Introduction
Multidrug resistance (MDR) refers to the tumor cells
having a resistance to a type of antitumor medicine, while also
having a cross resistance to the chemical structure and mechanism
of action of other completely different types of antitumor drugs
(1), which is a predominant cause in
the failure of chemotherapy. MDR can result in the overexpression
of transporter proteins, including P-glycoprotein (P-gp), multidrug
resistance-associated protein (MRP), lipoprotein receptor-related
protein (LRP) and others proteins (2–4), which
belong to the ATP-binding cassette (ABC) transporters. The ABC
transporters are a superfamily of transmembrane proteins that
transport a wide variety of substrates across the extracellular and
intracellular membranes (5).
Furthermore, MDR is also closely linked to the tumor
microenvironment, especially the immune system (6). However, cytokines that are produced by
the immune system have been studied by more and more people
(7,8).
Cytokines are small proteins or small molecular
peptides, such as interleukin 3 (IL-3) mainly secreted by T
lymphocytes (9), interferon-γ
(IFN-γ) secreted by T lymphocytes and natural killer cells
(10) and tumor necrosis factor-α
(TNF-α)secreted by macrophages (11). They can transmit information between
the cells and serve an important role in immune regulation and
anti-tumor effects amongst others (12). The immune cells that secrete
cytokines can recognize the abnormaly high expression of P-gp,
MDR-1 and MRP-1 in MDR cells and react with them. Proteins such as
P-gp and MRP, which offer chemoresistance, were overexpressed in
tumor cells. Furthermore, their growth status is different from
that of normal cells. May be when resistant proteins increase in
tumor cells, some immune cells can recognize and react to them, for
example by secreting cytokines, in order to reverse drug resistance
(13–15). This part of the study has attracted
increasingly more attention from scholars and thus revealing the
mechanism will help to solve the effective treatment of tumor
MDR.
It was recently revealed that two compounds called
verapamil (VER) (16) and
cyclosporin A (CsA) (17) amongst
other chemical reversal agents were capable of improving the
sensitivity of tumor cells to a certain extent, which can improve
the clinical effect of chemotherapy when combined with the use of
anti-tumor chemotherapeutic drugs. However, CsA has
immunosuppressive properties and can cause renal toxicity which
restricts its application. VER, a calcium antagonist, has a
relatively small adverse reaction, but due to the lack of its
antitumor effects doctors rarely use it in chemotherapy regimens.
Therefore it is important to identify novel and effective clinical
reversal agents.
Experimental studies have demonstrated that GBEE has
anti-tumor, anti-metastasic and synergistic attenuated effects that
could improve the body's immune function in different conditions
(18–21). Clinical studies demonstrated that
GBEE capsules could shrink gastric cancer tumors and improved the
quality of life of many kinds of cancer patients. Furthermore it
has been used for many years in the hospital and no adverse
reactions have been reported (22).
In addition, the possibility of using GBEE to reverse multidrug
resistance of tumors and its clinical application have not yet been
reported.
In the present study, in order to simulate the
commonly clinical PFC [(cis-dichlorodiamineplatinum, cisplatin
(DDP)+fluorouracil (FU), FU+cyclophosphamide (CPA)] scheme, a
gradually increasing dose in a phased induction was used in
vivo to induce S180 cells and make them have MDR. S180 MDR
ascites tumor mouse models were established in order to study the
reversal effects and mechanism of GBEE in MDR of S180 mice tumor
cells in vitro and in vivo.
Materials and methods
Ginkgo biloba exocarp extracts (GBEE)
preparation
GBEEs is a Chinese traditional medicine Ginkgo fruit
that was collected in October 2013 in Taixing, China. GBEE was
prepared by water extraction and alcohol precipitation (23). Infrared spectroscopy qualitative
analysis shows that it contains protein polysaccharide, the total
content being 66.4%, which was identified using the phenol-sulfuric
acid and Coomassie brilliant blue methods. It was extracted from
the exopleura of the Chinese traditional medicine Ginkgo
biloba (identified by Yin Meng, a senior pharmacist, at the
Yangzhou Institute for Drug Control, Yangzhou, China).
Cell culture and establishment of an
S180 MDR cell line in mice
Mouse S180 cell line (Chinese Academy of Sciences,
Institute of Cell Biology, Shanghai, China) was inoculated in the
abdominal cavity of ICR mice (Comparative Medicine Center of
Yangzhou University, Yangzhou, China) after the in vitro
amplification was done once every seven to eight days. The S180
cells of ascites tumor in mice were extracted under aseptic
conditions and seeded at an initial concentration of
1×106 cells/ml. A total of 0.2 ml of cell suspension was
injected into the abdominal cavity of mice from the right lower
abdomen. After 24 h, in order to simulate the commonly clinical PFC
scheme, DDP (Jiangsu Howson Pharmaceutical Co., Ltd, Jiangsu,
China) was injected intraperitoneally (ip), once a week; FU
(Tianjin Jin Yao Amino Acid Co., Ltd, Tianjin, China),
intragastrically (ig) once a day and CPA (Jiangsu Hengrui Medicine
Co., Ltd, Jiangsu, China), ig once a day. The gradually increasing
dose at different stages was used to induce S180 cells and make
them obtain MDR, which was named as the S180 MDR cell line
(24,25). At the first stage 3.0 mg/kg DDP + 3.0
mg/kg FU + 3.0 mg/kg CPA was given and the induction time was two
generations; At the second stage 4.0 mg/kg DDP + 6.0 mg/kg FU + 6.0
mg/kg CPA and an induction time of two generations was given;
Finally, at the third stage 5.0 mg/kg DDP + 12.0 mg/kg FU + 12.0
mg/kg CPA and an induction time of two generations was given. This
study was approved by the Ethics committee of the Medical College
of Yangzhou University.
Reverse index determined by the MTT
assay
The S180MDR cells were seeded in RPMI 1640 medium
(Gibco-BRL, Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% heat-inactivated new born bovine serum
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd,
Hangzhou, China) at an initial concentration of 1×105
cells/ml with 100 µl/well in 96-well culture plates. A blank and a
control group, various concentrations of DDP groups, various
concentrations of GBEE + DDP groups, a VER group (Xuzhou
Pharmaceutical Co., Ltd, Xuzhou, China) and a VER + DDP group were
all set up. A cell inhibitory rate less than 10% was named as
nontoxic dose, and the nontoxic dose of GBEE to S180 MDR cells was
15 µg/ml which was obtained by preliminary experiments. Therefore,
GBEE concentrations of 7.5, 15 and 30 µg/ml were selected as the
reversal doses in vitro. Following administration of the
compound the culture plate was placed in 5% CO2 in a
37°C incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 44 h. After each well had absorbed the supernatant (100 µl), 10
µl MTT solution was added (0.5 mg/ml; Sigma-Aldrich, St. Louis, MO,
USA) for 4 h before the end of incubation. Next, 100 µl acidified
isopropanol was added to each well and quickly mixed in the
solution. The absorbance at 570 nm (26,27) was
measured using a Enzyme-linked immune detector (BioTek Instruments,
Inc., Winooski, VT, USA). The half maximal inhibitory concentration
(IC50) and the resistance index (RI) were
calculated.
Flow cytometry for accumulation of ADR
in cells
Parent S180 cells and S180 MDR cells were seeded in
RPMI 1640 complete medium containing 5.0 µg/ml Adriamycin (ADR;
Zhejiang Haizheng Pharmaceutical Co., Ltd, Zhejiang, China) at an
concentration of 1×106 cells/ml in 24-well culture
plates. The parent S180 was set as the cell blank group and S180
MDR cells as the control group. Furthemore, other groups were those
with GBEE at 7.5, 25 and 30 µg/ml concentrations and the VER group
with a concentration of 5.0 µg/ml. Finally, the final volume of
each well was 2.0 ml and three parallel samples of each group were
set up. Following 3 h of incubation at 5% CO2 and 37°C,
the cells were washed three times with ice-cold phosphate-buffered
saline (PBS). Finally, the cells were resuspended in 1.0 ml
ice-cold PBS and uniformly mixed. The mean fluorescence intensity
(MFI) of ADR in 10,000 cells was detected by flow cytometry (BD
FACSCalibur flowcytometer, BD Biosciences Franklin Lakes, NJ, USA),
and the excitation and emission wavelengths of ADR were 488 and 575
nm, respectively.
