Introduction
Preeclampsia is a pregnancy-associated disorder,
characterized by hypertension and proteinuria. It affects
approximately 3% of pregnant women, and contributes substantially
to maternal and fetal morbidity and mortality worldwide (1,2).
Pathophysiology of the disease mainly occurs in early pregnancy due
to a variety of immune and genetic factors, placental ischemia,
oxidative stress and other factors resulting in vascular anomalies
and placental site trophoblastic cell dysfunction, shallow
implantation of the placenta, and increased resistance of uterine
artery blood flow (3,4). This leads to placental hypoperfusion
and functional decline, and hypoxic-ischemic placental oxidative
stress occurs locally, producing a variety of active polypeptide
substances into the maternal circulation, causing maternal systemic
small artery spasm (5). In China,
the incidence of preeclampsia ranges from 9.4 to 10.4% and it
seriously affects preterm labor and fetal growth restriction (FGR)
(6). Inadequate trophoblast invasion
of the maternal spiral arteries during early gestation is thought
to contribute to its etiology.
Currently, women at risk are mainly identified based
on clinical history. Nulliparity is one of the major clinical risk
factors for the development of preeclampsia. Other relevant factors
include an increased body mass index and other medical conditions
such as prepregnancy diabetes, previous preeclampsia, or chronic
hypertension (7,8). However, screening by maternal history
alone detects only 30% of women who may develop preeclampsia. The
clinical risk-based strategy is not effective for nulliparous women
without other risk factors. With increased understanding of the
pathogenesis of preeclampsia, clinical prediction models of the
disease have gradually diversified (9). Doppler has more recently shown some
promise. Indeed, Campbell first introduced color Doppler ultrasound
to investigate the uteroplacental circulation. Uterine artery
pulsatility index (PI) has been used as a marker of preeclampsia
and FGR, in the presence of which PI increases due to the elevation
in uterine artery impedance (10).
Although much research into the mechanism of
preeclampsia has been carried out, its exact pathogenesis remains
poorly known. Recently, studies have found that preeclampsia is
associated with a decreased level of serum placental growth factor
(PlGF), as well as increased levels of inhibin A and activin A
(11,12), although a systematic review of
screening tests for preeclampsia have concluded that no single test
is yet available to provide good diagnostic accuracy (7,13). A
combined screening involving several relevant markers is more
likely to provide the best prediction. First-trimester screening
would represent a major advantage over a second-trimester approach
since it opens prospects for early and more efficient interventions
(14,15). Accordingly, we aimed at evaluating
whether the measurement of maternal serum inhibin A, activin A and
PlGF at 3–4 months' gestation or a combination of these biochemical
markers with the second-trimester uterine artery PI are useful in
predicting preeclampsia in a group of nulliparous women.
Subjects and methods
Ethics statement
The Medical Ethics Committee of the Zhejiang
Provincial People's Hospital (Zhejiang, China) approved this study.
Written informed consent conforming to the tenets of the
Declaration of Helsinki were obtained from each participant prior
to the study.
Participants
In this prospective cohort study, a total of 800
pregnant women at the time of screening for Down syndrome at 11–13
weeks, and 100 gestational age-matched normal pregnancies as
controls were recruited at the Department of Urology, The First
Hospital of Zhejiang Province, from January 2013 to January 2015.
For cases in which no major fetal defect was detected, women were
invited to undergo an additional screening study for preeclampsia
and blood samples were taken, centrifuged to extract the serum and
stored at −80°C for subsequent analysis. Multiparous women,
multiple gestations, and pregnancies with a major fetal chromosomal
or structural anomaly were excluded from further analysis.
Doppler measurement
An ultrasound examination was carried out for
diagnosis of major fetal defects, measurement of nuchal
translucency thickness, and crown-rump length (CRL), which was used
to determined gestational age. Furthermore, participants underwent
Doppler measurement of the uterine artery PI at 22–24 weeks,
performed by experienced sonographers who had obtained the Fetal
Medicine Foundations Certificate of Competence in Placental and
Fetal Doppler. Uterine artery PI was calculated as the mean PI from
three similar consecutive waveforms. All examinations were
performed transabdominally using a C3-5-MHz curvilinear transducer
(Envisor 2540A; Philips Medical Systems, Shenyang, China). The
results of trimester Doppler were not communicated to the
physicians in charge of the follow-up and were not recorded in the
ultrasound report.
