. 128:e240–e327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee DS, Tu JV, Juurlink DN, Alter DA, Ko
DT, Austin PC, Chong A, Stukel TA, Levy D and Laupacis A:
Risk-treatment mismatch in the pharmacotherapy of heart failure.
JAMA. 294:1240–1247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Poldermans D, Boersma E, Bax JJ, et al:
Expression of concern relating to: ‘Bisoprolol reduces cardiac
death and myocardial infarction in high-risk patients as long as 2
years after successful major vascular surgery’. Eur Heart J. doi:
10.1093/eurheartj/ehu397.
|
|
27
|
van der Wall EE: Beta-blocking agents in
cardiovascular disease; are they here to stay? Neth Heart J.
22:481–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imamura T, Kinugawa K, Hatano M, Fujino T,
Muraoka H, Inaba T, Maki H, Kagami Y, Endo M, Kinoshita O, et al:
Preoperative beta-blocker treatment is a key for deciding left
ventricular assist device implantation strategy as a bridge to
recovery. J Artif Organs. 17:23–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel K, Fonarow GC, Ekundayo OJ, Aban IB,
Kilgore ML, Love TE, Kitzman DW, Gheorghiade M, Allman RM and Ahmed
A: Beta-blockers in older patients with heart failure and preserved
ejection fraction: Class, dosage, and outcomes. Int J Cardiol.
173:393–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacob SM, Chandy TT and Cherian VT: Oral
bisoprolol improves surgical field during functional endoscopic
sinus surgery. J Anaesthesiol Clin Pharmacol. 30:59–64. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka H, Matsumoto K, Sawa T, Miyoshi T,
Motoji Y, Imanishi J, Mochizuki Y, Tatsumi K and Hirata K:
Evaluation of global circumferential strain as prognostic marker
after administration of β-blockers for dilated cardiomyopathy. Int
J Cardiovasc Imaging. 30:1279–1287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cruickshank JM: Are we misunderstanding
beta-blockers. Int J Cardiol. 120:10–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakaya M, Chikura S, Watari K, Mizuno N,
Mochinaga K, Mangmool S, Koyanagi S, Ohdo S, Sato Y, Ide T, et al:
Induction of cardiac fibrosis by β-blocker in G protein-independent
and G protein-coupled receptor kinase 5/β-arrestin2-dependent
signaling pathways. J Biol Chem. 287:35669–35677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berk BC, Fujiwara K and Lehoux S: ECM
remodeling in hypertensive heart disease. J Clin Invest.
117:568–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burchfield JS, Xie M and Hill JA:
Pathological ventricular remodeling: Mechanisms: Part 1 of 2.
Circulation. 128:388–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bujak M and Frangogiannis NG: The role of
TGF-beta signaling in myocardial infarction and cardiac remodeling.
Cardiovasc Res. 74:184–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang XR, Chung AC, Yang F, Yue W, Deng C,
Lau CP, Tse HF and Lan HY: Smad3 mediates cardiac inflammation and
fibrosis in angiotensin II-induced hypertensive cardiac remodeling.
Hypertension. 55:1165–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li JM and Brooks G: Differential protein
expression and subcellular distribution of TGFbeta1, beta2 and
beta3 in cardiomyocytes during pressure overload-induced
hypertrophy. J Mol Cell Cardiol. 29:2213–2224. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pauschinger M, Knopf D, Petschauer S,
Doerner A, Poller W, Schwimmbeck PL, Kühl U and Schultheiss HP:
Dilated cardiomyopathy is associated with significant changes in
collagen type I/III ratio. Circulation. 99:2750–2756. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fukui M, Goda A, Komamura K, Nakabo A,
Masaki M, Yoshida C, Hirotani S, LeeKawabata M, Tsujino T, Mano T
and Masuyama T: Changes in collagen metabolism account for
ventricular functional recovery following beta-blocker therapy in
patients with chronic heart failure. Heart Vessels. 31:173–182.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lenneman AJ and Birks EJ: Treatment
strategies for myocardial recovery in heart failure. Curr Treat
Options Cardiovasc Med. 16:2872014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lund LH, Benson L, Dahlström U, Edner M
and Friberg L: Association between use of β-blockers and outcomes
in patients with heart failure and preserved ejection fraction.
JAMA. 312:2008–2018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Apostolovic S, Stanojevic D, Lainscak M,
Gelbrich G, JankovicTomasevic R, Pavlovic M, DjordjevicRadojkovic
D, SalingerMartinovic S, Putnikovic B, Radovanovic S, et al:
Regional differences among female patients with heart failure from
the cardiac insufficiency bisoprolol study in ELDerly (CIBIS-ELD).
Cardiol J. 21:265–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fazio G, Vernuccio F, Lo Re G, Grutta G
and Mongiovì M: Role of bisoprolol in patients with long QT
syndrome. Ann Noninvasive Electrocardiol. 18:467–470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sorrentino SA, Doerries C, Manes C, Speer
T, Dessy C, Lobysheva I, Mohmand W, Akbar R, Bahlmann F, Besler C,
et al: Nebivolol exerts beneficial effects on endothelial function,
early endothelial progenitor cells, myocardial neovascularization,
and left ventricular dysfunction early after myocardial infarction
beyond conventional β1-blockade. J Am Coll Cardiol. 57:601–611.
2011. View Article : Google Scholar : PubMed/NCBI
|