Introduction
Chronic osteomyelitis is a complication resulting
from trauma, which involves the combination of disruption of the
blood supply to the bone and soft tissue destruction (1). The treatment of chronic osteomyelitis
is challenging, due to prolonged therapy and recurrence (2). Debridement and antibiotic therapy are
common treatments for osteomyelitis. In recent years, the Ilizarov
method involving radical excision of the infected bone and
distraction osteogenesis with bone transport has shown promising
results in the treatment of chronic osteomyelitis (3,4).
However, scars occurring in chronic osteomyelitis can result in
deformities and restriction of the mobility of joints and
extremities. The pathogenesis of hypertrophic scarring is not fully
understood, and clinical treatment has yet to be clearly
defined.
Hypertrophic scarring mainly results from disorders
of the collagen metabolism (5). Type
I collagen is the major fibrous collagen, which is regulated by the
transforming growth factor β (TGF-β) signaling pathway at the
translational level (6). Three
isoforms of TGF-β have been identified in mammals, including
TGF-β1, TGF-β2 and TGF-β3. Active TGF-β1 is a major cytokine that
stimulates the gene transcription of type I collagen (7). Therefore, blocking the TGF-β1 signaling
pathway may theoretically suppress the production of collagen I,
thus preventing the formation of hypertrophic scars.
Decorin is a natural inhibitor of TGF-β1, which has
been demonstrated to have a beneficial effect of anti-fibrosis in
hypertrophic scars formed after burns (8). However, to the best of our knowledge,
no studies have investigated the effect of decorin on hypertrophic
scars in chronic osteomyelitis. Thus, the present study was
designed to investigate the in vivo effect of decorin in an
osteomyelitis rat model. It was hypothesized that the TGF-β1
cascade is involved in scar formation, and decorin was found to
have an inhibitory effect on scarring in osteomyelitis through the
TGF-β1 signaling pathway.
Materials and methods
Treatment groups and treatment of
animal model
A total of 72 Wistar rats 3 months of age with a
weight of 300–350 g were provided by the Animal Center of Shandong
University (Jinan, China). The study was approved by the Ethics
Committee of Shandong Provincial Hospital Affiliated to Shandong
University. The animals were kept and fed under standard conditions
of temperature (22±2°C, humidity (45±5)% and 12 h light.. Animals
were randomly divided into three groups: Group A, control rats;
group B, osteomyelitis model rats; and group C, decorin-treated
osteomyelitis rats.
Animals were given an intraperitoneal injection of
90 mg/kg 10% ketamine (Jiangsu Hengrui Medicine Co., Ltd.,
Lianyungang, China) to anesthetize for 30 min. The left leg of the
rats was shaved and disinfected, and soft tissue was dissected from
the lateral region of the femur. In group A (control group), the
femur was exposed and subsequently the wound was sutured, with no
further intervention. In group B, the chronic osteomyelitis model
was established according to the model described by Inanmaz et
al (9). The fracture of the
femur was performed using vascular forceps and was fixed by
Kirschner wire through the bone cavity. A saline solution
containing 106 colony-forming units/ml
methicillin-sensitive Staphylococcus aureus (MSSA; Nanjing
Bianzhen Biotechnology Co., Ltd., Nanjing, China) was injected into
the medullary cavity for bacterial inoculation, and the skin was
then closed and disinfected. In group C (decorin-treated group),
the animals were treated with decorin subsequent to the bacterial
inoculation in osteomyelitis model rats. Recombinant mouse decorin
was purchased from R&D Systems Inc. (Minneapolis, MN, USA), and
a dose of 50 µg was injected in the muscle tissue surrounding the
fracture on post-wounding days 0, 1, and 2. Subsequent to the
surgery, the animals were closely followed, and their weight and
temperature were monitored regularly.
Groups A and B were designed to examine the
formation of hypertrophic scarring in osteomyelitis and to assess
the involvement of TGF-β1 signaling in scar formation. Muscle
tissues from these groups were harvested on days 7, 14 and 28 after
surgery. Group C was designed to evaluate the efficacy and
mechanism of decorin in treating scars formed as a result of
osteomyelitis. Muscle tissues in group C were harvested on days 14
and 28 after surgery.
At the designated times, the animals were sacrificed
by decapitation following the intraperitoneal injection of 90 mg/kg
10% ketamine, and muscle tissues surrounding the femur were
harvested. The tissues were washed in normal saline and bisected.
One of the two tissue sections was fixed in 4% formaldehyde for
hematoxylin-eosin (H&E) or Masson's trichrome staining. The
other part of each tissue was snap-frozen in liquid nitrogen and
stored at −80°C for immunoblotting.
Radiography
Radiographs of the femur were obtained on
post-wounding days 7, 14 and 28 in order to evaluate alterations of
osteomyelitis, such as periosteal reaction, sclerosis and
osteolysis (10).
Histological analysis
H&E and Masson's trichrome staining were
performed to examine the fibrosis of muscles. Tissue samples were
fixed in 4% formaldehyde for 24 h. Routine dehydration, paraffin
embedding and serial sectioning into 5-mm samples were sequentially
performed. Subsequent to staining using H&E or Masson's
trichrome, the sections were mounted and observed for histology
under a light microscope.
Immunoblotting analysis
The protein expression levels of TGF-β1, TGF-β
receptor I (TβRI), TβRII, phosphorylated-Smad2 (pSmad2), pSmad3,
Smad2/3 and type I collagen were determined by western blot
analysis. Muscle tissue was ground and solubilized in lysis buffer
(Wuhan Boster Biological Technology, Ltd., Wuhan, China).
