Introduction
Spinal anesthesia is a potent anesthetic procedure
and is widely used as it has a number of advantages compared with
general anesthesia, such as reduction of stress responses, reduced
amount of blood loss, low cost and decreased morbidity and
mortality rates in high-risk patients (1). It is used for both emergency and
elective surgeries, and involves the injection of a local
anesthetic agent into the cerebrospinal fluid, thereby blocking
nerve transmission (2). Regional
anesthesia has been suggested as an alternative to systemic
anesthesia in order to evade or decrease general anesthetic
exposure (3). In addition, spinal
anesthetics have very short action times with infrequent
complications such as shivering (4,5). Several
adjunct drugs have been added to spinal anesthetics due to concerns
over toxicity and the duration of action (6,7).
Adjuncts such as benzodiazepines (8), opioids (9), neostigmine (10) and α2-receptor agonists (11,12) have
been employed.
Ketamine, an N-methyl-D-aspartate (NMDA) receptor
blocker, has an anesthetic effect when injected intrathecally and
is synergic with bupivacaine (13).
Ketamine is a phencyclidine derivative with potent analgesic
properties, which has various advantages over other local
anesthetics, as it tends to stimulate the cardiovascular system and
maintains respiratory response to carbon dioxide. Intrathecally
administered ketamine is advantageous as its beneficial effects on
the cardiovascular system and respiratory functions may be combined
with the analgesic effects of spinal anesthesia (14). The primary mechanism of action of the
spinal anesthetic ketamine is noncompetitive blocking of the NMDA
ionophore.
Widespread use of bupivacaine for pain management
has been largely based on the assumption that it is safe.
Bupivacaine is a local anesthetic that is employed in nerve block,
epidural and intrathecal anesthesia and is often administered to
control pain prior to, during and following spinal surgery
(15,16). Although extensively used in pain
control, bupivacaine has been reported to be cardiotoxic,
neurotoxic and the most myotoxic of the local anesthetics (17).
Levobupivacaine and ropivacaine, new long-acting
local anesthetics are S(−) enantiomers of two structurally similar
molecules, 1-butyl-2′,6′-pipecoloxylidide and
1-propyl-2′,6′-pipecoloxylidide, respectively (18,19) and
are been developed as safer alternatives to bupivacaine. Though
less lipid-soluble than bupivacaine, ropivacaine is a long-acting
spinal anesthetic. Levobupivacaine exhibits similar efficacy to
both ropivacaine and bupivacaine of sensory block for the sciatic
nerve (20,21), spinal block (22–24) and
epidural block (25,26), both in duration and intensity.
Studies have demonstrated that levobupivacaine and ropivacaine are
less neurotoxic than racemic bupivacaine, as evaluated by the
production of seizures in rats (27,28).
With these considerations, the present study has been designed to
evaluate the effects of intrathecal ketamine on spinal anesthesia
with levobupivacaine or ropivacaine.
Materials and methods
Animals
This study was approved by the Institutional Animal
Care Committee of Shandong University and performed in accordance
with the National Institutes of Health Guide for the Use of
Laboratory Animals (29). Female
Sprague-Dawley rats (Guangdong Medical Laboratory Animal Center,
Foshan, China) were used. A total of 70 rats were used that were
housed in a room on a 12 h light/dark cycle with free access to
water. Rats at post-natal day 21 (P21) were used for the study.
Injections of levobupivacaine and
ropivacaine
The rats were anesthetized with isoflurane (3–5%) in
oxygen and air. Percutaneous intrathecal injections were
administered at the low lumbar level (intervertebral space L4-5 or
L5-L6) with a 30-gauge needle perpendicular to the skin. Injection
volumes of 0.5 µl/g bodyweight, previously determined to produce
spread across lumbar and low thoracic segments in rat pups
(30), were delivered using a 50-µl
Hamilton syringe with a 22s ga gauge needle (Sigma-Aldrich, St.
Louis, MO, USA). A 0.5% concentration of levobupivacaine (31) or ropivacaine (32,33) was
administered to the rats. Ketamine was administered at 5 or 10
mg/kg (34). Control rats received
no anesthesia. The rats in the treatment groups received
levobupivacaine or ropivacaine alone, ketamine (5 or 10 mg/kg) and
levobupivacaine, or ketamine (5 or 10 mg/kg) and ropivacaine. A
total of 10 rats were used in each group.
Behavioral assessments for sensory and
motor blockade
The P21 rats underwent baseline measurement of hind
paw thermal withdrawal latencies immediately prior to spinal
injection. Blockade of thermal nociception was assessed using a
modified hot plate test as described previously (35,36).
Hind paws were exposed (left then right) to a hot plate (model 39D
hot plate analgesia meter; IITC Inc., Woodland Hills, CA, USA) at
53±1°C. The time (thermal withdrawal latency) until the rats lifted
their paws was measured using a stop clock. After 12–13 sec, the
tested paw was removed to avoid injury to the animal or the
development of hyperalgesia. The test was repeated thrice (with a
10-sec interval between tests) for each rat at every time point.
Thermal withdrawal latencies were measured every 10 min for ≥60 min
after the intrathecal injections.
Blockade of mechanical nociception was also assessed
in rats following exposure to anesthesia. Mechanical blockade was
determined using hind paw withdrawal with the aid of von Frey
filaments, used to apply logarithmically increasing pressure. The
P21 rats were lightly restrained on a flat surface and a
well-calibrated von Frey hairs device (Ugo Basile Electronic von
Frey device; Stoelting Co., Wood Dale, IL, USA) that delivers
increasing mechanical stimuli was applied to the dorsal surface of
the hindpaw of each rat, five times with 1-sec intervals (37). The number of evoked withdrawal
responses to each stimulus of increasing intensity was recorded
until a given stimulus evoked five responses, or a suprathreshold
cut-off pressure was reached (37).