Diagnostic of preeclampsia
Preeclampsia was defined as systolic blood pressure
≥140 mmHg and/or diastolic blood pressure ≥90 mmHg on at least two
occasions 4 h apart, developing after 20 weeks of gestation in
previously normotensive women with proteinuria of 300 mg or more in
24 h, or two readings of at least ++ on dipstick analysis of
midstream or catheter urine specimens if 24-h urine collection was
not available.
Measurement of serum markers
Maternal nonfasting blood samples were collected,
immediately centrifuged for 10 min at 3,800 × g, divided into 4
aliquots, and frozen at −80°C until used for the analyses. The
samples were tested for inhibin A, activin A and PlGF levels using
solid-phase sandwich enzyme linked immunosorbent assay (ELISA)
(R&D Systems, Minneapolis, MN, USA) by technicians who were
blinded to the clinical outcome. According to the manufacturer,
fresh quality-control samples were used at concentrations of 50 and
1000 pg/ml for PlGF, 50 and 1000 pg/ml for inhibin A, and 50 and
2500 pg/ml for activin A. The interassay coefficients of variation
for the respective low and high concentrations were 5.64 and 7.96%
for PlGF, 6.89 and 11.56% for inhibin A and 4.27 and 9% for activin
A.
Statistical analysis
The inhibin A, activin A and PlGF results are
expressed as multiples of the median (MoM). Basic demographic
characteristics including weight and height as well as serum
inhibin A, activin A and PlGF results in pregnancies with
preeclampsia and normal pregnancies were compared by unpaired
t-test. The sensitivity and specificity for different cut-offs of
each variable in detecting preeclampsia were calculated and
depicted as receiver operating characteristic (ROC) curves.