Homogenates were centrifuged at 62,000 × g at 4°C for 30 min and
the supernatant was collected. The protein concentration of the
samples was determined using a Bradford assay (11), with bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA) as the protein standard.
Proteins in the homogenate fraction were separated by 8% SDS-PAGE
and transferred to polyvinylidene difluoride membranes.
Immunoblotting was subsequently performed with the following
antibodies: Anti-TGF-β1, anti-TβRI, anti-TβRII, anti-pSmad2,
anti-pSmad3, anti-Smad2/3 and anti-collagen I. The antibodies
anti-TGF-β1 (cat. no. ab92486), anti-TβRI (cat. no. ab31013),
anti-TβRII (cat. no. ab61213) and anti-collagen I (ab34710), all
were purchased from Abcam (Cambridge, UK) and used at a dilution of
1:2,000, while anti-pSmad2 (cat. no. 3101), anti-pSmad3 (cat. no.
9520), anti-Smad2/3 (cat. no. 3102), and anti-GAPDH (cat. no.
2118), were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and used at a dilution of 1:1,000. Horseradish
peroxidase (HRP)-conjugated Goat Anti-Rabbit IgG H&L (cat. no.
ab6721) was purchased from Abcam, and used as the secondary
antibody at a dilution of 1:2,000. Immunoreactive bands were
visualized with an enhanced chemiluminescence kit (cat. no. ab5801;
Abcam). The bands on the western blots were scanned and the
intensity of the bands was determined from optical density
measurements using Gel-Pro Analyzer 4.0 software (Media
Cybernetics, Inc, Rockville, MD, USA).
Statistical analysis
Data were analyzed with the SPSS version 20.0
software (IBM Corp., Armonk, NY, USA). The results were presented
as the mean ± standard deviation. Differences between each group
were analyzed with two-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Radiography
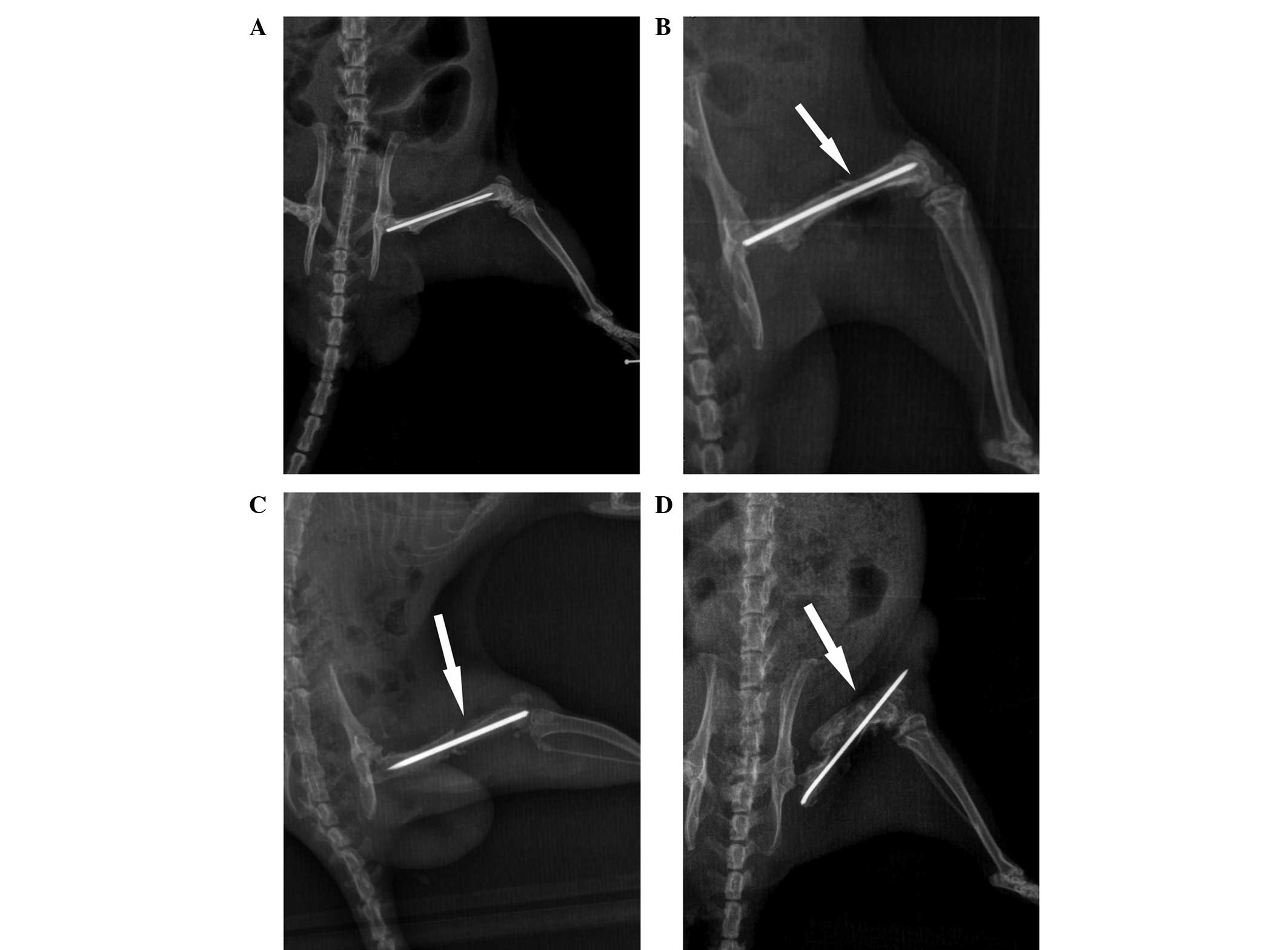
X-ray scans of the femur were performed, and
representative images at various time points are shown in Fig. 1. The scans provided evidence of
osteomyelitis on post-wounding days 14 and 28 in group B. A minimal
periosteal bone response was visible at day 7, while signs of
infected tissue cortical bone response were observed at day 14.