Mechanical withdrawal thresholds were recorded at baseline and
every 10 min for ≥60 min after the intrathecal injection.
Furthermore, for thermal and mechanical withdrawal tests, the rats
were observed for the possibility of exhibiting motor blockade
without sensory blockade, that is, by absence of lower limb
movement accompanied by vocalization or signs of upper body escape
responses. However, this was not observed.
The motor performance of the lower extremities was
assessed by a qualitative pinch score. For each leg, if there was
no spontaneous or evoked movement, the contribution to the score
was zero. If there was partial movement, the contribution was one
and if there was normal movement, the contribution to the score was
two. Thus, in summing the values for the two legs, the score could
range from zero (complete blockade) to four (normal).
Motor behavior on P22
Motor impairment of the rats that had undergone
spinal levobupivacaine or ropivacaine and/or ketamine injections on
P21 was assessed on P22. These rats were introduced to a dual
species Economex Rotarod (Columbus Instruments, Columbus, OH, USA)
using a spindle rotating at 10 rpm (38). Each rat was tested thrice. A time
interval of 10 min was used between each assessment. The maximal
latency for each trial was 300 sec before removal from the spindle.
The average of the three assessments was used for data
analysis.
Gait analysis at P23
In separate experiments, gait analysis was performed
to determine the gait of the rats. The analysis was conducted on
P23 following intrathecal injection on P21. Gait analysis was
performed as the animal crossed the glass runway of the CatWalk
system (Noldus Information Technology, Wageningen, The Netherlands)
where the paw print area (surface area of floor contacted by
hindpaw) and the paw print intensity (intensity of pixels forming
area of paw contact) was measured. The regularity index (index for
degree of interlimb coordination during gait), stability of gait
(distance between two hindpaws measured perpendicular to walking
direction), stride length (distance between placement of hindpaw