Multiple logistic regression analysis was used to model the
combination of inhibin A, activin A, PlGF and uterine artery PI.
The statistical software package SPSS 18.0 (SPSS, Inc., Chicago,
IL, USA) was used for all data analysis.
Results
A total of 800 pregnant women were recruited for the
study. Forty women (5.0%) did not deliver at our hospital and were
lost to follow-up, leaving a study cohort of 760 pregnancies.
Thirty-eight women developed preeclampsia, giving an incidence of
5.2%. There were 100 gestational age-matched normal pregnancies as
controls. At the time of blood sampling there was no statistically
significant difference in maternal age, body mass index and
gestational age, between the women that developed preeclampsia and
the normal controls (Table I).
However, gestational age at delivery and birth weight were lower in
pregnancies that developed preeclampsia compared with the
controls.
 | Table I.Demographic characteristics of the
women with preeclampsia in the study group and the normal
controls. |
Table I.
Demographic characteristics of the
women with preeclampsia in the study group and the normal
controls.
| Characteristics | Cases (39) | Controls (100) | P-value |
|---|
| Total no. | 39 | 100 | – |
| Primiparous | 38 | 95 | – |
| Pluriparous | 1 | 5 | – |
| Maternal age
(years) | 27.12±3.12 | 28.67±3.51 | 0.06 |
| Maternal BMI
(kg/m2) | 21.61±2.17 | 21.43±2.52 | 0.21 |
| GA at blood sample
(days) | 101.01±6.23 | 102.49±5.86 | 0.73 |
| Systolic BP
(mmHg) | 151.03±8.13 | 121.33±9.91 | <0.001 |
| Diastolic BP
(mmHg) | 98.17±4.34 | 72.51±6.99 | <0.001 |
| GA at delivery
(weeks) | 35.12±2.34 | 39.51±1.24 | <0.001 |
| Birth weight (g) | 2,715.11±576.72 | 3,243.51±324.21 | <0.001 |