Evident diaphyseal osteolysis and calcification were present at day
28 (Fig. 1), which are
characteristics of chronic osteomyelitis, thus indicating that the
model of osteomyelitis was successfully established.
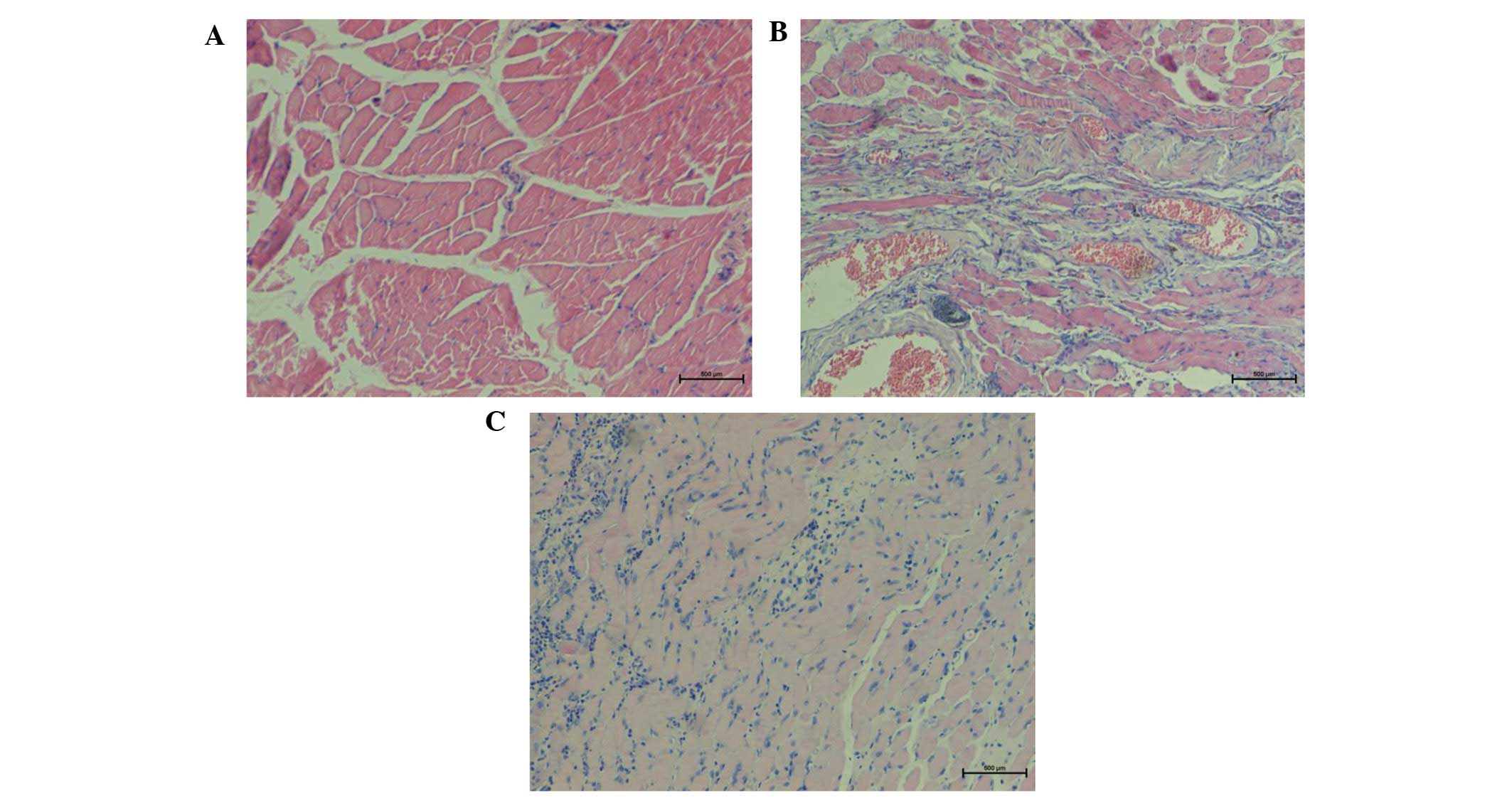
H&E staining
The results of H&E staining indicated a smaller
number of fibroblasts in group A, while the muscle fibers were
well-arranged (Fig. 2A). In group B,
H&E staining of muscle tissue revealed a high degree of chronic
inflammation, an increased number of blood vessels and disarranged
collagen tissue with a vortex-like distribution (Fig. 2B). By contrast, in group C, H&E
staining of decorin-treated tissues demonstrated a gradual
reduction in blood vessels, fibroblasts and collagen fibers, as
well as a reduced number and ordered arrangement of collagen fibers
(Fig. 2C).
