Introduction
Necrotizing enterocolitis (NEC) is a severe
gastrointestinal tract disease that affects newborn babies. Despite
years of investigation, the etiology remains unclear, and accepted
prevention and treatment strategies are lacking (1). The immature intestinal barrier function
and immune/inflammatory responses have been partly linked to NEC
(2). Loss of mucosal integrity due
to a variety of factors (ischemia, infection and inflammation) and
the host response to injury (circulatory, immunologic and
inflammatory) are the leading causes of necrosis of the affected
area (3). In infants with NEC, a
compromised epithelial barrier and immature immune response can
lead to exaggerated inflammation and oxidative stress damage. The
excessive inflammatory and oxidative stress is implicated in the
pathogenesis of NEC (4).
Vitamin D3-upregulated protein 1 (VDUP1) is a 46-kDa
intracellular protein that was initially isolated in HL-60 cells,
and its expression is upregulated by vitamin D3 administration
(5). VDUP1 interacts with the
antioxidant thioredoxin; hence, it is considered to increase the
vulnerability of cells to oxidative stress (6). VDUP1 is upregulated by various
stresses, including H2O2, irradiation, heat
shock, serum starvation and transforming growth factor-β (7). Although deficits in antioxidants and
increased oxidative stress accompanying NEC rat have been directly
implicated in the pathogenesis, a possible role for VDUP1 in the
adverse outcomes accompanying NEC has not yet been
investigated.
The nuclear factor (NF)-κB transcription factor
family is a crucial mediator of the inflammatory process and is
important in other processes, including cell development, growth,
survival and proliferation. NF-κB can be activated by numerous
stimuli including inflammatory factors, microbial molecules and
genotoxic tension (8). Recent
studies have demonstrated the pathological involvement of the
immune system in NEC (2,9). The levels of pro-inflammatory cytokines
are increased in the intestines in NEC patients and animals
(10). It has also been reported
that pro-inflammation may be significant in the pathogenesis of NEC
(11). In addition, correlative
studies have demonstrated that antioxidants reduced NEC severity in
in vivo experimental models (12). There is a close association between
oxidative stress and inflammation in NEC and we hypothesize that an
increase in oxidative stress-derived inflammation is one of the
primary mechanisms underlying the pathogenesis and progression of
NEC.
Astragaloside IV (AS-IV) is a major saponin purified
from Astragalus membranaceus (Fisch) Bge, and has been
widely used as a herbal prescription in traditional chinese
medicine for its anti-inflammatory, antioxidative, carioprotective
and immune-system stimulatory activities (13,14).
Recent evidence suggests that AS-IV is able to ameliorate ischemic
brain injury, diabetic peripheral neuropathy, hepatic fibrosis and
myocardial injury (15). In
addition, AS-IV has been demonstrated to inhibit the
pro-inflammatory transcriptional factor, NF-κB, tumor necrosis
factor (TNF)-α and mediate the p38 mitogen-activated protein kinase
signaling pathway (16).
However, the beneficial effect of AS-IV on NEC
remains to be investigated. In the present study, we hypothesized
that AS-IV, which has protective effects in an NEC murine model,
may also be associated with the inhibition of inflammation and
antioxidative effects (15,17). The present study was undertaken to
evaluate the protective effects of AS-IVs and to elucidate the
mechanism underlying these protective effects in rats.