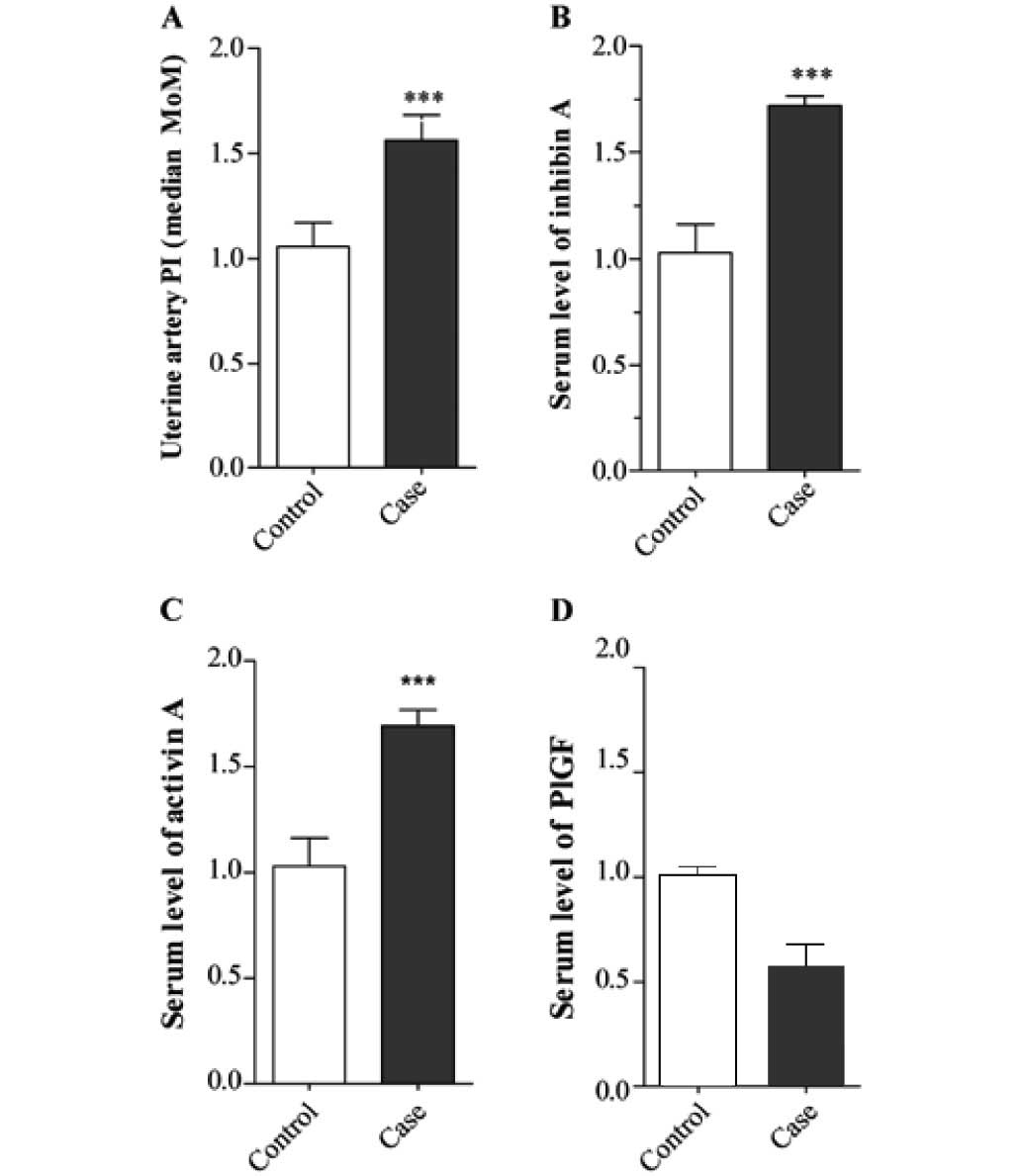
In pregnancies that developed preeclampsia, the
uterine artery PI was increased (1.61±0.047 vs. 1.02±0.049,
P<0.001; Fig. 1A), as was the
level of inhibin A (1.72±0.023 vs. 1.03±0.063, P<0.001; Fig. 1B) and the level of activin A as
compared with the controls (1.68±0.38 vs. 1.06±0.42, P<0.001;
Fig. 1C). In contrast, the level of
PlGF was decreased in pregnancies that developed preeclampsia
compared with the controls (0.69±0.23 vs. 1.00±0.26, P<0.001;
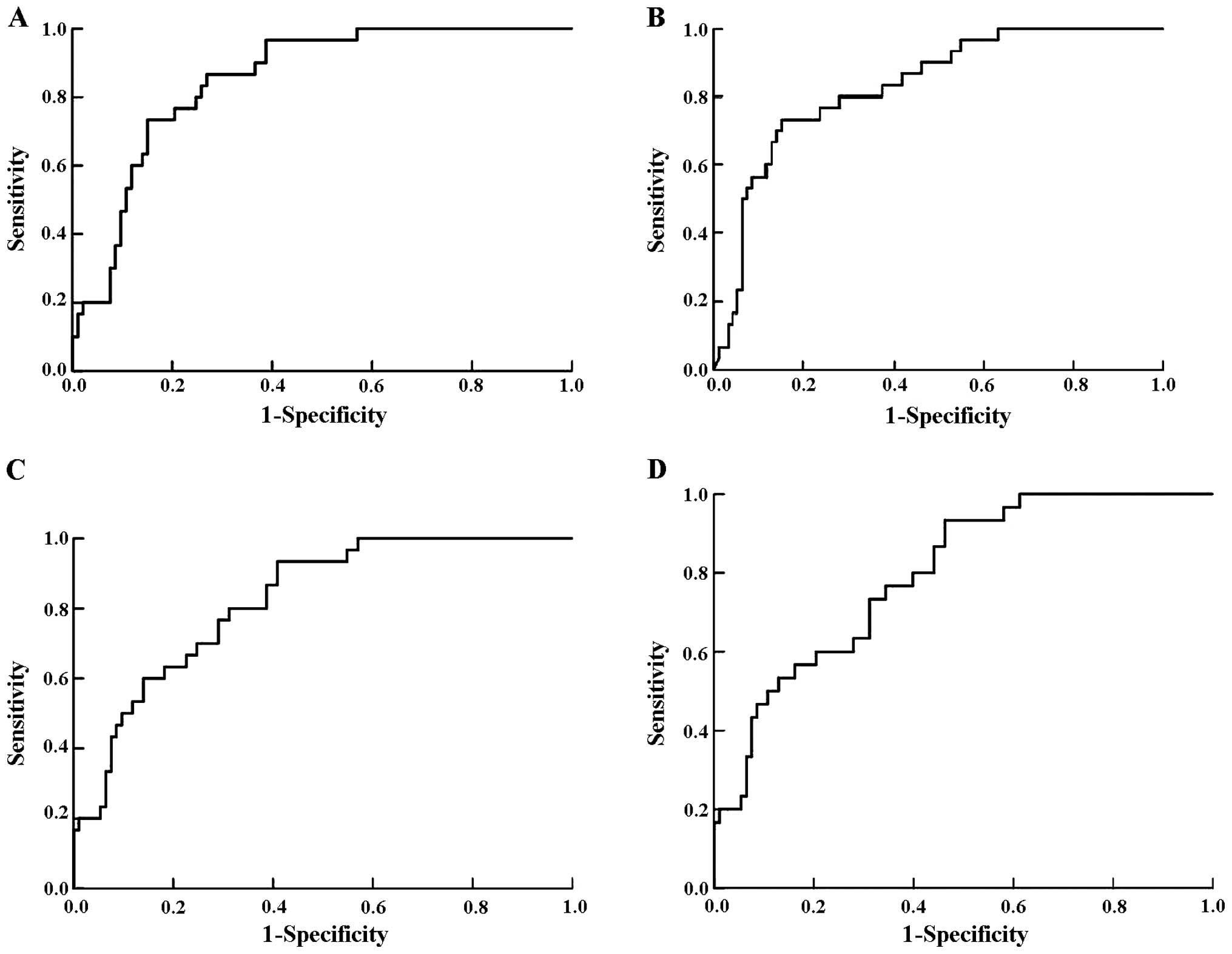
Fig. 1D). Fig. 2 shows the ROC curves for each
marker.
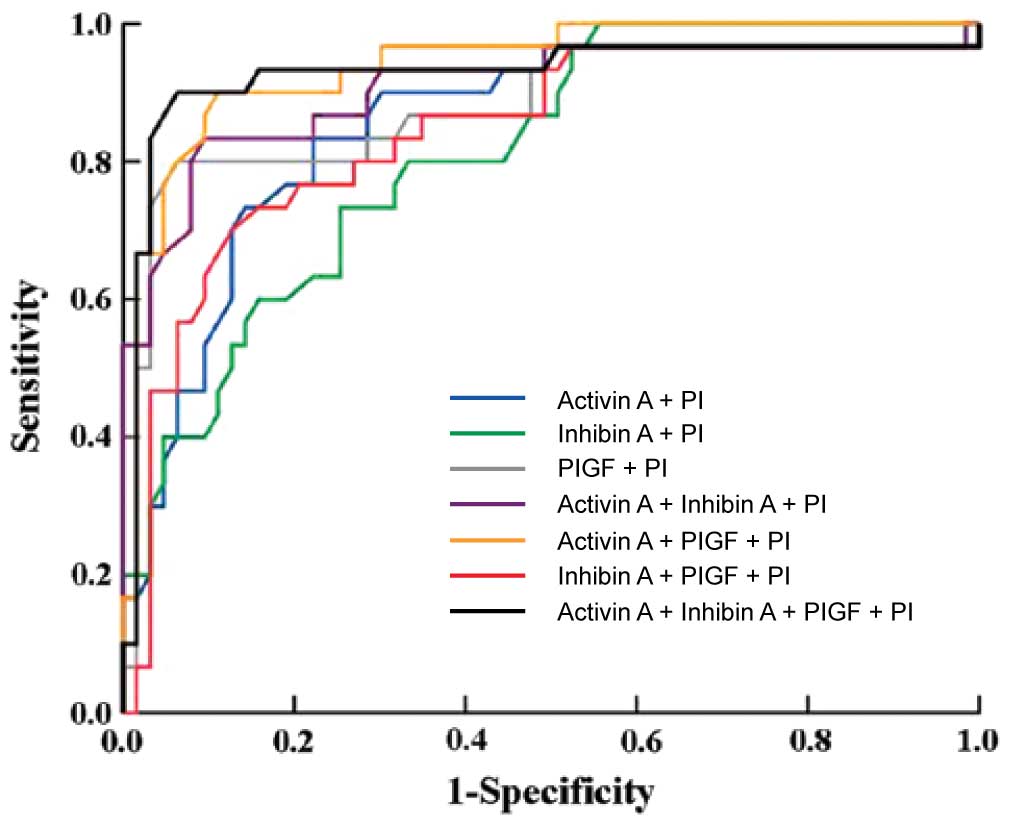
The ROC curves for combinations of the markers in
the prediction of preeclampsia are shown in Fig. 3. Combining activin A, inhibin A and
uterine artery PI using logistic regression analysis yielded an
area under the curve (AUC) of 0.917 [95% confidence interval (CI),
0.820–0.918; P<0.001] with a sensitivity of 84% at a specificity
of 81%. A combination of activin A, PlGF and uterine artery PI gave
an AUC of 0.915 (95% CI, 0.812–0.928; P<0.001) with a
sensitivity of 91% at a specificity of 82%. The combination of all
four markers gave an AUC of 0.942 (95% CI, 0.82–0.991; P<0.001),
with a sensitivity of 92% at a specificity of 81%.
Discussion
Preeclampsia is a disorder of pregnancy
characterized by high blood pressure and a large amount of protein
in the urine (16,17). The disorder usually occurs in the
third-trimester of pregnancy and gets worse over time. In severe
disease there may be red blood cell breakdown, a low blood platelet
count, impaired liver function, kidney dysfunction, swelling,
shortness of breath due to fluid in the lungs, or visual