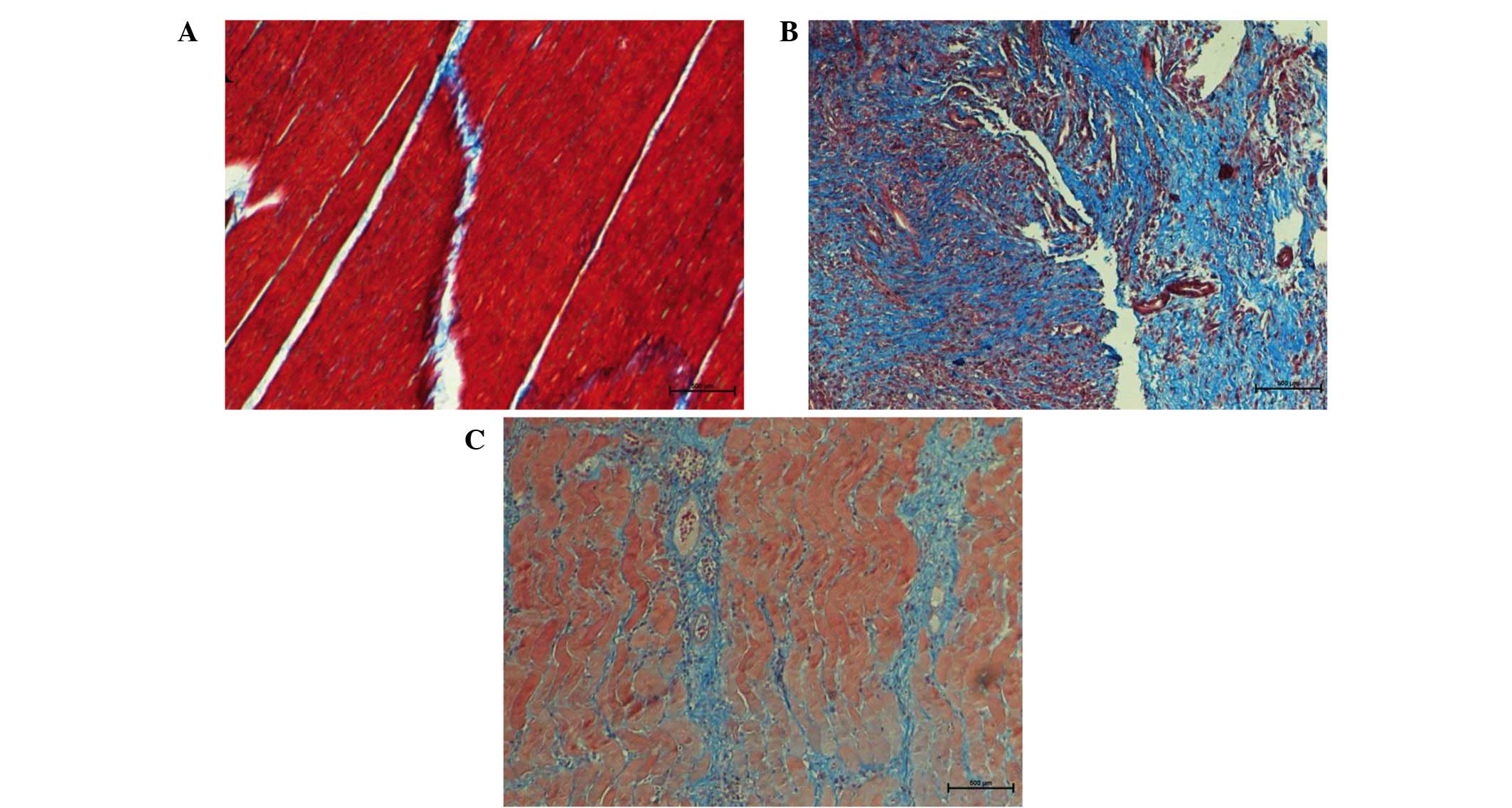
Masson's trichrome staining
Under Masson's trichrome staining, skeletal muscle
fibers appeared red and cell nuclei appeared dark blue, while
collagen fibers appeared light blue. Progressively accumulation of
collagen was considered as a hallmark of tissue fibrosis. In group
A, muscle fibers were regularly arranged with presence of few
detached collagen deposits among muscle fibers (Fig. 3A). In group B, a large amount of blue
proliferating collagen around atrophic muscle fibers was observed,
indicating fibrosis alterations in the skeletal muscle (Fig. 3B). Following treatment with decorin,
collagen production was significantly reduced in group C as
compared with that observed in group B (Fig. 3C).