Materials and methods
Materials
AS-IV (pure: 98%) was purchased from the National
Institutes for Food and Drug Control (Beijing, China). The
enzyme-linked immunosorbent assay (ELISA) kits for determination of
interleukin (IL)-6 (cat. no. R6000B), IL-1β (cat. no. RLB00) and
TNF-α (cat. no. RTA00) were purchased from R&D Systems
(Minneapolis, MN, USA). The diagnostic kits for glutathione (GSH;
cat. no. A006-2), malondialdehyde (MDA; cat. no. A003-1),
superoxide dismutase (SOD; cat. no. A001-1) and myeloperoxidase
(MPO; cat. no. A044) were purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Primary antibodies for
IκBα (1:1,000; mouse monoclonal; cat. no. 4814), phosphorylated
(p)-IκBα (1:1,000; mouse monoclonal; cat. no. 9246), NF-κBp65
(1:1,000; mouse monoclonal; cat. no. 6956), p-NF-κBp65 (1:1,000;
rabbit monoclonal; cat. no. 3039) and VDUP1 (1:1,000; rabbit
monoclonal; cat. no. 14715) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). In addition, secondary
antibodies were acquired from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Animals
Animal experiments were approved by the
Institutional Animal Care and Use Committee, Nanjing Medical
University (Nanjing, China). A total of 40 Sprague-Dawley murine
neonates (15 days old, 20–35 g) were used in the present study and
kept under controlled conditions. The murine neonates were kept
with their mothers in indistinguishable cages (1 cage per group of
10 neonates). Tap water and standard rodent food supplement were
used to feed the mothers. During the study period, the neonates
were fed freely on breast milk by their mothers. The experimental
animals were maintained in a controlled environment (12:12±1-h
light/dark cycle; temperature, 22±3°C; relative humidity, 55%).
Furthermore, the rats were allowed to acclimatize to the laboratory
for ≥7 days under climate-controlled conditions. The experiments
were performed in adherence to the guidelines for the Care and Use
of Laboratory Animals of the National Institute of Health.
Experimental design
Experimental NEC was induced by asphyxia (breathing
100% nitrogen gas for 2 min) and hypothermia (4°C for 10 min) twice
daily for 3 consecutive days. Age-matched normal rats served as a
control. Neonatal rats were divided into the following experimental
groups (n=10 for all groups): NEC control rats treated with saline
solution for 4 days (group 1), NEC rats treated with 25 mg/kg/d
AS-IV for 4 days (group 2), NEC rats treated with 50 mg/kg/d AS-IV
for 4 days (group 3) and NEC rats treated with 75 mg/kg/d AS-IV for
4 days (group 4). On the fourth day, the animals were sacrificed
via decapitation, and the biochemical estimations were completed on
the same day.
Histopathologic examination
To investigate the effects of AS-IV on the
protection against NEC, hematoxylin and eosin staining (H&E)
was performed. Upon sacrifice, the intestines from the rats in all
of the groups were resected. For each tissue, corresponding blocks
of H&E were scored (18).
Pathological changes in intestinal architecture were evaluated
using the NEC histological injury scoring system described by
Nadler et al (19). The
grading system was as follows: Grade 0, normal; grade 1 (mild),
separation of villous core, without other abnormalities; grade 2
(moderate), villous core separation, submucosal edema and
epithelial sloughing; and grade 3 (severe), denudation of
epithelium with loss of villous, full-thickness necrosis or
perforation.
GSH, MDA, SOD and MPO
measurements
Partial distal ileum tissue samples were taken and
rapidly homogenized with ice-cold normal saline, and the tissue
homogenate (10% w/v) was prepared for further biochemical
experiments. SOD activity was assessed using the xanthine oxidase
method by using commercially available kits and MDA content was
quantified using a thiobarbituric acid assay with 1,1,3,
3-tetramethoxypropane as an external standard at 532 nm according
to the manufacturer's instructions. GSH activity was measured with
a dithio-dinitrobenzoic acid kit at the absorbance of 412 nm, and
MPO activity was measured as previously described by Hillegass
et al (20). Each measurement
was performed in duplicate.
Measurement of cytokine levels
Serum TNF-α, IL-1β, and IL-6 concentration were
determined from samples performed in parallel and repeated three
times using ELISA kits according to the manufacturer's
instructions. The optical density of each well was read at 450
nm.
Quantification of mRNA expression
Total RNA was isolated from the intestinal tissue
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions.