and subsequent placement of same paw) and the duty cycle (ratio
between stance duration and full stepcycle duration) was also
measured. Animals underwent a daily training paradigm for 2 days on
P22-23, with runway crossings toward food rewards at the distant
end. On P23, runway crossings were recorded and included in
analysis if the maximal time for crossing the 60 cm long section of
the runway used for gait recording was ≤2 sec and there were no
intermediate stops during the crossing. Three crossings per animal
were analyzed using CatWalk®7.1.6 software (Noldus
Information Technology).
Statistical analysis
All the values are represented as the mean±standard
deviation. One-way analysis of variance was used and the values
were analyzed using SPSS software version 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
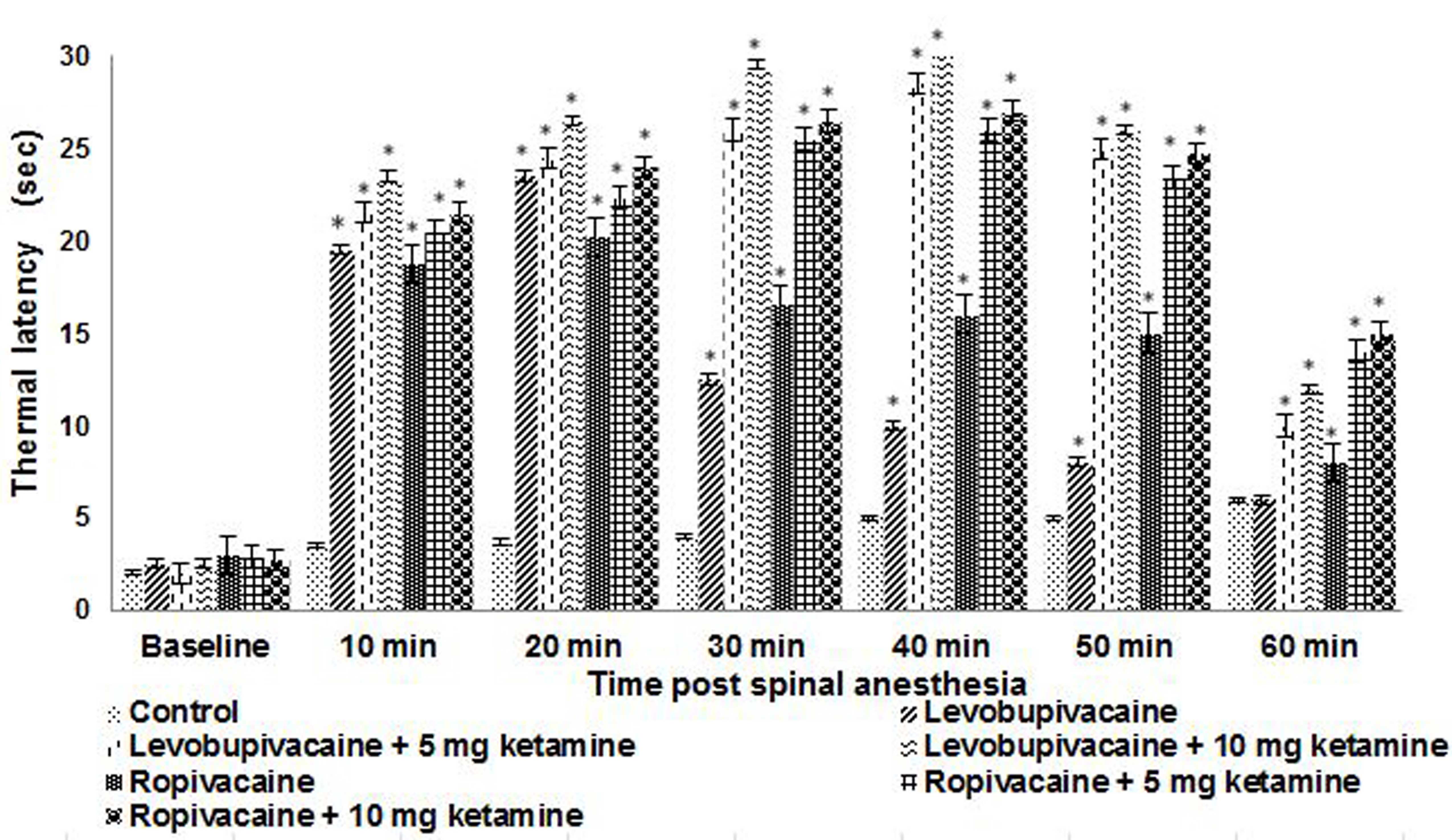
Response to thermal stimulus
Hind paw thermal withdrawal latencies were
determined on P21 for rats in the groups receiving spinal
anesthesia (Fig. 1). Levobupivacaine
and ropivacaine produced dense thermal nociceptive blockade at the
first measurement at 10 min following injection compared with
control and remained dense at 20 min. The co-administration of
ketamine was found to further enhance the blockade to 40–45 min,
with a peak observed at ~40 min. The thermal withdrawal latencies
remained significantly greater than control values at 50 min for
the animals that received ketamine plus either ropivacaine or
levobupivacaine; however, thermal withdrawal latencies remained
higher and for a longer time for ropivacaine than for bupivacaine,
irrespective of whether ketamine was co-administered (Fig. 1). The latencies were observed to be
markedly higher and of longer duration in the rats that received a
10-mg dose of ketamine with levobupivacaine or ropivacaine compared
with rat pups that received 5 mg ketamine.
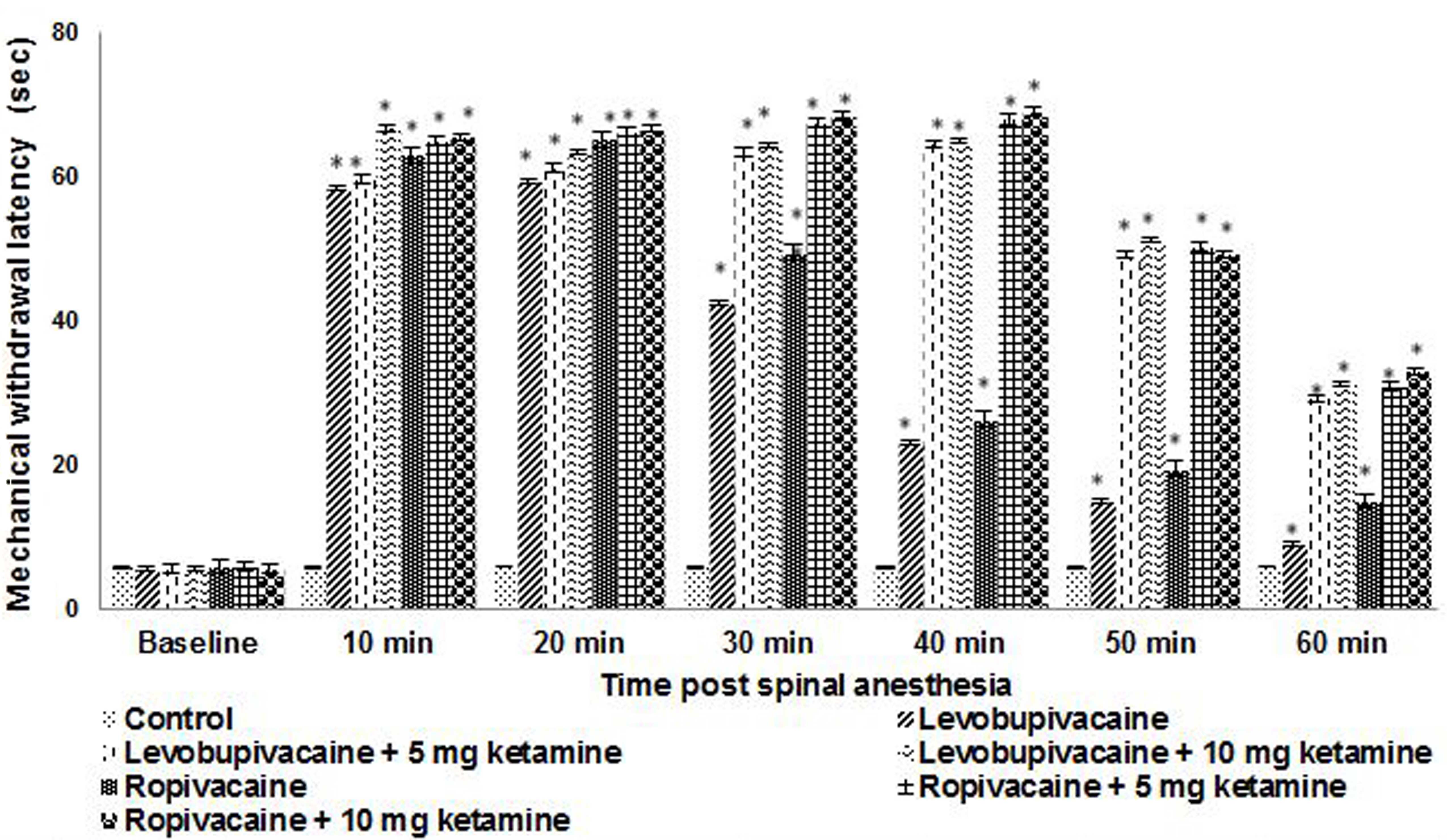
Response to mechanical stimulus
Mechanical withdrawal responses to von Frey
filaments are presented in Fig. 2
for rats at P21 that received spinal anesthesia. Threshold values
were higher following intrathecal injections compared with control.