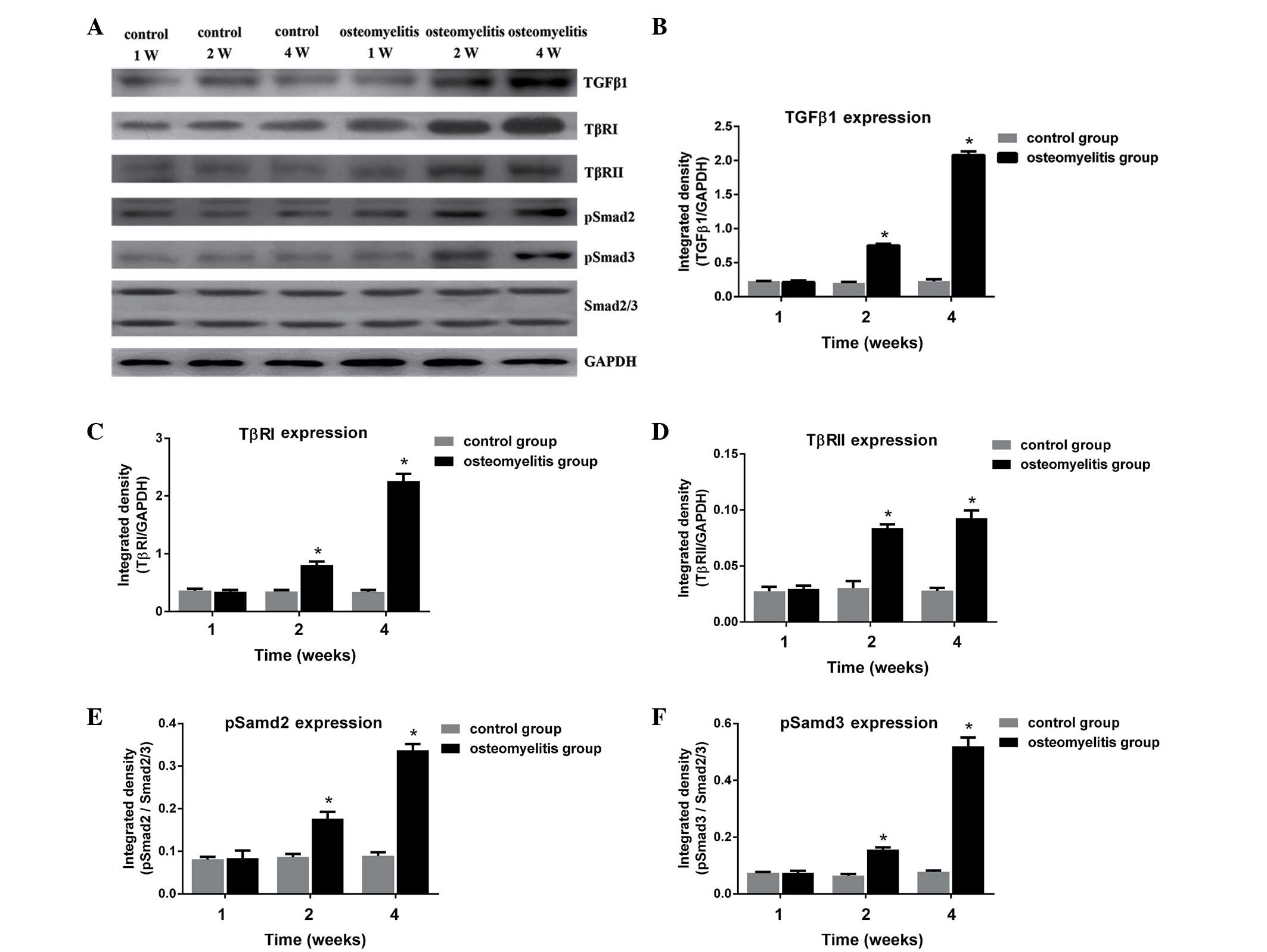
Expression of TGF-β1 and activation of
the TGF-β1/Smad signaling pathway
To determine whether activation of TGF-β1 serves a
role in the fibrosis typically observed in osteomyelitis, the
expression of TGF-β1 and activation of the TGF-β1/Smad signaling
pathway in the rat osteomyelitis model were investigated. As shown
in Fig. 4, TGF-β1, TβRI, TβRII,
pSmad2 and pSmad3 proteins in the control group were expressed at
similar levels at different time points (1, 2 and 4 weeks after
wounding). In group B, the expression levels of TGF-β1, TβRI,
TβRII, pSmad2 and pSmad3 were significantly increased at days 14
and 28 after surgery, when compared with the control group for each
protein (P<0.05). This suggests that the TGF-β1/Smad signaling
pathway served an important role in the fibrosis observed in the
osteomyelitis model group. Furthermore, it indicates that scar
formation occurred at days 14 and 28 after bacterial
inoculation.
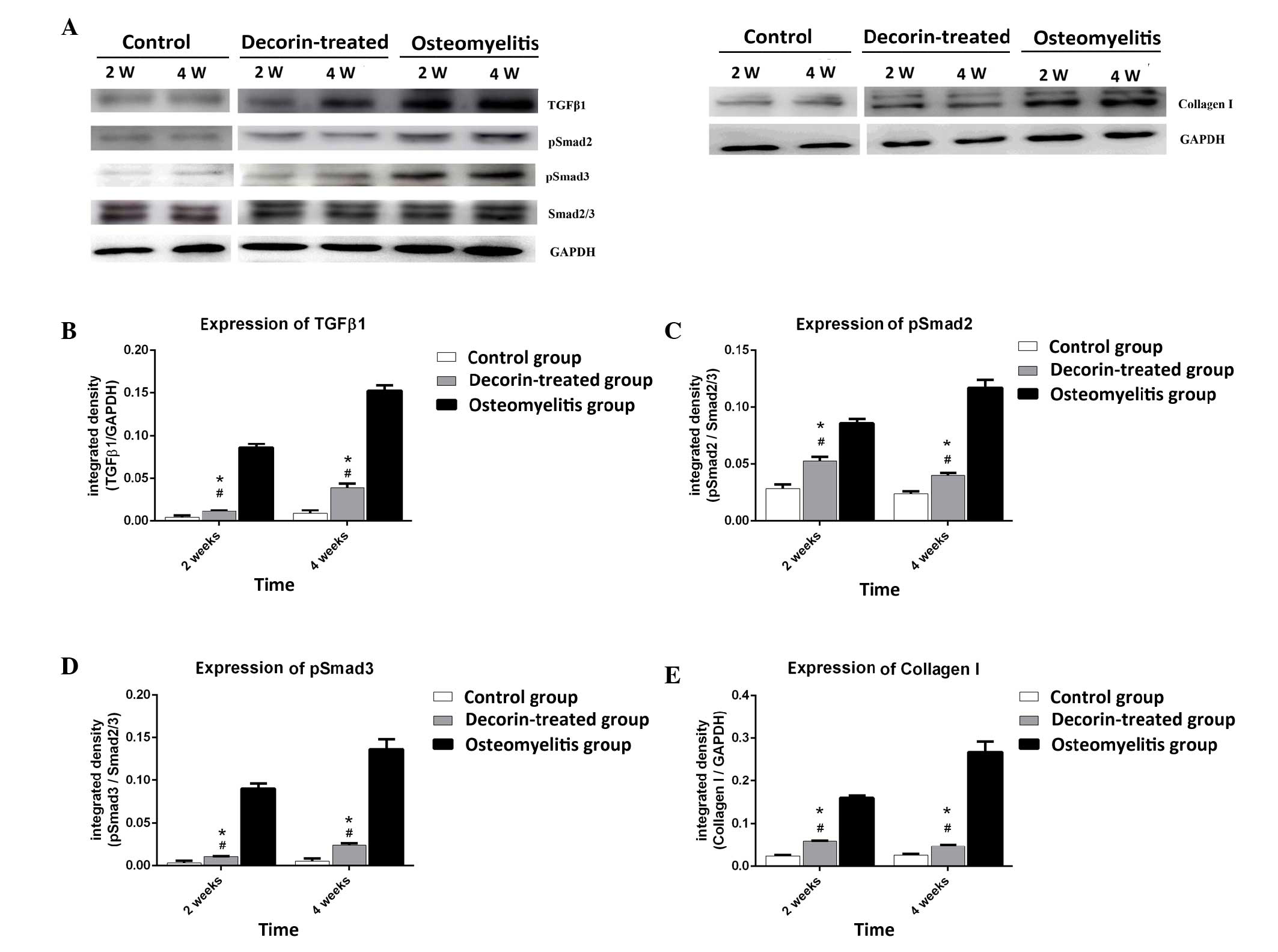
Effect of decorin in blocking muscle
scar formation in osteomyelitis model
In order to further determine whether decorin is
able to reduce muscle scarring in osteomyelitis, the study examined
the expression levels of TGF-β1, pSmad2, pSmad3 and collagen I at
post-wounding days 14 and 28. As shown in Fig. 5, TGF-β1, pSmad2, pSmad3 and collagen
I expression levels in the decorin-treated group were higher than
those in the control group at days 14 and 28 (P<0.05). However,
decorin injection resulted in significant downregulation of these
proteins at days 14 and 28, when compared with the levels in the
osteomyelitis group (P<0.05). This suggests that decorin is able
to reduce the formation of muscle scarring in osteomyelitis through
inhibition of the TGF-β1 signaling pathway.
Discussion
Chronic osteomyelitis is an infection of the bone,
which frequently results in unusual formation of hypertrophic scars
in the surrounding soft tissue (12). Treatment for hypertrophic scarring in
osteomyelitis is challenging, and a good understanding of
underlying molecular processes is essential for the optimal
treatment of such hypertrophic scars (13).
Collagen is the predominant component of scars,
which are elaborated by fibroblasts. The pathogenesis of scar
formation is regulated through a balance between collagen synthesis
and degradation (14). Collagen is a
molecule composed of three polypeptide chains. Cross-links between
staggered collagen molecules provide stability and strength to the
extracellular matrix (15). The scar
tissue is characterized by the overproduction and deposition of