P-gp activity study
Activity experiments were performed by measuring the
accumulation and efflux of Rhodamine 123 (Rho 123, Sigma-Aldrich),
a well-known P-gp substrate, which is inversely proportional to the
P-gp activity (28,29). When measuring the accumulation of Rho
123, parent S180 cells and S180 MDR cells were seeded in RPMI 1640
complete medium containing 2.0 µg/ml Rho 123 at an concentration of
1×106 cells/ml in 24-well culture plates. The experiment
groups were the same as above. The final volume of each well was
2.0 ml and there were a total of three parallel samples of each
group. Following 3 h of incubation at 5% CO2 and 37°C,
the cells were washed three times with ice-cold PBS. Finally, the
cells were resuspended in 1.0 ml ice-cold PBS and uniformly mixed.
The MFI of Rho 123 in 10,000 cells was detected by flow
cytometry.
When measuring the efflux of Rho 123, the early
steps used involved seeding parent S180 and MDR cells in RPMI 1640
complete medium containing 2.0 µg/ml Rho 123 at a concentration of
1×106 cells/ml and in 24-well culture plates. The final
volume was 2.0 ml and the culture was incubated at 37°C and 5%
CO2 in a constant temperature incubator. When measuring
the efflux of Rho 123, the early steps were the same as those
obtained when measuring the accumulation of Rho 123. Following 2 h
of incubation with 5% CO2 at 37°C, the cells were washed
three times with ice-cold phosphate-buffered saline (PBS), then
resuspended in RPMI 1640 complete medium without Rho 123 and again
incubated for 1 h. Finally, the cells were resuspended in 1.0 ml
ice-cold PBS and were uniformly mixed after being washed three
times with ice-cold PBS. At last, the MFI of Rho 123 in 10,000
cells was detected by flow cytometry. The excitation and emission
wavelengths of Rho 123 were 488 and 530 nm, respectively.
Determination of life-prolonging rate
in vivo
The S180 MDR cells of ascites tumor in mice were
extracted under aseptic conditions and seeded at an initial
concentration of 1×106 cells/ml. A 0.2 ml cell
suspension was injected into the abdominal cavity of mice from the
right lower abdomen to establish the model of S180 MDR ascites
tumor in mice. After 24 h, the mice were randomly divided into a
blank and a control group, 3.0 mg/kg cis-dichlorodiamineplatinum
(DDP), 12.5, 25 and 50 mg/kg GBEE medicine concentration groups,
12.5, 25 and 50 mg/kg GBEE + 3.0 mg/kg DDP combination groups, 15
mg/kg VER, 15 mg/kg VER + 3.0 mg/kg DDP combination group, and
there were 10 mice in each group. DDP was administered ip every two
days, and GBEE and VER were administered intragastrically once
daily. The tumor inhibitory rate less than 10% was defined as the
nontoxic dose, and the nontoxic dose of GBEE to S180 MDR ascites
tumor in mice was 25 mg/kg, which obtained by preliminary
experiments. Therefore GBEE at doses of 12.5, 25 and 50 mg/kg were
selected as the reversal dose in vivo. After administering
the drugs for one week, the mice were normally fed until they had a
natural death. The survival of tumor-bearing mice and the survival
time of the mice were observed daily. The maximum survival time
recorded was 60 days, where mice living for more than 60 days was
recorded as 60 d (30). At that
point the life-prolonging rate of mice was calculated.
Determination of tumor inhibitory rate
in vivo
The cells in S180 MDR ascites tumor in mice were
obtained under aseptic conditions and seeded at an initial
concentration of 1×106 cells/ml. A 0.2 ml cell
suspension was injected into the right forelimb armpit in mice in
order to establish the model of S180 MDR transplantable solid tumor
mice. The experiment groups and the method of drug delivery were
the same as above. After stopping the drug delivery for 7 days, all
the mice were sacrificed, the tumors were excised and weighed and
the tumor inhibition rate was weighted.
Expression analysis of MDR-1 mRNA and
MRP-1 mRNA by quantitative polymerase chain reaction (qPCR)
The total RNA was extracted from the S180 MDR
ascites tumor cells from alive mice of the different groups
(treated with various concentrations of GBEE, VER or combinations
of DDP) using TRIzol Reagent (Sangon Biotech, Co., Ltd, Shanghai,
China) according to the manufacturer's instructions. The
supernatant was removed by centrifugation after each group of cells
were treated by the drugs. 1 ml TRIzol was added to each group and
the solution was pipetted at room temperature for 5 min several
times. In total 0.2 ml chloroform (Chinese Medicine Group Chemical
Reagent Co., Ltd.) was added, and the reaction was vortexed for 15
sec at room temperature for 3 min. The solution was centrifuged at
9,180 × g for 15 min at 4°C and the upper colorless aqueous phase
was carefully removed (~600 µl) and transferred to an RNase-free
1.5 ml Eppendorf tube. An equal volume of isopropanol (Chinese
Medicine Group Chemical Reagent Co., Ltd.) was added, then mixed
and placed at room temperature for 20 mins. The solution was then
centrifuged at 9,180xg for 10 min at 4°C and the supernatant was
removed. 1 ml of 75% ethanol (National Pharmaceutical Group
Chemical Reagent Co., Ltd.) was added and the precipitate was
pelleted by centrifugation at 2,683xg for 3 min, 4°C and the
supernatant was carefully separated. The pellet was placed at room
temperature to dry for 2–3 min and 30 µl RNase-Free
ddH2O was added and stirred for several times until the
RNA was fully dissolved. 2 µl were used in order to determine the
OD260/OD280 ratio and the RNA concentration in the final solution
was determined by UV spectrophotometry. The remaining samples were
kept at −80°C.
Thereafter, 2.0 µg total RNA was used to perform
first-strand cDNA synthesis (Takara Biotechnology, Co., Ltd.,
Dalian, China) and SYBR Green fluorescence qPCR analysis (Takara
Biotechnology, Co. Ltd.) was performed using an Applied Biosystems
7500 real time-PCR analyzer (Carlsbad, CA, USA). The primer
sequences were as follows: Forward, 5′-ATGACACCCCTGAAATCCA-3′ and
reverse, 5′-CGCTCCTGTGGTGTTTTTA-3′ for the MDR-1 gene, and the
length of the PCR product was 215 bp. The second primer sequence
was as follows: Forward, 5′-CCTACTACCCCAGCATTGT-3′ and reverse,
5′-TATTCCTTCAGTCTCTCCAC-3′ for the MRP-1 gene, and the length of
the PCR product was 238 bp. The third primer sequence was as
follows: Forward, 5′-CTTAGCACCCCTGGCCAAG-3′ and reverse,
5′-GATGTTCTGGAGAGCCCCG-3′ for the housekeeping gene GAPDH, the
length of the PCR product was 450 bp. The above primers were
synthesized by Sangon Biotech, Co., Ltd. The following reagents
were used in the reverse transcription reaction: 2 µl 5X
Primescript RT master mix, 1 µg total RNA and RNase-free
ddH2O up to 10 µl. SYBR Premix Taq II DNA polymerase
(Takara Biotechnology, Co., Ltd.) was used. The reverse
transcriptase reaction conditions were as follows: 37°C for 15 min,
85°C for 5 sec and a cooling step at 4°C. qPCR was conducted using
the following conditions: 95°C for 30 sec followed by 40 cycles of
95°C for 5 sec and 60°C for 34 sec, and a dissociation curve
analysis of the amplification products was performed at the end of
each PCR reaction to confirm that only one product was amplified
and detected. The relative mRNA levels were calculated by the
2−∆∆Cq method (31), and
the experiment was repeated three times. Furthermore, a negative
control was used that consisted of mice inoculated with parental
and drug resistant cells, which were fed under the same conditions
as the other groups. However, the same volume of normal saline was
administered instead of drugs.