disturbances. Preeclampsia increases the risk of poor outcomes for
both the mother and the baby. If left untreated, it results in
seizures at which point it is known as eclampsia.
There have been many assessments of tests aimed at
predicting preeclampsia, although no single biomarker is likely to
be sufficiently predictive of the disorder (18–20).
Predictive tests that have been assessed include those related to
placental perfusion, vascular resistance, kidney dysfunction,
endothelial dysfunction, and oxidative stress. A combined screening
involving several relevant markers is more likely to provide the
best prediction. First-trimester screening would represent a major
advantage over a second-trimester approach since it opens prospects
for early and more efficient interventions. The aim of this study
was to evaluate, in a group of nulliparous women, whether the
measurement of maternal serum inhibin A, activin A and PlGF at 3–4
months gestation or a combination of these biochemical markers with
the second-trimester uterine artery PI are useful in predicting
preeclampsia.
Uterine artery PI is an important marker for the
prediction of preeclampsia. In our study, the sensitivity was 76%
with the specificity set at 80% and it dropped slightly to 57% for
a specificity of 90%. Consistent with the literature, we showed an
increased uterine artery PI during the late second-trimester in
patients who developed preeclampsia (14,21–25).
The clinical manifestations of preeclampsia are
associated with general endothelial dysfunction, including
vasoconstriction and end-organ ischemia. Implicit in this
generalized endothelial dysfunction may be an imbalance of
angiogenic and anti-angiogenic factors (26–28).