type I and III collagen by fibroblasts (16).
Various cytokines are important components in the
process of collagen synthesis. Among these, TGF-β is a collagen
synthesis stimulator and the most potent inducers of fibrosis
(17). The TGF-β family members
control cell proliferation, differentiation, apoptosis, migration
and extracellular matrix production (18). There are three isoforms of TGF-β
expressed in mammalian tissues: TGF-β1 and TGF-β2 have been
identified as serving major role in tissue fibrosis (19,20),
while TGF-β3 has been shown to reduce scar formation (21). All three isoforms are considered to
function through the TGF-β signaling cascade pathway in wound
healing and abnormal scar formation. TGF-β signaling is initiated
by its binding to membrane receptor type II (namely TβRII).
Subsequent to binding of the ligand, TβRII forms a serine/threonine
kinase complex with TβRI, which leads to the subsequent
intracellular Smad cascade. This initiates the phosphorylation of
Smad2 and Smad3 by TGF-β receptors, resulting in the formation of
complexes with Smad4 (22). The
Smad2-Smad3-Smad4 complex then translocates into the nucleus as a
transcription factor and binds to promoters of type I, II and VII
collagen genes in order to initiate collagen synthesis (23,24). An
increasing number of studies have strongly suggested a correlation
among TGF-β receptor, Smad activity, and fibroblast
overproliferation and collagen production that leads to abnormal
scar formation and pathologic fibrogenesis (25,26).
Due to the close association between TGF-β signaling
and the production of collagen, blocking the TGF-β signaling
pathway may prevent the formation of scars. There are various
negative regulators of TGF-β signaling, including decorin, SnoN and
Ski (27). Decorin is a small
leucine-rich proteoglycan that serves an important role in
regulating a myriad of functions in the extracellular matrix. It
consists of a 40 kD core protein and a glycosaminoglycan side chain
(28,29). Decorin has been shown to bind TGF-β
via the core protein and not the side chain, leading to an
anti-fibrotic response (30).
Previous studies have demonstrated that decorin has a
downregulatory effect on TGF-β production and collagen synthesis in
hypertrophic scar fibroblasts in vitro (31,32).
In vivo experiments, in which decorin was injected or
synthesized from an expression vector, revealed that this
proteoglycan had a beneficial anti-fibrotic effect (33). The role of decorin in anti-fibrotic
activity and differentiation in skeletal muscle by binding TGF-β
and myostatin has been well established (34,35).