Determination of cytokines serum
levels
The serum was obtained from S180 MDR tumor-bearing
mice and the IL-3, IL-18 and IFN-γ levels were measured by a double
antibody sandwich ELISA method (Bio-Swamp, Chinese Bang Yi Trading
Co., Ltd, Shanghai, China). All the assays were performed according
to the manufacturer's instructions.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical significance was assessed using one-way
analysis of variance with SPSS 16.0 software (SPSS Inc., Chicago,
IL, USA) where P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of GBEE on reversing resistance
activity in S180 MDR cells
The IC50 of DDP on parent S180 cells was
0.068 µg/ml, while the IC50 of DDP on S180 MDR cells was
0.724 µg/ml. The RI of S180 MDR cells after DDP was 10.90.
Following treatment of the cells with DDP + GBEE at 7.5, 15 and 30
µg/ml and VER at 5.0 µg/ml, respectively, there was a significant
decrease of the IC50 of DDP on S180 MDR cells (Table I). The results indicated that GBEE
could effectively reverse the resistance of S180 MDR cells to
DDP.
 | Table I.Effect of GBEE on reversing
resistance in S180 MDR cells. |
Table I.
Effect of GBEE on reversing
resistance in S180 MDR cells.
|
| IC50
(µg/ml) |
|
|
|---|
|
|
|
|
|
|---|
| Groups | S180 | S180 MDR | RF | RI |
|---|
| DDP (µg/ml) | 0.068±0.018 | 0.724±0.071 | 10.90 |
|
| DDP+GBEE
(µg/ml) |
|
|
|
|
| 30 | 0.048±0.013 | 0.085±0.009 |
1.77 |
8.73 |
| 15 | 0.054±0.027 | 0.063±0.017 |
1.17 | 11.49 |
|
7.5 | 0.075±0.005 | 0.073±0.021 |
0.97 | 10.16 |
| DDP+VER
(µg/ml) |
|
|
|
|
| 5 | 0.065±0.014 | 0.098±0.045 |
1.51 |
7.39 |
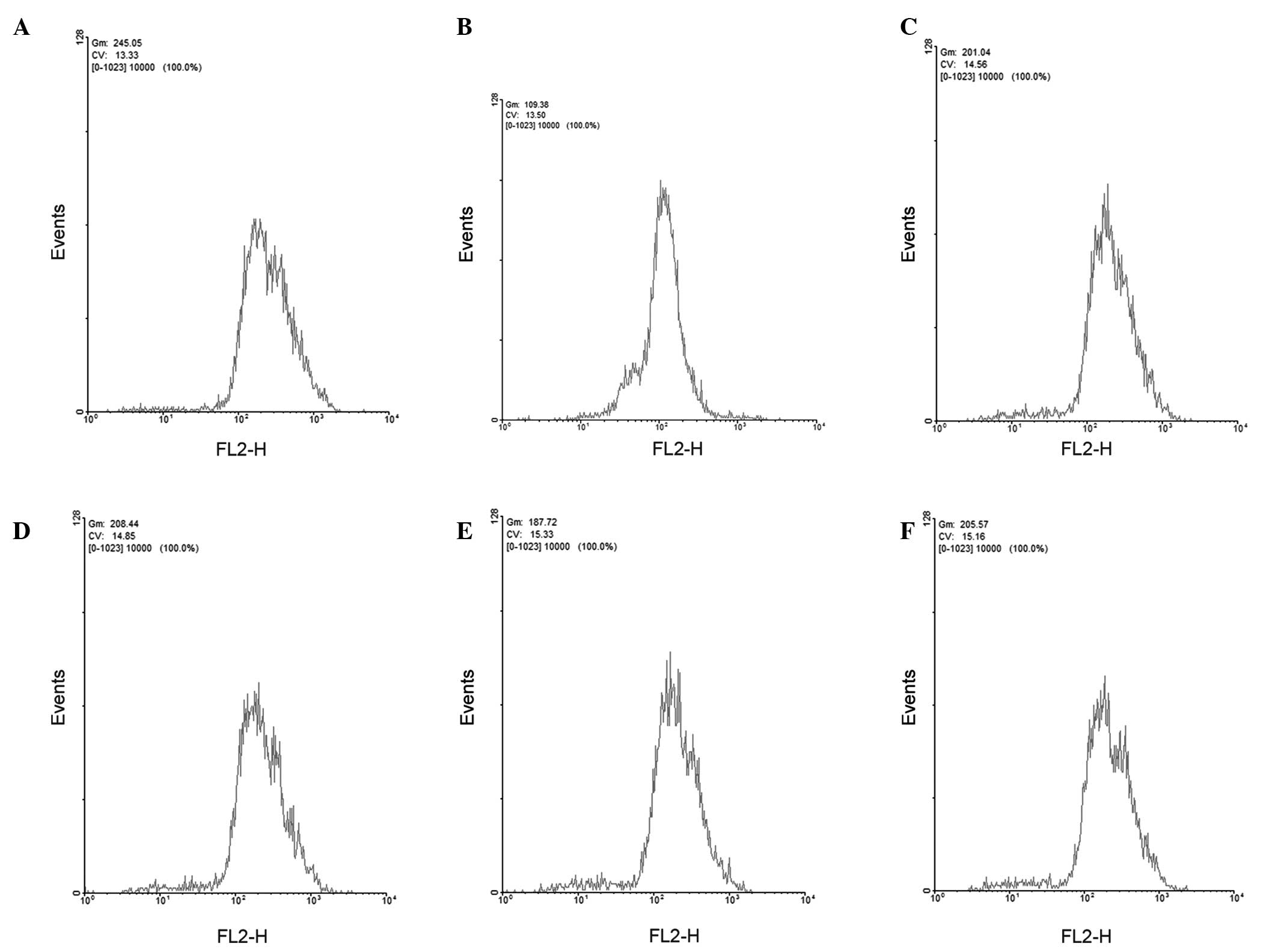
GBEE increased intracellular
accumulation of ADR
The capability of GBEE to affect the accumulation of
ADR within S180 MDR cells was examined by flow cytometry (Fig. 1). The accumulation of ADR within
parent S180 and S180 MDR cells was 250.82±5.86 and 113.37±7.26
(mean fluorescence intensity), respectively. After adding GBEE at
7.5, 15 and 30 µg/ml concentrations and VER at 5.0 µg/ml, the
residual amount of ADR within S180 MDR cells increased to
204.38±4.89, 211.89±11.21, 188.92±3.57, 208.52±8.22, respectively.
In comparison with the S180 MDR control group the differences were
significant (P<0.001).
GBEE improved functional activity of
P-gp
The effect of GBEE on the accumulation and efflux of
Rho 123 in S180 and S180 MDR cells was determined by flow
cytometry. As shown in Fig. 2,
compared with the parent cells, the accumulation of Rho 123 in S180
MDR cells was significantly reduced, and the efflux of Rho 123 was
markedly increased (P<0.05). However, GBEE and VER inhibited the
activity of P-gp. Fig. 2
demonstrated that GBEE markedly increased the accumulation of Rho
123 and induced the efflux of Rho 123 in S180 MDR cells. GBEE at 15
and 7.5 µg/ml were more effective.