Next, the gene expression levels of IL-1β, IL-6, TNF-α and the p65
subunit of NF-κB in the distal ileum were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) with
the use of a PrimeScript RT Reagent kit (Takara Bio, Inc., Otsu,
Japan), StepOnePlus Real-Time PCR system (Thermo Fisher Scientific
Inc.) and SYBR Premix Ex Taq II (Takara Bio, Inc.). The
primers used have been reported previously (21,22). The
specific PCR primer pairs (purchased from GenScript Corporation,
Piscataway Township, NJ, USA) for the target genes were as follows:
Glyceraldehyde 3-phosphate dehydrogenase forward, 5′-CCA TGG AGA
AGG CTG GGG-3′ and reverse, 5′-CAA AGT TGT CAT GGA TGA CC-3′; NF-κB
forward, 5′-CAT TGA GGT GTA TTT CAC GG-3′ and reverse, 5′-GGC AAG
TGG CCA TTG TGT TC-3′; IL-6 forward, 5′-GGA GTT CCG TTT CTA CCT
GG-3′ and reverse, 5′-GCC GAG TAG ACC TCA TAG TG-3′; TNF-α forward,
5′-CCA CGT CGT AGC AAA CCA CCA AG-3′ and reverse, 5′-CAG GTA CAT
GGG CTC ATA CC-3′; IL-1β forward, 5′-TTG GGA TCC ACA CTC TCC AG-3′
and reverse, 5′-AGA AGC TGT GGC AGC TAC CT-3. The following thermal
cycling conditions were performed: 95°C for 10 min; 95°C for 15
sec; 60°C for 30 sec; and 72°C for 30 sec for 40 cycles. Samples
were quantified according to the 2−ΔΔCq method (23).
Western blotting of the VDUP1/NF-κB
signaling pathway
Distal ileum tissue samples were homogenized using
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Haimen, China) and centrifuged (12,000 × g, 20 min,
4°C) for western blot analysis. Equal amounts (50 µg) of protein
were separated by a 10% SDS-PAGE gel, and transferred onto a
polyvinylidene difluoride membrane using standard procedures
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following blocking
with 1% bovine serum albumin (Beyotime Institute of Biotechnology)
in phosphate-buffered saline for 1.5 h at 37°C, the blots were
incubated for 1 h with the appropriate concentration of specific
antibody at 37°C, followed by horseradish peroxidase-conjugated
anti-rabbit (1:5,000; cat. no. sc-2030) or anti-mouse (1:5,000;
cat. no. sc-2302) secondary antibody for 1 h at 37°C. After the