Thresholds were maximized between 5 to 40 min following injections
in rats receiving ropivacaine or levobupivacaine. In the rats that
received ketamine along with ropivacaine, the threshold remained
higher at 40 min as compared with that in rats exposed to
ropivacaine alone. The 10 mg dose of ketamine exhibited a higher
mechanical blockade even at 50 min compared with the 5-mg dose,
with fading of the effect from 40 min. The combination of ketamine
and ropivacaine resulted in blocks of longer duration as compared
with levobupivacaine, which exhibited more dense blocks.
Motor block scores
Motor block (pinch) scores are shown in Table I. Rats at P21 that received either
ropivacaine or levobupivacaine showed no signs of motor impairment.
Levobupivacaine and ropivacaine produced dense motor block in all
animals, which recovered almost completely by 40 min in all
animals. Animals that received intrathecal injections of ketamine
presented blocks even at 50 min. At 40 min, rats that received 10
mg ketamine exhibited motor blocks more than those that received 5
mg ketamine (Table I).
 | Table I.Hind leg motor response of rats
assessed using pinch scores following spinal anesthesia. |
Table I.
Hind leg motor response of rats
assessed using pinch scores following spinal anesthesia.
|
| Time (min) |
|---|
|
|
|
|---|
| Group | 0 | 5 | 10 | 20 | 30 | 40 | 50 |
|---|
| Control | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
|
Levobupivacaine | 0 | 0 | 0 | 2 | 2 | 4 | 4 |
| Levobupivacaine + 5
mg ketamine | 0 | 0 | 0 | 1 | 2 | 2 | 4 |
| Levobupivacaine +
10 mg ketamine | 0 | 0 | 0 | 1 | 1 | 2 | 2 |
| Ropivacaine | 0 | 0 | 0 | 0 | 2 | 2 | 4 |
| Ropivacaine + 5 mg
ketamine | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Ropivacaine + 10 mg
ketamine | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
All control animals that received no anesthesia
responded to pinching with forceps on the skin of the back with
startled jerks and exhibited substantial escape behaviors. All
animals that received spinal injections of levobupivacaine or
ropivacaine did not exhibit behavioral response to pinching over
the skin of the back at the lumbar and lower thoracic levels
(between 8–10 min after injection); however, they showed slight
withdrawal behaviors to pinching at upper thoracic levels and on
the forepaws at 15–20 min after injections. Withdrawal behaviors
were absent for a longer period of time in animals that received
ketamine and ropivacaine compared with animals that received
levobupivacaine and ketamine.
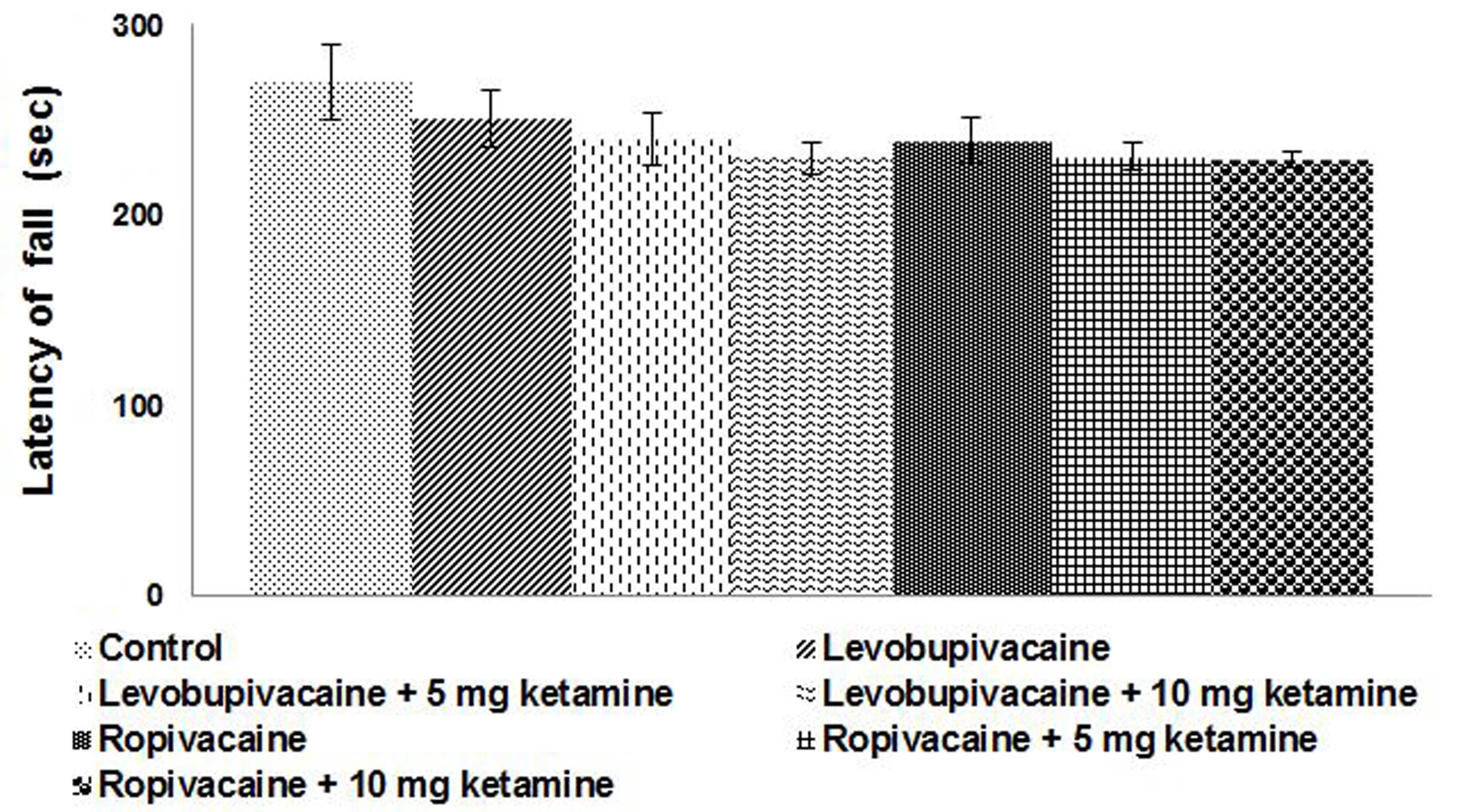
Motor performance in adult rats with
postnatal anesthetic exposures
P22 rats that were exposed to intrathecal injections
on P21 were tested for motor performance using a rotarod apparatus.