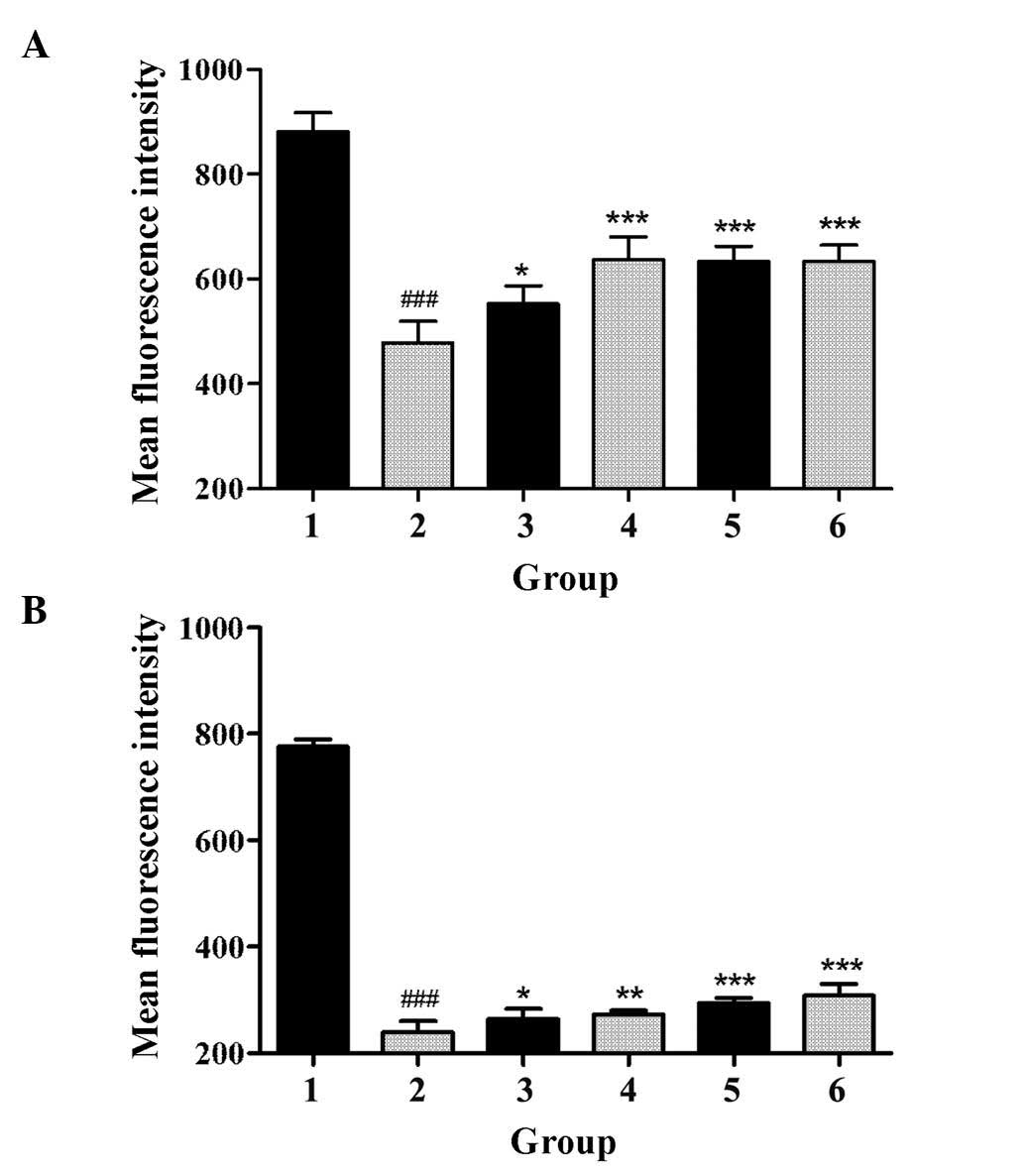 | Figure 2.Effect of GBEE on the accumulation and
efflux of Rho 123 in S180 MDR cells. (A) The accumulation and (B)
efflux of Rho 123 in S180 and S180 MDR cells. Group 1, mean
fluorescence intensity of Rho 123 in parent S180 cells; Group 2,
mean fluorescence intensity of Rho 123 in S180 MDR cells; Groups 3,
4, 5 and 6, S180 MDR cells treated with GBEE at 7.5, 15 and 30
µg/ml + VER 5 µg/ml, respectively; ###P<0.001 vs.
parent cells; *P<0.05, **P<0.01 and ***P<0.001 vs. S180
MDR cells. Data are the mean ± standard deviation of at least three
independent experiments performed in triplicate. GBEE, Ginkgo
biloba exocarp extracts; Rho 123, Rhodamine 123; VER,
verapamil; S180 MDR, mice S180 sarcoma cell line with kinds of
chemotherapeutic drugs resistance. |
GBEE enhanced the efficacy of DDP in
vivo to prolong the survival time of S180 MDR cell of ascites tumor
in mice
An established S180 MDR cell ascites tumor mouse
model was used to evaluate the efficacy of GBEE to enhance the
sensitivity of DDP in S180 MDR cells and to prolong the survival
time in vivo. There was no significant difference in the
survival time between animals treated with DDP 3.0 mg/kg and GBEE
12.5, 25 and 50 mg/kg. However, the combination of GBEE and DDP
could effectively reverse the drug resistance of S180 MDR ascites
tumor cells and prolong the survival time of mice. The
life-prolonging rate of the DDP group was 7.89%, while the
life-prolonging rate after the combined treatment was 25.00, 39.58,
29.17 and 33.59%, respectively, which showed a significant
difference compared with animals treated with DDP and GBEE alone
(P<0.05; Table II).
 | Table II.The life-prolonging rate by a
combination of GBEE and DDP in a S180 MDR ascites tumor mouse
model. |
Table II.
The life-prolonging rate by a
combination of GBEE and DDP in a S180 MDR ascites tumor mouse
model.
| Group | Surviving time
(day) | Life-prolonging
rate (%) |
|---|
| Blank |
| 12.88±1.17 |
| Control |
| 12.00±1.99 |
| DDP | 12.81±1.04 |
7.89 |
| GBEE (mg/kg) |
|
|
| 50 | 12.46±1.79 |
3.87 |
| 25 | 12.47±1.26 |
3.92 |
|
12.5 | 12.21±0.53 |
1.74 |
| GBEE+DDP
(mg/kg) |
|
|
|
50+3 |
15.00±1.55a | 25.00 |
|
25+3 |
16.75±1.81b | 39.58 |
|
12.5+3 |
15.50±0.86a | 29.17 |
| VER | 12.82±2.20 |
6.85 |
| VER+DDP |
16.03±0.92b | 33.59 |
GBEE enhanced the efficacy of DDP in
vivo to inhibit tumor growth in S180 MDR transplantable solid tumor
mice
The efficacy of GBEE to reverse the sensitivity of
DDP in S180 MDR cells and to inhibit tumor growth in vivo
was evaluated by a S180 MDR transplantable solid tumor mouse model.
GBEE at 12.5, 25 and 50 mg/kg and DDP 3.0 mg/kg alone did not show
a significant tumor inhibitory effect on S180 MDR transplantable
solid tumor growth. However, the combination of DDP 3.0 mg/kg and
GBEE 12.5, 25 and 50 mg/kg markedly decreased the tumor mass, and
the tumor inhibitory rate of DDP was increased from 19.92 to 61.60,
65.43 and 46.09%, respectively, with a significant differences
(P<0.001; Table III).
Meanwhile, the tumor inhibitory rate of DDP 3.0 mg/kg + VER 15
mg/kg was 60.76%. The effect observed was equal to that of the
combination of DDP 3.0 mg/kg and GBEE 12.5 and 25 mg/kg, and the
results indicated that GBEE could effectively reverse the
sensitivity of DDP in S180 MDR cells and enhance the tumor
inhibitory effect.
 | Table III.Inhibition of tumor growth by the
combination of GBEE and DDP in a S180 MDR solid tumor mouse
model. |
Table III.