Both circulating and placental levels of soluble fms-like tyrosine
kinase-1 (sFlt-1) are higher in women with preeclampsia than in
women with normal pregnancy (29–31).
sFlt-1 is an anti-angiogenic protein that antagonizes vascular
endothelial growth factor (VEGF) and PIGF (32–34),
both of which are pro-angiogenic factors. Soluble endoglin (sEng)
has also been shown to be elevated in women with preeclampsia and
has anti-angiogenic properties, similar to sFlt-1. Both sFlt-1 and
sEng are upregulated in all pregnant women to some extent,
supporting the idea that hypertensive disease in pregnancy is a
normal pregnancy adaptation gone awry. As natural killer cells are
intimately involved in placentation and placentation involves a
degree of maternal immune tolerance for a foreign placenta, it is
not surprising that the maternal immune system may respond more
negatively to the arrival of some placentae under certain
circumstances, such as a placenta which is more invasive than
normal. Initial maternal rejection of the placental
cytotrophoblasts may be the cause of the inadequately remodeled
spiral arteries in those cases of preeclampsia associated with
shallow implantation, leading to downstream hypoxia and the
appearance of maternal symptoms in response to upregulated sFlt-1
and sEng.
Inhibin A has been reported as an early marker in
predicting preeclampsia. Roes et al took blood samples at
7–13 weeks gestation from 90 pregnant women, 30 who later developed
preeclampsia and 60 controls, and found that inhibin A was
increased 5-fold in the women with preeclampsia. Another study
(35) reported a sensitivity of
inhibin A in the prediction of preeclampsia of 32% at a specificity
of 90%, concluding that the sensitivity of inhibin A was too low
for it to be useful as a marker for predicting preeclampsia, but
that, combined with other markers, it could play an important role.
Activin A has also been proposed as a marker for predicting
preeclampsia. The level was elevated at 10–14 weeks gestation in
women with established preeclampsia (36). In a case-control study, the
sensitivity was 82% at a specificity of 91%. Published data
regarding PlGF in the prediction of preeclampsia are controversial.
A sensitivity of 80.4% at a specificity of 78% has been reported. A
prospective study documented that PlGF was decreased distinctly in
early pregnancy in women who subsequently developed preeclampsia,
and that the degree of decrease was related to the severity of the
disease (37).
In our study, we found that both serum inhibin A and
activin A levels were increased, while the PlGF level was decreased
in the early second-trimester in women who developed preeclampsia.
It should be borne in mind that the population of our study was
entirely ethnic Chinese, and further study is required to determine
whether levels of these biochemical markers differ among different
ethnic groups. Furthermore, while it seems that the prediction of
preeclampsia is technically feasible using inhibin A, activin A,
PlGF and uterine artery Doppler, the cost-benefit balance of this
screening needs to be evaluated. In order to accommodate the
numerous scans required to perform uterine artery Doppler, more
qualified sonographers would need to be trained.
In conclusion, early second-trimester serum inhibin
A, activin A, PlGF and second-trimester uterine artery Doppler PI
may add further information for the prediction of preeclampsia. The
combination of the three serum markers and uterine artery Doppler
PI has the highest prediction value for preeclampsia.
Acknowledgements
This study was supported by grants from Zhejiang
Medical Science and Technology Project.
References
|
1
|
Hodgins S: Pre-eclampsia as underlying
cause for perinatal deaths: time for action. Glob Health Sci Pract.
3:525–527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim J, Cloete G, Dunsmuir DT, Payne BA,
Scheffer C, von Dadelszen P, Dumont GA and Ansermino JM: Usability
and feasibility of PIERS on the move: an mHealth App for
pre-eclampsia triage. JMIR Mhealth Uhealth. 3:e372015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaiworapongsa T, Chaemsaithong P, Yeo L
and Romero R: Pre-eclampsia part 1: current understanding of its
pathophysiology. Nat Rev Nephrol. 10:466–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cornelius DC and Lamarca B: TH17- and
IL-17-mediated autoantibodies and placental oxidative stress play a
role in the pathophysiology of pre-eclampsia. Minerva Ginecol.