Furthermore, the number of fibroblasts in decorin-deficient mice
has been shown to increase by two-fold as compared with that in
wild-type mice (36).
In the present study, an animal model mimicking the
biological development of osteomyelitis was used to determine the
effect of decorin in inhibiting scar formation in osteomyelitis.
The study initially examined the alterations in the expression
levels of TGF-β1 to determine whether the activation of TGF-β1 is
involved in the fibrosis observed in osteomyelitis. The results
revealed an increased expression of TGF-β1 in osteomyelitis. In
order to better understand the possible mechanisms of fibrosis, the
activities of TβRI, TβRII, Smad2 and Smad3 were examined, which are
downstream targets of TGF-β1 mediated signaling. The results
demonstrated the involvement of TGF-β1 signaling in the
transmission of fibrosis signals in osteomyelitis. To further
investigate the effect and mechanism of decorin on reducing scar
formation, the degree of fibrosis in the surrounding tissue was
investigated by Masson's trichrome staining, while the activation
of TGF-β1 signaling was examined, in the decorin-treatment group.
Following decorin treatment, fibrotic changes in the surrounding
tissue were reduced and the activation of TGF-β1 signaling
significantly decreased. This indicated that one of the mechanisms
through which decorin decreased scarring in osteomyelitis was by
inhibiting the TGF-β1 signaling pathway.
However, there are certain limitations to this
study. Although animals received the same dose of MSSA for
osteomyelitis or decorin for treatment, intersubject variability of
the animals may have potentially affected the degree of scarring.
In addition, the anti-fibrotic effect of decorin in muscle tissue
seemed to be dose-dependent and time-dependent. Fukushima et
al (37) revealed that an
injection of 50 µg decorin at 10 and 15 days after injury
significantly decreased the amount of fibrosis. In the present
study, three injections of decorin at a dose of 50 µg each time
were performed, and a significant reduction in fibrosis was
observed. Future studies should investigate the dose and time
responses of the anti-fibrotic effect of decorin, as well as the
underlying molecular mechanism in vitro.
In conclusion, the present study demonstrated the
involvement of the TGF-β1 cascade in scar formation in
osteomyelitis. Decorin was shown to reduce type I collagen
expression in osteomyelitis rats in vivo through the
inhibition of TGF-β1 signaling pathway. Therefore, suppressing
TGF-β1 activity by decorin treatment may be a feasible method to
limit the negative effects of scar formation and promote muscle
functional recovery in osteomyelitis. The use of gene therapy and
cell therapy procedures to deliver a high level of decorin
expression to reduce scar in osteomyelitis will be further
investigated.
References
|
1
|
Lazzarini L, De Lalla F and Mader JT: Long
bone osteomyelitis. Curr Infect Dis Rep. 4:439–445. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eid AJ and Berbari EF: Osteomyelitis:
Review of pathophysiology, diagnostic modalities and therapeutic
options. J Med Liban. 60:51–60. 2012.PubMed/NCBI
|
|
3
|
Papakostidis C, Bhandari M and Giannoudis
PV: Distraction osteogenesis in the treatment of long bone defects
of the lower limbs: Effectiveness, complications and clinical
results; a systematic review and meta-analysis. Bone Joint J 95-B.
1673–1680. 2013. View Article : Google Scholar
|
|
4
|
Lowenberg DW, Buntic RF, Buncke GM and
Parrett BM: Long-term results and costs of muscle flap coverage
with Ilizarov bone transport in lower limb salvage. J Orthop
Trauma. 27:576–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao Z and Xi C: Hepatocyte growth factor
reduces hypertrophy of skin scar: In vivo study. Adv Skin Wound
Care. 26:266–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sriram S, Robinson P, Pi L, Lewin AS and
Schultz G: Triple combination of siRNAs targeting TGFβ1, TGFβR2,
and CTGF enhances reduction of collagen I and smooth muscle actin
in corneal fibroblasts. Invest Ophthalmol Vis Sci. 54:8214–8223.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cutroneo KR, White SL, Phan SH and Ehrlich
HP: Therapies for bleomycin induced lung fibrosis through
regulation of TGF-beta1 induced collagen gene expression. J Cell
Physiol. 211:585–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Z, Li XJ, Liu Y, Zhang X, Li YY and
Xu WS: Recombinant human decorin inhibits cell proliferation and
downregulates TGF-beta1 production in hypertrophic scar
fibroblasts. Burns. 33:634–641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inanmaz ME, Uslu M, Isik C, Kaya E, Tas T
and Bayram R: Extracorporeal shockwave increases the effectiveness
of systemic antibiotic treatment in implant-related chronic
osteomyelitis: Experimental study in a rat model. J Orthop Res.
32:752–756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kishor C, Mishra RR, Saraf SK, Kumar M,
Srivastav AK and Nath G: Phage therapy of staphylococcal chronic
osteomyelitis in experimental animal model. Indian J Med Res.
143:87–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Y, Wei H, Sun R, Tian Z and Zheng X:
Rapid method for protein quantitation by Bradford assay after
elimination of the interference of polysorbate 80. Anal Biochem.
494:37–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orimolade EA, Olabanji JK, Oladele AO and
Yusuf MB: Chronic osteomyelitis in the lower extremity predisposing
to the unusual formation of keloids. Singapore Med J. 52:e190–e193.