Inhibition of tumor growth by the
combination of GBEE and DDP in a S180 MDR solid tumor mouse
model.
| Group | Tumor mass (g) | Inhibition rate
(%) |
|---|
| Blank |
| 1.861±0.81 |
| Control |
| 2.257±0.32 |
| DDP | 1.807±0.50 | 19.92 |
| GBEE (mg/kg) |
|
|
| 50 | 1.918±0.62 | 15.01 |
| 25 | 2.010±0.46 | 10.93 |
|
12.5 | 1.958±0.39 | 13.26 |
|
50+3 |
1.217±0.27a | 46.09 |
| GBEE+DDP
(mg/kg) |
|
|
|
25+3 |
0.780±0.18a | 65.43 |
|
12.5+3 |
0.867±0.28a | 61.60 |
| VER | 1.867±0.34 | 17.29 |
| VER+DDP |
0.886±0.24a | 60.76 |
GBEE induced the expression level of
MDR-1 mRNA, MRP-1 mRNA in S180 MDR ascites tumor mouse cells in
vivo
In order to determine whether GBEE mediated reversal
effects on ABC transporters in vivo, the cellular RNAs were
isolated from S180 MDR ascites tumor mouse cells and the MDR-1 mRNA
and MRP-1 mRNA expression was analyzed by qPCR. As shown in
Fig. 3A and B, two mRNA expression
levels of S180 MDR cells of the control group were 3.448, 3.906
times higher than parent S180 cells from the blank group.
Nevertheless, the two gene expression levels of GBEE 12.5, 25 and
50 mg/kg alone and its combinational groups were decreased.
Furthermore, there were significant changes compared with control
and DDP 3.0 mg/kg groups. These results indicated that GBEE could
overcome the DDP resistance of S180 MDR cells of ascites tumor mice
via the downregulation of MDR-1 and MRP-1. As shown in Fig. 3C and D, the melting curve of the
MDR-1 mRNA, MRP-1 mRNA and GAPDH amplification products in cells of
different groups had a single peak and no other impurity peak. This
showed that the fluorescent signals from SYBR Green were all PCR
amplification products without any primer dimer and non-specific
products.
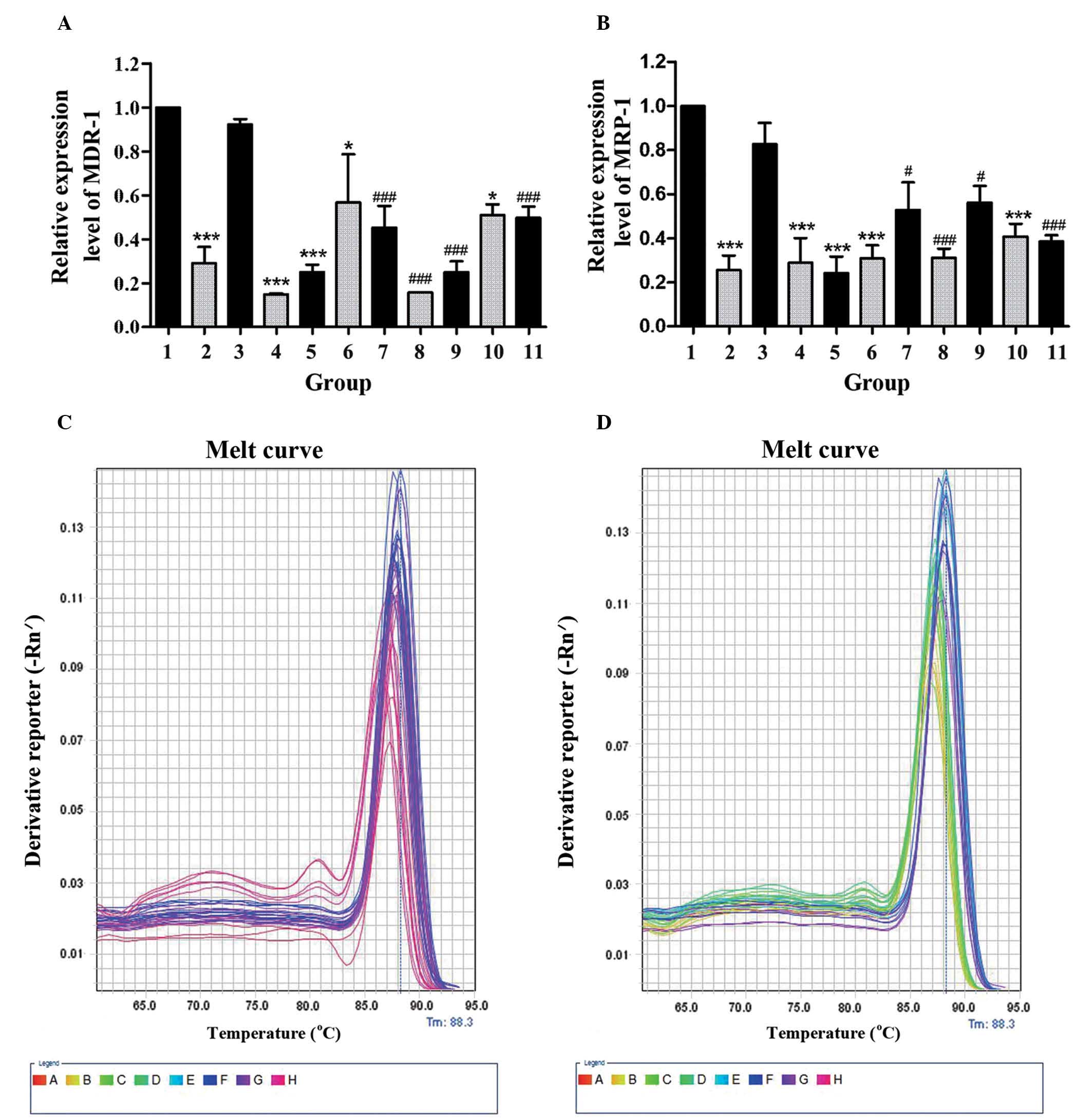 | Figure 3.Results of GBEE on the expression of
MDR-1 mRNA and MRP-1 mRNA in S180 MDR ascites tumor cells. (A) The
MDR-1 mRNA and (B) MRP-1 mRNA expression was analyzed by
quantitative polymerase chain reaction. The melting curve of (C)
MDR-1 mRNA, (D) MRP-1 mRNA and the GAPDH amplification product in
cells of different groups was with a single peak. The data are the
mean ± standard deviation of at least three independent experiments
performed in triplicate. Group 1, control group consisting of S180
MDR cells without any treatment; Group 2, blank group consisting of
parent S180 cells without any treatment; Group 3, DDP 3.0 mg/kg was
given intraperintoneally every two days, Groups 4, 5, 6, 10, GBEE
12.5, 25 and 50 mg/kg + VER 15 mg/kg was given intragastrically
once daily. Groups 7, 8, 9, 11, the combination of DDP 3.0 mg/kg
and GBEE 12.5, 25, 50 mg/kg + VER 15 mg/kg. GAPDH was used as an
internal control. The mRNA expression levels of MDR1 and MRP1 were
quantified by the 2−∆∆Ct method and the control group
was set as a standard. *P<0.05 and ***P<0.001 vs. the control
group; #P<0.05 and ###P<0.001 vs. the
DDP group. GBEE, Ginkgo biloba exocarp extracts; MDR-1,
multidrug resistance-1; MRP-1, multidrug resistance-associated
protein-1; S180MDR, mice S180 sarcoma cell line with
chemotherapeutic drug resistance; VER, verapamil; DDP,
cis-dichlorodiamineplatinum. |
GBEE promoted cytokine serum levels of
S180 MDR tumor-bearing mice
The effect of GBEE on the IL-3, IL-18 and IFN-γ
serum levels of S180 MDR tumor-bearing mice was determined by
ELISA. The levels of each cytokine are shown in Fig. 4. It was observed that the serum
levels of three cytokines in the blank group mice were
significantly higher than the control. The reason for this may be
that MDR inhibited the production of cytokines. Compared with the
blank and control groups respectively, GBEE 12.5, 25 and 50 mg/kg
alone and its combinational groups could significantly enhance the
serum levels of IL-3, IL-18 and IFN-γ in S180 MDR tumor-bearing
mice. The levels of these three cytokines in DDP 3 mg/kg group were
1,625±70.71, 33.27±0.47 and 124.50±19.80 pg/ml. Under the effect of
GBEE 25 mg/kg, the levels increased to 3,079±262.34, 121.94±20.27
and 409.67±8.73 pg/ml, respectively.