66:243–249. 2014.PubMed/NCBI
|
|
5
|
Craici IM, Wagner SJ, Weissgerber TL,
Grande JP and Garovic VD: Advances in the pathophysiology of
pre-eclampsia and related podocyte injury. Kidney Int. 86:275–285.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu J, Shixia CZ, Wu Y and Duan T: Inhibin
A, activin A, placental growth factor and uterine artery Doppler
pulsatility index in the prediction of pre-eclampsia. Ultrasound
Obstet Gynecol. 37:528–533. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghosh SK, Raheja S, Tuli A, Raghunandan C
and Agarwal S: Is serum placental growth factor more effective as a
biomarker in predicting early onset preeclampsia in early second
trimester than in first trimester of pregnancy? Arch Gynecol
Obstet. 287:865–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weed S, Bastek JA, Anton L, Elovitz MA,
Parry S and Srinivas SK: Examining the correlation between
placental and serum placenta growth factor in preeclampsia. Am J
Obstet Gynecol. 207:140.e1–6. 2012. View Article : Google Scholar
|
|
9
|
Woodham PC, Brittain JE, Baker AM, Long
DL, Haeri S, Camargo CA Jr, Boggess KA and Stuebe AM: Midgestation
maternal serum 25-hydroxyvitamin D level and soluble fms-like
tyrosine kinase 1/placental growth factor ratio as predictors of
severe preeclampsia. Hypertension. 58:1120–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shokry M, Bedaiwy MA, Fathalla MM,
Alsemary A, Elwakil S and Murphy A: Maternal serum placental growth
factor and soluble fms-like tyrosine kinase 1 as early predictors
of preeclampsia. Acta Obstet Gynecol Scand. 89:143–146. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai J, Larroca S Garcia-Tizon, Peeva G,
Poon LC, Wright D and Nicolaides KH: Competing risks model in
screening for preeclampsia by serum placental growth factor and
soluble fms-like tyrosine kinase-1 at 30–33 weeks gestation. Fetal
Diagn Ther. 35:240–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanita O, Alia NN, Zaleha AM and Nor Azlin
MI: Serum soluble FMS-like tyrosine kinase 1 and placental growth
factor concentration as predictors of preeclampsia in high risk
pregnant women. Malays J Pathol. 36:19–26. 2014.PubMed/NCBI
|
|
13
|
Ghosh SK, Raheja S, Tuli A, Raghunandan C
and Agarwal S: Serum placental growth factor as a predictor of
early onset preeclampsia in overweight/obese pregnant women. J Am
Soc Hypertens. 7:137–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Odibo AO, Rada CC, Cahill AG, Goetzinger
KR, Tuuli MG, Odibo L, Macones GA and England SK: First-trimester
serum soluble fms-like tyrosine kinase-1, free vascular endothelial
growth factor, placental growth factor and uterine artery Doppler
in preeclampsia. J Perinatol. 33:670–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh SK, Raheja S, Tuli A, Raghunandan C
and Agarwal S: Can maternal serum placental growth factor
estimation in early second trimester predict the occurrence of
early onset preeclampsia and/or early onset intrauterine growth
restriction? A prospective cohort study. J Obstet Gynaecol Res.
39:881–890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demers S, Bujold E, Arenas E, Castro A and
Nicolaides KH: Prediction of recurrent preeclampsia using
first-trimester uterine artery Doppler. Am J Perinatol. 31:99–104.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai J, Poon LC, Pinas A, Bakalis S and
Nicolaides KH: Uterine artery Doppler at 30–33 weeks gestation in
the prediction of preeclampsia. Fetal Diagn Ther. 33:156–163. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kafkaslı A, Türkçüoğlu I and Turhan U:
Maternal, fetal and perinatal characteristics of preeclampsia cases
with and without abnormalities in uterine artery Doppler indexes. J
Matern Fetal Neonatal Med. 26:936–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou A, Dekker GA, Lumbers ER, Lee SY,
Thompson SD, McCowan LM and Roberts CT: SCOPE consortium: The
association of AGTR2 polymorphisms with preeclampsia and uterine
artery bilateral notching is modulated by maternal BMI. Placenta.
34:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Llurba E, Sánchez O, Domínguez C, Soro G,
Goya M, Alijotas-Reig J and Cabero L: Smoking during pregnancy:
changes in mid-gestation angiogenic factors in women at risk of
developing preeclampsia according to uterine artery Doppler
findings. Hypertens Pregnancy. 32:50–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gallo DM, Poon LC, Akolekar R, Syngelaki A
and Nicolaides KH: Prediction of preeclampsia by uterine artery
Doppler at 20–24 weeks gestation. Fetal Diagn Ther. 34:241–247.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi K, Ohkuchi A, Suzuki H, Usui R,
Kuwata T, Shirasuna K, Matsubara S and Suzuki M: Biophysical
interaction between blood pressure and uterine artery Doppler for
the occurrence of early-onset preeclampsia: a prospective cohort
study. Pregnancy Hypertens. 3:270–277. 2013.PubMed/NCBI
|
|
23
|
Goetzinger KR, Zhong Y, Cahill AG, Odibo
L, Macones GA and Odibo AO: Efficiency of first-trimester uterine
artery Doppler, a-disintegrin and metalloprotease 12,
pregnancy-associated plasma protein a, and maternal characteristics
in the prediction of preeclampsia. J Ultrasound Med. 32:1593–1600.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weintraub AY, Aricha-Tamir B, Steiner N,
Hamou BE, Baron J and Hershkovitz R: Postpartum uterine artery
Doppler velocimetry among patients following a delivery complicated
with preeclampsia. Hypertens Pregnancy. 32:450–458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diguisto C, Le Gouge A, Piver E, Giraudeau
B and Perrotin F: Second-trimester uterine artery Doppler, PlGF,
sFlt-1, sEndoglin, and lipid-related markers for predicting
preeclampsia in a high-risk population. Prenat Diagn. 33:1070–1074.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lehnen H, Mosblech N, Reineke T, Puchooa
A, Menke-Möllers I, Zechner U and Gembruch U: Prenatal clinical
assessment of sFlt-1 (soluble fms-like tyrosine kinase-1)/PlGF
(placental growth factor) ratio as a diagnostic tool for
preeclampsia, pregnancy-induced hypertension, and proteinuria.