Notable differences were observed in rats that received anesthesia
as compared with control rats that were not exposed to anesthesia.
Rats that received either ropivacaine or levobupivacaine along with
ketamine exhibited falls at a much higher frequency and with
shorter time intervals between the falls (Fig. 3). Ketamine injections resulted in
shorter falling latency than that of the rats that received
levobupivacaine or ropivacaine alone.
Gait analysis at P23
Gait analysis was performed on the rat pups on P23
following anesthesia exposure on P21. The influence of the
anesthetics on the walking gait of the rat pups was analyzed using
the CatWalk® runway system. The system recorded static
and dynamic parameters, including paw pressure, print area, duty
cycle (ratio between stance duration and full step cycle duration),
stride length as well as interlimb coordination. Ketamine
injections at P21 resulted in a significant (P<0.05) reduction
in paw print area and paw print intensity in rats that were exposed
to spinal anesthesia with ropivacaine and levobupivacaine as
compared with the intensities in control rats that were not exposed
to anesthetics. The reduction in dynamic parameters was observed to
be more marked in rats that received ketamine along with
levobupivacaine or ropivacaine compared with those that received
the latter two anesthetics without ketamine. More pronounced
alterations in gait were observed in rat pups injected with the 10
mg ketamine dose as compared with 5 mg (Table II). The treatment with intrathecal
injections of ketamine plus ropivacaine or levobupivacaine resulted
in a greater anesthetic effect than ropivacaine or levobupivacaine
alone.
 | Table II.Gait analysis of rats following
spinal anesthesia. |
Table II.
Gait analysis of rats following
spinal anesthesia.
|
| Static
parameters | Dynamic
parameters |
|---|
|
|
|
|
|---|
| Group | Paw print area | Paw print
intensity | Regulatory
index | Duty cycle | Stride length | Stability of
gait |
|---|
| Control |
44.13±1.21 |
171.20±10.20 |
100.30±2.16 |
60.16±1.14 |
110.33±9.36 |
34.19±0.77 |
|
Levobupivacaine |
22.25±1.68a |
121.25±7.15a |
90.38±1.69a |
53.35±2.18a |
100.10±6.10a |
28.22±1.56a |
| Levobupivacaine + 5
mg ketamine |
18.50±1.22a |
118.80±9.21a |
88.11±2.53a |
51.20±2.05a |
95.16±4.81a |
20.15±1.00a |
| Levobupivacaine+10
mg ketamine |
15.10±1.56a |
110.10±6.01a |
85.22±4.37a |
46.75±2.10a |
90.25±5.05a |
19.80±0.87a |
| Ropivacaine |
21.20±0.54a |
123.56±5.96a |
89.21±4.16a |
53.44±1.36a |
102.21±3.74a |
27.80±0.54a |
| Ropivacaine + 5 mg
ketamine |
17.51±0.66a |
119.56±5.07a |
87.20±2.90a |
50.23±2.22a |
93.25±2.90a |
24.10±1.36a |
| Ropivacaine + 10 mg
ketamine |
14.88±0.60a |
113.24±4.29a |
81.06±5.06a |
48.68±3.12a |
90.20±4.60a |
20.0
5±1.01a |
Discussion
Local anesthetics and spinal analgesics are commonly
co-administered to improve analgesia or reduce local anesthetic
requirements (34,39). The use of the stereoisomers
levobupivacaine or ropivacaine, which have wider therapeutic
windows than racemic bupivacaine, is increasing (40,41).
However, to prolong the duration of action, additional modalities
are being sought. Ketamine is reported to act as an antagonist of
the NMDA receptor in the spinal cord, and also to affect
voltage-sensitive calcium channels, and opiate and monoaminergic
receptors and thereby causes analgesic and anesthetic effects
(42). Thus, the effect of ketamine
is considered to be synergic with that of intrathecal bupivacaine
because combined administration demonstrates better blocking with a
longer duration (13,29).
Several in vitro and in vivo studies
have demonstrated the potential of ketamine for producing
neuroprotective effects (43–45).
This ability is indicated by the observations that ketamine blocks
NMDA-receptor activation, mediates beneficial changes in
apoptosis-regulating proteins and interferes with the inflammatory
response to injury when administered in typical sedative or
anesthetic doses. Cardiovascular stimulation caused by ketamine may
also improve cerebral perfusion and this action may be advantageous
in patients, particularly after brain injury (46).