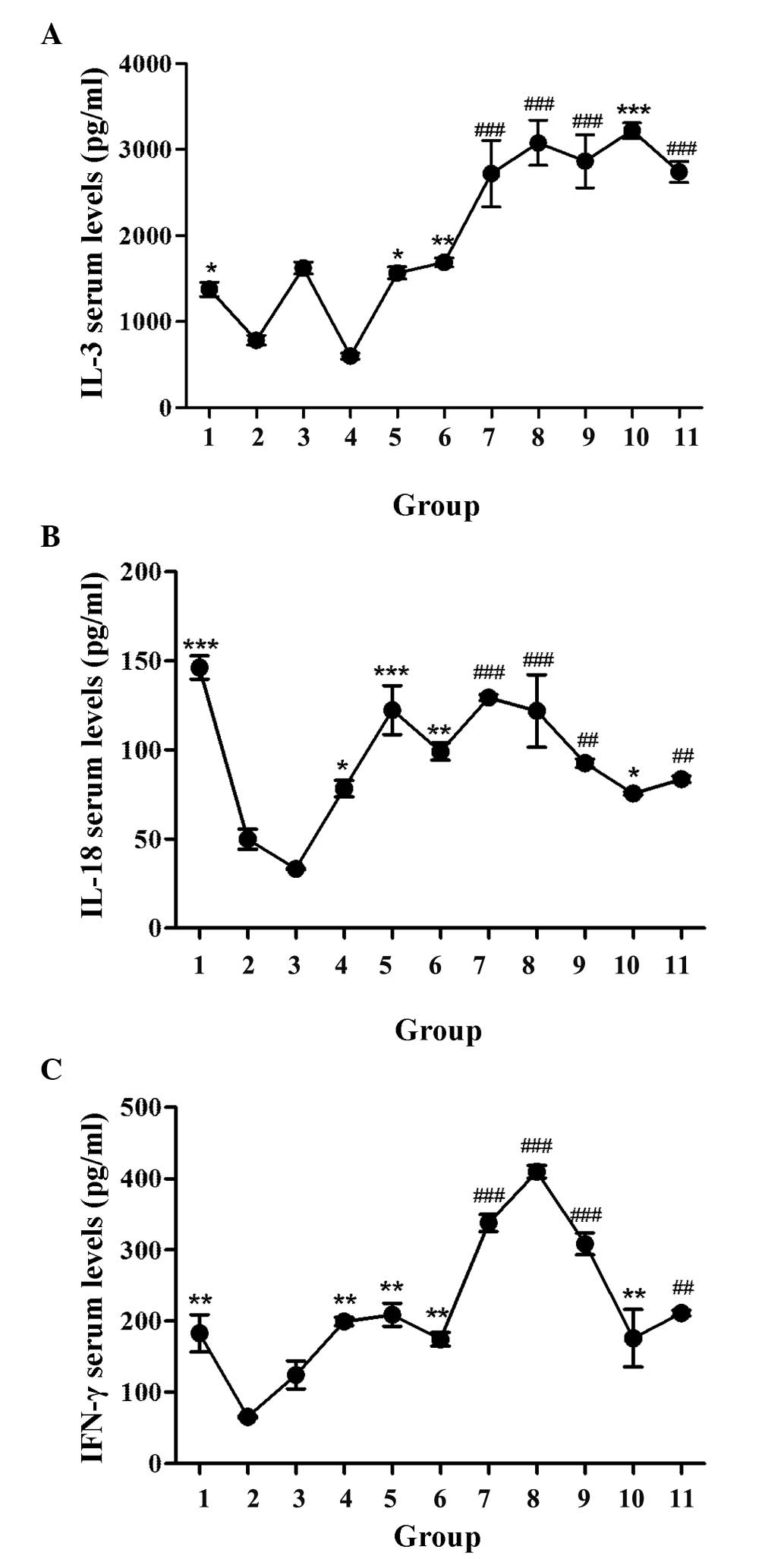 | Figure 4.Effect of GBEE on the serum levels of
cytokines in S180 MDR tumor-bearing mice. (A) IL-3, (B) IL-18 and
(C) IFN-γ serum levels of S180 MDR tumor-bearing mice were
determined by ELISA. Data are the mean ± standard deviation of at
least three independent experiments performed in triplicate. Group
1, parent S180 cells without any treatment (blank group); Group 2,
S180 MDR cells without any treatment (control group). Group 3, DDP
3.0 mg/kg was given intraperitoneally every two days, Groups 4, 5,
6 and 10, GBEE 12.5, 25, 50 mg/kg + VER 15 mg/kg was given
intragastrically once daily. Groups 7, 8, 9 and 11, the combination
of DDP 3.0 mg/kg and GBEE 12.5, 25, 50 mg/kg + VER 15 mg/kg.
*P<0.05, **P<0.01 and ***P<0.001 vs. control group;
##P<0.01 and ###P<0.001 vs. DDP group.
GBEE, Ginkgo biloba exocarp extracts; S180 MDR, mice S180
sarcoma cell line with chemotherapeutic drug resistance; IL,
interleukin; IFN, interferon; DDP, cis-dichlorodiamineplatinum;
VER, verapamil. |
Discussion
Currently researchers in China and around the world
have focused on finding highly-efficient and harmless MDR reversal
agents (with antitumor properties) from traditional Chinese
Medicine or natural plant medicine. According to the reported
literature, astragalus polysaccharides (32) and Lentinan (33) could are able to reverse MDR in some
tumor cell lines in vitro. The total alkaloid concentration
from Fritillaria thunbergii (34)
has also been shown to be able to enhance the MDR reversal effects
of DDP on A549/DDP cells in vitro and in vivo. A
clinical observation demonstrated that Brucea Javanica Oil Emulsion
(35), which is extracted from
Traditional Chinese Medicine plant Brucea Javanica, could enhance
the effect of Adramycin on refractory malignant pleural effusion.
One of its mechanism may be associated with the reversal effect on
patients with primary and secondary drug resistance. The present
study observed the effect of GBEE in S180 MDR cells and its
ascitic, transplantable solid tumor mouse models, which were
established by the simulation of a clinically chemotherapeutic PFC
scheme. Experimental results demonstrated that GBEE 7.5, 15 and 30
µg/ml could significantly decrease the IC50 of DDP on
S180 MDR cells in vitro and its RIs were 10.16, 11.49 and
8.73, respectively. In addition, GBEE 12.5, 25 and 50 mg/kg could
effectively reverse the resistance of S180 MDR cells to DDP in
vivo and then markedly prolong the survival time and increase
the tumor inhibitory rate of mice with GBEE treatment. The results
demonstrated that GBEE could reverse the resistance of S180 MDR
cells to DDP both in vitro and in vivo.
P-gp, encoded by the MDR-1 gene, is one of the ATP
dependent drug efflux pumps (36).
MRP is a type of glutathione and glucuronate conjugate pump
(37). They were all available to
use the energy of ATP to transport the medicine from the
intracellular to the extracellular space and as a consequence to
weaken the anti-tumor effect. The present study took advantage of
the fluorescent properties of ADR (38) and Rho 123 that were used for flow
cytometry. The experimental results demonstrated that GBEE 7.5, 15
and 30 µg/ml in vitro could inhibit the functional activity
of P-gp on drug-resistant cells. In addition, it could improve the
intracellular accumulation of ADR, Rho 123 and induce the efflux of
Rho 123. The results of qPCR showed that in vivo GBEE 12.5,
25 and 50 mg/kg could inhibit the expression of MDR-1 mRNA and
MRP-1 mRNA in S180 MDR cells of mice with ascites tumor. Two mRNA
expression levels of S180 MDR cells of the GBEE 50 mg/kg group were
6.711 and 3.455 times lower compared to the control group.