Geburtshilfe Frauenheilkd. 73:440–445. 2013.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hassan MF, Rund NM and Salama AH: An
elevated maternal plasma soluble fms-like tyrosine kinase-1 to
placental growth factor ratio at midtrimester is a useful predictor
for preeclampsia. Obstet Gynecol Int. 2013:2023462013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bdolah Y, Elchalal U, Natanson-Yaron S,
Yechiam H, Bdolah-Abram T, Greenfield C, Goldman-Wohl D, Milwidsky
A, Rana S, Karumanchi SA, et al: Relationship between nulliparity
and preeclampsia may be explained by altered circulating soluble
fms-like tyrosine kinase 1. Hypertens Pregnancy. 33:250–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rizos D, Eleftheriades M, Karampas G,
Rizou M, Haliassos A, Hassiakos D and Vitoratos N: Placental growth
factor and soluble fms-like tyrosine kinase-1 are useful markers
for the prediction of preeclampsia but not for small for
gestational age neonates: a longitudinal study. Eur J Obstet
Gynecol Reprod Biol. 171:225–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohkuchi A, Hirashima C, Takahashi K,
Suzuki H, Matsubara S and Suzuki M: Onset threshold of the plasma
levels of soluble fms-like tyrosine kinase 1/placental growth
factor ratio for predicting the imminent onset of preeclampsia
within 4 weeks after blood sampling at 19–31 weeks of gestation.
Hypertens Res. 36:1073–1080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang QT, Wang SS, Zhang M, Huang LP, Tian
JW, Yu YH, Wang ZJ and Zhong M: Advanced oxidation protein products
enhances soluble Fms-like tyrosine kinase 1 expression in
trophoblasts: a possible link between oxidative stress and
preeclampsia. Placenta. 34:949–952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ben Ali Gannoun M, Bourrelly S, Raguema N,
Zitouni H, Nouvellon E, Maleh W, Chemili A Brahim, Elfeleh R,
Almawi W, Mahjoub T, et al: Placental growth factor and vascular
endothelial growth factor serum levels in Tunisian Arab women with
suspected preeclampsia. Cytokine. 79:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moore GS, Allshouse AA, Winn VD, Galan HL
and Heyborne KD: Baseline placental growth factor levels for the
prediction of benefit from early aspirin prophylaxis for
preeclampsia prevention. Pregnancy Hypertens. 5:280–286.
2015.PubMed/NCBI
|
|
34
|
Tsiakkas A, Cazacu R, Wright A, Wright D
and Nicolaides KH: Maternal serum placental growth factor at 12,
22, 32 and 36 weeks gestation in screening for pre-eclampsia.
Ultrasound Obstet Gynecol. 47:472–477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roes EM, Gaytant MA, Thomas CM, Raijmakers
MT, Zusterzeel PL, Peters WH and Steegers EA: First trimester
inhibin-A concentrations and later development of preeclampsia.
Acta Obstet Gynecol Scand. 83:1172004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ong CY, Liao AW, Munim S, Spencer K and
Nicolaides KH: First-trimester maternal serum activin A in
pre-eclampsia and fetal growth restriction. J Matern Fetal Neonatal
Med. 15:176–180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taylor RN, Grimwood J, Taylor RS, McMaster
MT, Fisher SJ and North RA: Longitudinal serum concentrations of
placental growth factor: evidence for abnormal placental
angiogenesis in pathologic pregnancies. Am J Obstet Gynecol.
188:177–182. 2003. View Article : Google Scholar : PubMed/NCBI
|