Considering the effects of ketamine, the present
study probed whether intrathecal ketamine increases the blockade
induced by spinal levobupivacaine and ropivacaine. One of the most
important properties of a long-acting local anesthetic is the
reversible inhibition of nerve impulses, causing a prolonged
sensory or motor blockade appropriate for anesthesia in different
types of surgeries (47).
In the present study it was observed that a single
dose of intrathecal levobupivacaine and ropivacaine at 0.5%
produced reliable sensory and motor blockade in rat pups at P21.
The results of the assessment of sensory and motor blockade in rat
pups suggest that ropivacaine induced sensory and motor blocks for
a longer duration than levobupivacaine. Administration of ketamine
following levobupivacaine or ropivacaine resulted in stronger
blocks and extended the duration of the blocks as determined using
thermal and mechanical stimuli.
On further analysis of the extent of blockade, the
performance of the rats following 24 h of anesthesia was determined
using a rotarod apparatus. The latency to fall was recorded as the
efficiency of the rats in rotating the spindle. The rats exposed to
anesthesia presented a shorter period before falling, with the rats
that received ketamine falling much sooner. The results of rotation
of the spindle indicate that ketamine brought about stronger
blockade than either ropivacaine or levobupivacaine administered as
a single drug.
Earlier reports have demonstrated longer anesthetic
blocks in patients receiving ketamine administration along with
bupivacaine (42). Patients
undergoing cesarean section who received intrathecal ketamine along
with bupivacaine had a significantly prolonged duration of
anesthesia compared with the control group of patients who received
bupivacaine without ketamine (48).
Clinically, ketamine is administered via
intravenous, intramuscular, epidural, intrathecal, rectal,
subcutaneous, transdermal, topical, oral, intranasal, transmucosal
and sublingual routes. Epidural ketamine has been reported to
provide prolonged analgesia when administered alone or added to
local anesthetics (49–51). Epidural injection has an additional
benefit as inadvertent intravascular administration does not result
in cardiovascular side effects (52)
and intrathecal ketamine at doses of 3–10 mg/kg exhibits
dose-dependent antihyperalgesic effects in neonatal rats (34).
Gait analysis normally presents the stability of the
rats following anesthesia. The gait analysis of the experimental
animals was assessed using the CatWalk® system, which is
a video-based automated gait analysis system that evaluates the
changes in gait of rodents (53).
The CatWalk® system allows analysis of sensorimotor
co-ordination and both static and dynamic components of gait.
Intrathecal ketamine injection resulted in alterations in the gait
parameters in rats that were exposed to levobupivacaine or
ropivacaine.
Marked reductions in hindpaw print area and print
intensity were observed subsequent to ketamine administration. Duty
cycle was observed to decrease, as the time that the hind paw was
in contact with the surface during gait decreased. The results for
dynamic parameters such as stride length, gait regularity and
stability suggested that gait coordination was slightly decreased
in the levopubivacaine and ropivacaine groups compared with that in
the control rat pups. The changes in static and dynamic parameters
were more pronounced in rats that received 10 mg ketamine than in
the rats that received the lower dose.
The results of the present study suggest that
ketamine at doses of 5 and 10 mg/kg was effective in extending the
anesthetic effects of ropivacaine and levobupivacaine. Kim et
al (54) demonstrated the
synergistic effects of intravenous ketamine administration in
patients exposed to spinal bupivacaine. Prophylactic use of
intravenous ketamine has been found to significantly reduce the
frequency and the intensity of perioperative shivering associated
with spinal anesthesia (55).
Intraarticular ketamine and levobupivacaine when administered to
patients undergoing arthroscopic meniscectomy provided more
effective post-operative analgesia than ketamine alone (56).
The present study was conducted in an attempt to
evaluate the anesthetic effects of ketamine when used in
combination with ropivacaine or levobupivacaine. The observations
are suggestive of the potency of ketamine in extending the duration
and strength of spinal anesthesia. Furthermore, additional research
is necessary in order to fully understand the effects of ketamine
administration in combination with ropivacaine or
levobupivacaine.
Acknowledgements
This study was supported by Shandong Province
Science and Technology Agency funded projects (no.
ZR2009CM098).
References
|
1
|
Gaiser RR: Spinal, epidural and caudal
anesthesiaIntroduction to Anesthesia. Longnecker DE and Murphy FL:
9th. W.B. Saunders; Philadelphia, PA: pp. 230–231. 1997
|
|
2
|
Charles BB: Local anestheticsMiller's
Anesthesia. Miller RD, Fleisher LA, Savarese JJ, Wiener-Kronish J
and Young WL: 6th. Elsevier/Churchill Livingstone; Philadelphia,
PA: pp. 573–604. 2005
|
|
3
|
McGowan FX Jr and Davis PJ:
Anesthetic-related neurotoxicity in the developing infant: Of mice,
rats, monkeys and possibly, humans. Anesth Analg. 106:1599–1602.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sessler DI and Ponte J: Shivering during
epidural anaesthesia. Anesthesiology. 72:816–821. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Whitte J and Sessler DI: Perioperative
shivering: Physiology and pharmacology. Anaesthesiology.
96:467–484. 2002. View Article : Google Scholar
|
|
6
|
Sakura S, Kirihara Y, Muguruma T,
Kishimoto T and Saito Y: The comparative neurotoxicity of
intrathecal lidocaine and bupivacaine in rats. Anesth Analg.
101:541–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakura S: Research on local anesthetic
neurotoxicity using intrathecal and epidural rat models. J Anesth.