Furthermore, the two mRNA expression levels of the GBEE 25 mg/kg
combination group were 5.843 and 2.677 times lower than the DDP 3.0
mg/kg group. Therefore, it is possible that the reversal mechanism
of GBEE is related to the inhibition of the expression of MDR-1
mRNA and MRP-1 mRNA in drug-resistant tumor cells. Furthermore it
reduces the composition of the resistant protein and improves the
concentration of chemotherapeutic medicine in tumor cells by
suppressing the functional activity of P-gp.
A previous study has revealed that MDR-1 itself is a
target-antigen (also called ‘foreign’ element) that can induce
CD8+ T-cell increase and the secretion of reactive
molecules, and promote its lethal effect to MDR tumor cells
(39). Promotion of the body's
immune function is beneficial to the body in order to identify
‘foreign’ ABC-resistant proteins and reduce the function of
drug-resistant cell pumps that pump out chemotherapeutic drugs by
destroying their spatial structure, and then increasing the
accumulation of chemotherapeutic drugs in drug-resistant cells to
exert an antitumor effect. At the same time, the immune function of
the body is beneficial to immune cells that secrete cytokines, some
of which can directly or indirectly reverse drug-resistant effects.
As an example macrophages and lymphocytes secrete IL-1, IL-2 and
IFN-γ that can promote ADR and induce the apoptosis of MDR tumor
cells by inhibiting the expression of the P-gp protein (40); Cytokine-induced killer cells can
inhibit the expression of the MDR-1 gene or P-gp, MDR-1, MRP-1
proteins and reverse drug resistance (41,42).
Furthermore, IFN-α is produced by mononuclear cells and
lymphocytes, which can decrease the expression of resistant
proteins, including MRP, P-gp and GST and also reverse
drug-resistant effects (43). In
addition some of them can also promote the secretion of other
cytokines to create a synergy. For example IL-18 can facilitate the
secretion of IFN-γ, IFN-α and IL-2 (44). The majority of patients undergoing
chemotherapy have a weakened immune system that is an important
cause of MDR. Therefore, the need to find reversal agents that can
promote immune function is of great clinical significance. IL-3
produced by T-cells can support the proliferation and
differentiation of hematopoietic pluripotent stem and progenitor
cells by improving the immune function and promoting hematopoietic
function (45). The present study
showed that GBEE 12.5, 25 and 50 mg/kg could significantly increase
the serum levels of IL-3, IL-18, IFN-γ in S180 MDR tumor-bearing
mice (P<0.05). Furthermore, it improved the immune function,
which had a great significance in reversing MDR and enhancing
antitumor effects.
To conclude, the present study showed that GBEE
could inhibit cell proliferation in the S180 MDR cell line and
reverse its drug-resistance in vitro. The results also
demonstrated that in vivo GBEE could prolong the survival
time of S180 MDR ascites tumor mice and increase the tumor
inhibition rate of S180 MDR tumor-bearing mice by inhibiting the
expression of MDR-1 mRNA and MRP-1mRNA. Furthemore GBEE could
prolong the survival time by increasing the serum levels of IL-3,
IL-18 and IFN-γ, and by influencing the quantity and functional
activity of relative drug-resistant proteins. The previous research
results showed that GBEE could significantly increase the serum
levels of IL-2 and TNF-α in S180 MDR tumor-bearing mice (46). It is also significant to reverse the
drug resistance of the tumor. GBEE, therefore, is a promising
reversal agent that can be used in the development of treatments
for drug-resistant cancer, particularly in combination with other
chemotherapeutic drugs.
Acknowledgements
The present study was supported by the Medical
High-Tech Research projects of Jiangsu province (grant no.
BG2007609) and the Science and Technology Innovation Fund project
for College students in Yangzhou University (grant no.
x2014668).
References
|
1
|
Daniel C, Bell C, Burton C, Harguindey S,
Reshkin SJ and Rauch C: The role of proton dynamics in the
development and maintenance of multidrug resistance in cancer.
Biochim Biophys Acta. 1832:606–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang F, Wu XN, Chen J, Wang WX and Lu ZF:
Resveratrol reverses multidrug resistance in human breast cancer
doxorubicin-resistant cells. Exp Ther Med. 7:1611–1616.
2014.PubMed/NCBI
|
|
3
|
Molinas A, Sicard G and Jakob I:
Functional evidence of multidrug resistance transporters (MDR) in
rodent olfactory epithelium. PLoS One. 7:e361672012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu F, Shao ZY, Zhai BJ, Zhao CL and Shen
DM: Ultrasound reverses multidrug resistance in human cancer cells
by altering gene expression of ABC transporter proteins and Bax
protein. Ultrasound Med Biol. 37:151–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
6
|
Wan X and Sun G: The research progress of
immune mechanism of reverse tumor multi-drug resistance. An Hui Yi
Yao. 10:401–402. 2006.(In Chinese).
|
|
7
|
Gomes-Giacoia E, Miyake M, Goodison S,
Sriharan A, Zhang G, You L, Egan JO, Rhode PR, Parker AS, Chai KX,
et al: Intravesical ALT-803 and BCG treatment reduces tumor burden
in a carcinogen induced bladder cancer rat model; a role for
cytokine production and NK cell expansion. PLoS One. 9:e967052014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson SD, De Costa AM and Young MR:
Effect of the premalignant and tumor microenvironment on immune
cell cytokine production in head and neck cancer. Cancers (Basel).
6:756–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhen JH: Progress in research on
interleukin −2. Strait Pharmaceutical Journal. 18:1–3. 2006.
|
|
10
|
Liu YL, Chen TG and Liu XL: Research
progress of interferon. Progress in Veterinary Medicine. 29:81–84.
2008.
|
|
11
|
Fu S and Qian YK: Research progress of
tumor necrosis factor. International Journal of Immunology.
5:252–257. 1993.
|
|
12
|
Tu WQ and Yang XQ: Regulation of cytokine
immune response and its clinical significance. Foreign Medical
Sciences(Section of Pediatrics). 5:228–232. 1993.
|
|
13
|
Cun YM, Yang YX, Xiang Y, et al: IL-2 gene
transfection reversing multidrug resistance in choriocarcinoma cell
line. Basic and Clinical Medicine. 21:523–526. 2001.
|
|
14
|
Hu JW, Shen ZY, Yang JC, et al:
Combination of interferon gamma and Vera Pammy to reverse multidrug
resistance in human lung cancer cell line. Suzhou University
Journal of Medical Science. 23:16–18. 2003.
|
|
15
|
Malorni W, Rainaldi G, Tritarelli E, et
al: Tumor necrosis factor alpha is a powerful apoptotic inducer in
lymphoid leukemic cells expressing the P-170 glycoprotein.
International Journal of Cancer. 67:238–47. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Fu L and Liu Z: Reversal of
multidrug resistance in cancer cell by verapamil: A research
progress. Foreign Medical Science (Section of Pharmacy).
34:190–193. 2007.(In Chinese).
|
|
17
|
Jiao B: Experimental study of reversal of
multidrug resistance by cyclosporin a in various human gliom cell
lines. Zhong Liu Fang Zhi Yan Jiu. 28:457–459. 2001.(In
Chinese).
|
|
18
|
Xu AH, Chen HS and Zhuo C: The inhibitory
effect of Ginkgo biloba endocarp polysaccharides (GBEP) on human
cancer cell strains and its synergic effect in combination with
adriamycin. Zhong Guo Xin Yao Za Zhi. 9:753–755. 2000.(In
Chinese).
|
|
19
|
Chen Y, Mao LL, Hu BY, Chen HS and Xu AH:
Synergistic attenuated antitumor effect of Ginkgo biloba exocarp
extracts combined with cis-dichlorodiamineplatinum in S180
tumor-bearing mice. Zhong Guo Xin Yao Za Zhi. 23:1569–1573.