2011.PubMed/NCBI
|
|
13
|
Wolfram D, Tzankov A, Pülzl P and
Piza-Katzer H: Hypertrophic scars and keloids - a review of their
pathophysiology, risk factors, and therapeutic management. Dermatol
Surg. 35:171–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia S, Zhao Y, Law M, Galiano R and Mustoe
TA: The effects of collagenase ointment on the prevention of
hypertrophic scarring in a rabbit ear scarring model: A pilot
study. Wounds. 23:160–165. 2011.PubMed/NCBI
|
|
15
|
Boudko SP, Engel J and Bächinger HP: The
crucial role of trimerization domains in collagen folding. Int J
Biochem Cell Biol. 44:21–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhogal RK, Stoica CM, McGaha TL and Bona
CA: Molecular aspects of regulation of collagen gene expression in
fibrosis. J Clin Immunol. 25:592–603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gharaee-Kermani M, Hu B, Phan SH and
Gyetko MR: Recent advances in molecular targets and treatment of
idiopathic pulmonary fibrosis: Focus on TGFbeta signaling and the
myofibroblast. Curr Med Chem. 16:1400–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Whitman M: Smads and early developmental
signaling by the TGFbeta superfamily. Genes. 12:2445–2462. 1998.
View Article : Google Scholar
|
|
19
|
Joyce ME, Roberts AB, Sporn MB and
Bolander ME: Transforming growth factor-beta and the initiation of
chondrogenesis and osteogenesis in the rat femur. J Cell Biol.
110:2195–2207. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou XH, Cao B, Liu HQ, Wang YZ, Bai SF and
Chen H: Effects of osthole on apoptosis and TGF-beta1 of
hypertrophic scar fibroblasts. J Asian Nat Prod Res. 11:663–669.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waddington SN, Crossley R, Sheard V, Howe
SJ, Buckley SM, Coughlan L, Gilham DE, Hawkins RE and McKay TR:
Gene delivery of a mutant TGFβ3 reduces markers of scar tissue
formation after cutaneous wounding. Mol Ther. 18:2104–2111. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massagué J: TGFbeta signaling: Receptors,
transducers and Mad proteins. Cell. 85:947–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peltonen J, Kähäri L, Jaakkola S, Kähäri
VM, Varga J, Uitto J and Jimenez SA: Evaluation of transforming
growth factor beta and type I procollagen gene expression in
fibrotic skin diseases by in situ hybridization. J Invest Dermatol.
94:365–371. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Smith P, Pu LL, Kim YJ, Ko F and
Robson MC: Exogenous transforming growth factor beta(2) modulates
collagen I and collagen III synthesis in proliferative scar
xenografts in nude rats. J Surg Res. 87:194–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan C, Dong Y, Xie Y, Su Y, Zhang X,
Leavesley D and Upton Z: Shikonin reduces TGF-β1-induced collagen
production and contraction in hypertrophic scar-derived human skin
fibroblasts. Int J Mol Med. 36:985–991. 2015.PubMed/NCBI
|
|
26
|
Bai X, He T, Liu J, Wang Y, Fan L, Tao K,
Shi J, Tang C, Su L and Hu D: Loureirin B inhibits fibroblast
proliferation and extracellular matrix deposition in hypertrophic
scar via TGF-β/Smad pathway. Exp Dermatol. 24:355–360. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deheuninck J and Luo K: Ski and SnoN,
potent negative regulators of TGF-beta signaling. Cell Res.
19:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iozzo RV: Matrix proteoglycans: From
molecular design to cellular function. Annu Rev Biochem.
67:609–652. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ameye L and Young MF: Mice deficient in
small leucine-rich proteoglycans: Novel in vivo models for
osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular
dystrophy and corneal diseases. Glycobiology. 12:107R–116R. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miura T, Kishioka Y, Wakamatsu J, Hattori
A, Hennebry A, Berry CJ, Sharma M, Kambadur R and Nishimura T:
Decorin binds myostatin and modulates its activity to muscle cells.
Biochem Biophys Res Commun. 340:675–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed Z, Bansal D, Tizzard K, Surey S,
Esmaeili M, Gonzalez AM, Berry M and Logan A: Decorin blocks
scarring and cystic cavitation in acute and induces scar
dissolution in chronic spinal cord wounds. Neurobiol Dis.
64:163–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Honardoust D, Varkey M, Hori K, Ding J,
Shankowsky HA and Tredget EE: Small leucine-rich proteoglycans,
decorin and fibromodulin, are reduced in postburn hypertrophic
scar. Wound Repair Regen. 19:368–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kolb M, Margetts PJ, Sime PJ and Gauldie
J: Proteoglycans decorin and biglycan differentially modulate
TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung
Cell Mol Physiol. 280:L1327–L1334. 2001.PubMed/NCBI
|
|
34
|
Zhu J, Li Y, Shen W, Qiao C, Ambrosio F,
Lavasani M, Nozaki M, Branca MF and Huard J: Relationships between
transforming growth factor-beta1, myostatin and decorin:
Implications for skeletal muscle fibrosis. J Biol Chem.
282:25852–25863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kishioka Y, Thomas M, Wakamatsu J, Hattori
A, Sharma M, Kambadur R and Nishimura T: Decorin enhances the
proliferation and differentiation of myogenic cells through
suppressing myostatin activity. J Cell Physiol. 215:856–867. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hakkinen L, Strassburger S, Kähäri VM,
Scott PG, Eichstetter I, Lozzo RV and Larjava H: A role for decorin
in the structural organization of periodontal ligament. Lab Invest.
80:1869–1880. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fukushima K, Badlani N, Usas A, Riano F,
Fu F and Huard J: The use of an antifibrosis agent to improve
muscle recovery after laceration. Am J Sports Med. 29:394–402.
2001.PubMed/NCBI
|