21:533–544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bharti N, Madan R, Mohanty P and Kaul HL:
Intrathecal midazolam added to bupivacaine improves the duration
and quality of spinal anaesthesia. Acta Anaesthesiol Scand.
47:1101–1105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karaman S, Kocabas S, Uyar M, Hayzaran S
and Firat V: The effects of sufentanil or morphine added to
hyperbaric bupivacaine in spinal anaesthesia for caesarean section.
Eur J Anaesthesiol. 23:285–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan PH, Chia YY, Lo Y, Liu K, Yang LC and
Lee TH: Intrathecal bupivacaine with morphine or neostigmine for
postoperative analgesia after total knee replacement surgery. Can J
Anesth. 48:551–556. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al
Jazzar MD, Alameddine MM, Al-Yaman R, Bulbul M and Baraka AS:
Effect of low dose dexmedetomidine or clonidine on the
characteristics of bupivacaine spinal block. Acta Anaesthesiol
Scand. 50:222–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshitomi T, Kohjitani A, Maeda S, Higuchi
H, Shimada M and Miyawaki T: Dexmedetomidine enhances the local
anesthetic action of lidocaine via an −2A adrsenoceptor. Anesth
Analges. 107:96–101. 2008. View Article : Google Scholar
|
|
13
|
Togal T, Demirbilek S, Koroglu A, Yapici E
and Ersoy O: Effects of S (+) ketamine added to bupivacaine for
spinal anaesthesia for prostate surgery in elderly patients. Eur J
Anaesthesiol. 21:193–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schug SA, Buerkle H, Moharib M and
Cardwell HM: New drugs for neuraxial blockade. Curr Opin
Anaesthiol. 12:551–557. 1999. View Article : Google Scholar
|
|
15
|
Kotilainen E, Muittari P and Kirvel O:
Intradiscal glycerol or bupivacaine in the treatment of low back
pain. Acta Neurochir (Wien). 139:541–545. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sice PJ, Chan D and MacIntyre PA: Epidural
analgesia after spinal surgery via intervertebral foramen. Br J
Anaesth. 94:378–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgan GE, Mikhail MS and Murray MJ: Local
anestheticsClinical Anesthesiology. 4th. McGraw-Hill; New York, NY:
pp. 263–275. 2006
|
|
18
|
McClure JH: Ropivacaine. Br J Anaesth.
76:300–307. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burlacu CL and Buggy DJ: Update on local
anaesthetics: Focus on levobupivacaine. Ther Clin Risk Manage.
4:381–392. 2008.
|
|
20
|
Santorsola R, Casati A, Cerchierini E,
Moizo E and Fanelli G: Levobupivacaine for peripheral blocks of the
lower limb: A clinical comparison with bupivacaine and ropivacaine.
Minerva Anestesiol. 67(Suppl 1): S33–S36. 2001.(In Italian).
|
|
21
|
Casati A, Chelly JE, Cerchierini E,
Santorsola R, Nobili F, Grispigni C, Di Benedetto P and Torri G:
Clinical properties of levobupivacaine or racemic bupivacaine for
sciatic nerve block. J Clin Anesth. 14:111–114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alley EA, Kopacz DJ, McDonald SB and Liu
SS: Hyperbaric spinal levobupivacaine: A comparison to racemic
bupivacaine in volunteers. Anesth Analg. 94:194–198. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Glaser C, Marhofer P, Zimpfer G, Heinz MT,
Sitzwohl C, Kapral S and Schindler I: Levo-bupivacaine versus
racemic bupivacaine for spinal anesthesia. Anesth Analg.
94:194–198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casati A, Moizo E, Marchetti C and
Vinciguerra F: A prospective, randomized, double-blind comparison
of unilateral spinal anesthesia with hyperbaric bupivacaine,
ropivacaine, or levobupivacaine for inguinal herniorrhaphy. Anesth
Analg. 99:1387–1392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casati A, Santorsola R, Aldegheri G,
Ravasi F, Fanelli G, Berti M, Fraschini G and Torri G:
Intraoperative epidural anesthesia and postoperative analgesia with
levobupivacaine for major orthopedic surgery: A doubleblind,
randomized comparison of racemic bupivacaine and ropivacaine. J
Clin Anesth. 15:126–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peduto VA, Baroncini S, Montanini S,
Proietti R, Rosignoli L, Tufano R and Casati A: A prospective,
randomized, double blind comparison of epidural levobupivacaine
0.5% with epidural ropivacaine 0.75% for lower limb procedures. Eur
J Anaesthesiol. 20:979–983. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohmura S, Kawada M, Ohta T, Yamamoto K and
Kobayashi T: Systemic toxicity and resuscitation in bupivacaine-,
levobupivacaine-, or ropivacaine-infused rats. Anesth Analg.
93:743–748. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marganella C, Bruno V, Matrisciano F,
Reale C, Nicoletti F and Melchiorri D: Comparative effects of
levobupivacaine and racemic bupivacaine on excitotoxic neuronal
death in culture and N-methyl-D-aspartate-induced seizures in mice.
Eur J Pharmacol. 518:111–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. 8th. National Academies Press; Washington DC, USA:
2011
|
|
30
|
Westin BD, Walker SM, Deumens R, Grafe M
and Yaksh TL: Validation of a preclinical spinal safety model:
Effects of intrathecal morphine in the neonatal rat.