2014.(In Chinese).
|
|
20
|
Xu AH, Ren L, Zheng YY and Chen HS:
Immunomodulatory effect of Ginkgo biloba exocarp polysaccharides on
immunosuppressive mice induced by cyclophosphamide. Zhong Guo Yao
Li Xue Yu Du Li Xue Za Zhi. 22:69–72. 2008.(In Chinese).
|
|
21
|
Chen HS, Zhuo F and Chu YF: Clinical study
on treatment of patients with upper digestive tract malignant
tumors of middle and late stage with Ginkgo biloba exocarp
polysaccharides capsule preparation. Zhong Xi Yi Jie He Xue Bao.
1:189–191. 2003.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhai F and Chen HS: Ginkgo biloba exocarp
Polysaccharide preparation in the treatment of 84 cases of advanced
cancer. Liao Ning Zhong Yi Za Zhi. 29:5642002.
|
|
23
|
Shen TT, Xu AH, Zheng YY, et al:
Anti-metastasis effect of Ginkgo biloba exocarp extracts on
metastasis of Lewis lung cancer in C57BL /6J mice and its
mechanism. Chin J of Pharmacol Toxicol. 27:67–71. 2013.
|
|
24
|
Xu JL, Wang ZH and FJ S: Establishment of
tumor multi-drug resistance mice model induced by combination
chemotherapeutic in vivo. Shi Yan Dong Wu Yu Bi Jiao Yi Xue.
25:215–217. 2005.
|
|
25
|
Chen Q, Li GH and Sun FJ: Establishment of
obtained multidrug resistance model of S180 cell line mice used for
study on Chinese materia medica and its stability. Zhong Cao Yao.
37:1691–1694. 2006.(In Chinese).
|
|
26
|
Wu Q, Xu A, Yang Q, Wang LX and Chen HS:
Influence of effective part GBEE-2 of exocarpium ginkgo extracts on
apoptosis and apoptotic pathway of human gastric adenocarcinoma
cell line SGC-7901. Traditional Chinese Drug Research and Clinical
Pharmacology. 22:270–273. 2011.
|
|
27
|
Cao CJ: Inhibitory effect of extract of
Ginkgo biloba extract on liver cancer in mice and its effect on
survivin expression. Shi Zhen Guo Yi Guo Yao. 26:1322–1324.
2015.
|
|
28
|
Zrieki A, Farinotti R and Buyse M:
Cyclooxygenase-2 inhibitors prevent trinitrobenzene sulfonic
acid-induced P-glycoprotein up-regulation in vitro and in vivo. Eur
J Pharmacol. 636:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loetchutinat C, Saengkhae C, Marbeuf-Gueye
C and Garnier-Suillerot A: New insights into the
P-glycoprotein-mediated effluxes of rhodamines. Eur J Biochem.
270:476–485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu SY, Bian RL and Chen X: Screening
method of animal tumor in vivoExperimental Methodology of
Pharmacology. 3rd. People's Medical Publishing House; Beijing: pp.
1757–1784. 2002
|
|
31
|
Isabel D, Ferreira I, Virgílio E, do
Rosário I and Pedro VL: Cravo* 2: Real-time quantitative PCR with
SYBR Green I detection forestimating copy numbers of nine drug
resistance candidate genes in Plasmodium falciparum. Malaria
Journal. 5:1–6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Jia YJ and Li XJ: Resistance
reversal effect of astragalus polysaccharides for injection on
cisplatin-resistant human lung adenocarcinoma cell line A549/DDP.
Drug Evaluation Research. 35:417–419. 2012.(In Chinese).
|
|
33
|
Wu HY, Chen YM, Lin L, Qiu QA and Liu N:
Lentinan enhances cisplatin-mediated inhibition of cell
proliferation in human gastric cancer cell line SGC-7901. Shi Jie
Hua Ren Xiao Hua Za Zhi. 19:344–348. 2011.(In Chinese).
|
|
34
|
Li ZH, An C, Hu KW, Zhou KH, Duan HH and
Tang MK: Multidrug resistance reversal activity of total alkaloid
from Fritillaria thunbergii on cisplatin-resistant hum an lung
adenocarcinom a A549/DDP cells. Zhong Guo Yao Li Xue Yu Du Li Xue
Za Zhi. 27:315–320. 2013.(In Chinese).
|
|
35
|
Zhu C, Liu XF and Li G: Effect of Brucea
Javanica oil emulsion combined with adramycin for refractory
malignant pleural effusion. Shi Yong Quan Ke Yi Xue. 5:871–873.
2007.(In Chinese).
|
|
36
|
Hennessy M and Spiers JP: A primer on the
mechanics of P-glycoprotein the multidrug transporter. Pharmacol
Res. 55:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kruh GD and Belinsky MG: The MRP family of
drug efflux pumps. Oncogene. 22:7537–7552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng YM, Wang N, Wang YF, Han L, Zhang Y,
Jiang JH, Zhou YB and Wang QD: Correlation between reversing effect
of cepharanthine hydrochloride on multidrug resistance and
P-glycoprotein expression and function of K562/ADR cells. Yao Xue
Xue Bao. 47:594–599. 2012.(In Chinese). PubMed/NCBI
|
|
39
|
Niethammer AG, Wodrich H, Loeffler M, Lode
HN, Emmerich K, Abdollahi A, Krempien R, Debus J, Huber PE and
Reisfeld RA: Multidrug resistance-1 (MDR-1): A new target for T
cell-based immunotherapy. FASEB J. 19:158–159. 2005.PubMed/NCBI
|
|
40
|
Shi YJ, Ren HY, Cen XN, Zhu Q and Yu JR:
Immunological effector cells enhance apoptosis induced by
adriamycin in a multi-drug resistant human breast cancer cell line.
Zhonghua Zhong Liu Za Zhi. 28:188–191. 2006.(In Chinese).
PubMed/NCBI
|
|
41
|
Wang L, Deng Q, Wang J, Bai X, Xiao X, Lv
HR, Zhao MF and Liu PJ: Effect of CIK on multidrug-resistance
reversal and increasing the sensitivity of ADR in K562/ADR cells.
Oncol Lett. 8:1778–1782. 2014.PubMed/NCBI
|
|
42
|
Ping CQ, Zhang YJ, Sun GX, Dong M, Zhou
XL, Ping J, Liu Y Qm and Hong LJ: The expression effect of DC-CIK
on K562/A multidrug resistance gene mdr1. Xian Dai Sheng Wu Yi Xue
Jin Zhan. 14:2667–2671. 2014.(In Chinese).
|
|
43
|
Wang CH, Pan CX and Wang W: The reversal
study of alpha-interferon on human breast cancer multidrug
resistance cell lines MX-1/Taxo (MX-1/T). Dang Dai Yi Xue.
20:17–19. 2014.(In Chinese).
|
|
44
|
Li Y, Zou Y, Cai B, Yang B, Ying B, Shi Y
and Wang L: The associations of IL-18 serum levels and promoter
polymorphism with tacrolimus pharmacokinetics and hepatic allograft
dysfunction in Chinese liver transplantation recipients. Gene.
491:251–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu CF, Hong JH and Chiang CS: The roles of
macrophages and nitric oxide in interleukin-3-enhanced
HSV-Sr39tk-mediated prodrug therapy. PLoS One. 8:e565082013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li J, Fu EC, Xu AH, et al: Effect of GBEE
on the levels of IL-2, IL-12, TNF- and TGF- in tumor bearing mice.
Chinese Journal of Immunology. 28:415–417. 2012.
|