Anesthesiology. 113:183–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamurtekin E, Fitzsimmons BL, Shubayev VI,
Grafe MR, Deumens R, Yaksh TL and Walker SM: Evaluation of spinal
toxicity and long-term spinal reflex function after intrathecal
levobupivaciane in the neonatal rat. Anesthesiology. 119:142–155.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miller RD and Katzung BG: Local
anestheticsBasic and Clinical Pharmacology. Katzung BG: 8th. Lange
Medical Books/McGraw-Hill; New York, NY: pp. 436–445. 2001
|
|
33
|
Takenami T, Wang G, Nara Y, Fukushima S,
Yagishita S, Hiruma H, Kawakami T and Okamoto H: Intrathecally
administered ropivacaine is less neurotoxic than procaine,
bupivacaine and levobupivacaine in a rat spinal model. Can J
Anesth. 59:456–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Walker SM and Yaksh TL: Neuraxial
analgesia in neonates and infants: A review of clinical and
preclinical strategies for the development of safety and efficacy
data. Anesth Analg. 115:638–662. 2012.PubMed/NCBI
|
|
35
|
Hu D, Hu R and Berde CB: Neurologic
evaluation of infant and adult rats before and after sciatic nerve
blockade. Anesthesiology. 86:957–965. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kohane D, Sankar W, Shubina M, Hu D, Nader
R and Berde C: Sciatic nerve blockade in infant, adolescent and
adult rats: A comparison of ropivacaine with bupivacaine.
Anesthesiology. 89:1199–1208. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walker SM, Howard RF, Keay KA and
Fitzgerald M: Developmental age influences the effect of epidural
dexmedetomidine on inflammatory hyperalgesia in rat pups.
Anesthesiology. 102:1226–1234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sanders RD, Xu J, Shu Y, Fidalgo A, Ma D
and Maze M: General anesthetics induce apoptotic neurodegeneration
in the neonatal rat spinal cord. Anesth Analg. 106:1708–1711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Walker SM, Goudas LC, Cousins MJ and Carr
DB: Combination spinal analgesic chemotherapy: A systematic review.
Anesth Analg. 95:674–715. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gunter J: Benefit and risks of local
anesthetics in infants and children. Paediatr Drugs. 4:649–672.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ivani G and Mossetti V: Continuous central
and perineural infusions for postoperative pain control in
children. Curr Opin Anaesthesiol. 23:637–642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kathirvel S, Sadhasivam S, Saxena A,
Kannar TR and Ganjoo P: Effects of intrathecal ketamine added to
bupivacaine for spinal anaesthesia. Anaesthesia. 55:899–904. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonzales JM, Loeb AL, Reichard PS and
Irvine S: Ketamine inhibits glutamate-, N-methyl-D-aspartate- and
quisqualate-stimulated cGMP production in cultured cerebral
neurons. Anesthesiology. 82:205–213. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Himmelseher S, Pfenninger E and Georgieff
M: The effects of ketamine-isomers on neuronal injury and
regeneration in rat hippocampal neurons. Anesth Analg. 83:505–512.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Himmelseher S, Pfenninger E, Kochs E and
Auchter M: S(+)-ketamine up-regulates neuronal regeneration
associated proteins following glutamate injury in cultured rat
hippocampal neurons. J Neurosurg Anesthesiol. 12:84–94. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hudetz JA and Pagel PS: Neuroprotection by
Ketamine: A review of the experimental and clinical evidence. J
Cardiothorac Vasc Anesth. 24:131–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hansen TG: Ropivacaine: A pharmacological
review. Expert Rev Neurother. 4:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Khezri MB, Ghasemi J and Mohammadi N:
Evaluation of the analgesic effect of ketamine as an additive to
intrathecal bupivacaine in patients undergoing cesarean section.
Acta Anaesthesiol Taiwan. 51:155–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xie H, Wang X, Liu G and Wang G: Analgesic
effects and pharmacokinetics of a low dose of ketamine
preoperatively administered epidurally or intravenously. Clin J
Pain. 19:317–322. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Martindale SJ, Dix P and Stoddart PA:
Double-blind randomized controlled trial of caudal versus
intravenous S(+)-ketamine for supplementation of caudal analgesia
in children. Br J Anaesth. 92:344–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schnabel A, Poepping DM, Kranke P, Zahn PK
and Pogatzki-Zahn EM: Efficacy and adverse effects of ketamine as
an additive for paediatric caudal anaesthesia: A quantitative
systematic review of randomized clinical trials. Br J Anaesth.
107:601–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vranken JH, Troost D, de Haan P, Pennings
FA, van der Vegt MH, Dijkgraaf MG and Hollmann MW: Severe toxic
damage to the rabbit spinal cord after intrathecal administration
of preservative-free S(+)-ketamine. Anesthesiology. 105:813–818.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hamers FP, Lankhorst AJ, van Laar TJ,
Veldhuis WB and Gispen WH: Automated quantitative gait analysis
during overground locomotion in the rat: Its application to spinal
cord contusion and transection injuries. J Neurotrauma. 18:187–201.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim MH, Jung SY, Shin JD, Lee SH, Park MY,
Lee KM, Lee JH, Cho K and Lee W: The comparison of the effects of
intravenous ketamine or dexmedetomidine infusion on spinal block
with bupivacaine. Korean J Anesthesiol. 67:85–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hidayah MN, Liu CY and Joanna OS: Ketamine
and tramadol for the prevention of shivering during spinal
anaesthesia. Clin Ter. 165:193–198. 2014.PubMed/NCBI
|
|
56
|
Isik C, Demirhan A, Yetis T, Oktem K,
Sarman H, Tekelioglu UY and Duran T: Efficacy of intraarticular
application of ketamine or ketamine-levobupivacaine combination on
post-operative pain after arthroscopic meniscectomy. Knee Surg
Sports Traumatol Arthrosc. 23:2721–2726. 2015. View Article : Google Scholar : PubMed/NCBI
|