bands were washed, the immune-reactive proteins were visualized by
enhanced chemiluminescence reagent (EMD Millipore, Billerica, MA
USA). The band intensities were quantified using the ChemiDoc MP
system (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are reported as means ± standard error of the
mean. Statistical analysis of the results was performed by one-way
analysis of variance followed by Fisher's protected least
significant difference using the statistical program StatView for
Macintosh computers (Abacus Concepts, Berkeley, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
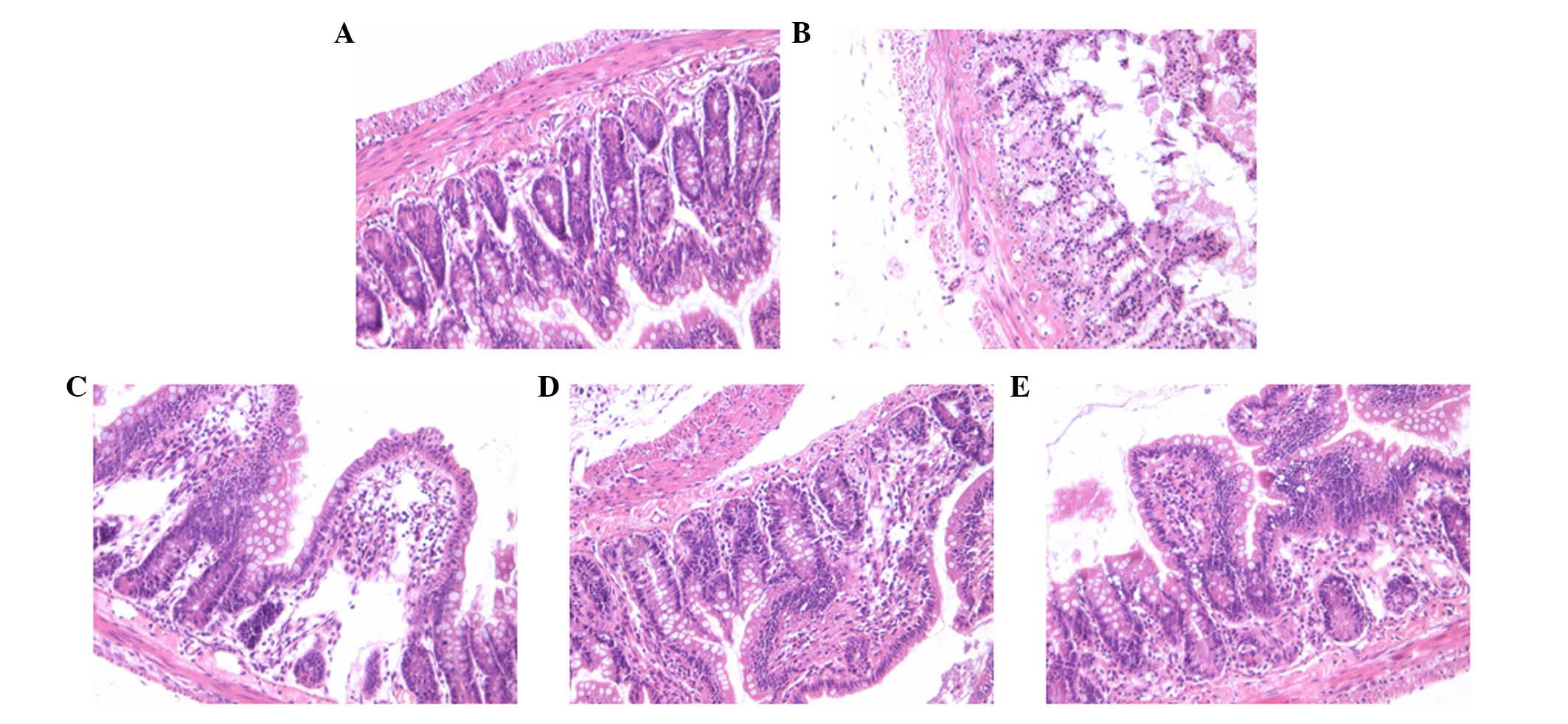
Effect of AS-IV on the incidence of
NEC
In the present study, the severity of the
histological changes of the ileal segments and the incidence of NEC
in all of the groups were determined using a scoring system between
0 and 3. The histologic grading of intestinal injury is presented
in Table I. In the normal control
group, no evident histological alteration was observed in the ileal
specimens. By contrast, ileal tissue samples obtained from the NEC
group rats demonstrated significant pathological changes, such as
pneumatosis intestinalis, fragility, edema, discoloration and
weakness of tissue integrity. However, NEC rat pathological changes
were significantly ameliorated by AS-IV as demonstrated in the
representative images, particularly at a high dose (75 mg/kg;
Fig. 1).
 | Table I.Comparison of histopathological
features in each group. |
Table I.
Comparison of histopathological
features in each group.
| Group | Intestinal injury
scoring |
|---|
| Control (n=12) | 0 |
| NEC (n=7) |
2.6±0.53a |
| NEC + AS-IV (25
mg/kg; n=8) |
2.1±0.83 |
| NEC + AS-IV (50
mg/kg; n=10) |
1.9±0.87b |
| NEC + AS-IV (75
mg/kg; n=10) |
1.4±0.51c |
Antioxidant parameters
The GSH levels in the NEC group were significantly
lower, as compared with the controls (P=0.0004). Following
treatment with AS-IV, the GSH levels in three treatment groups were
increased. The 75 mg/kg group was closest to the mean value in the
normal control group, which represents a significant increase in
the GSH levels (P=0.0094; Fig.
2A).
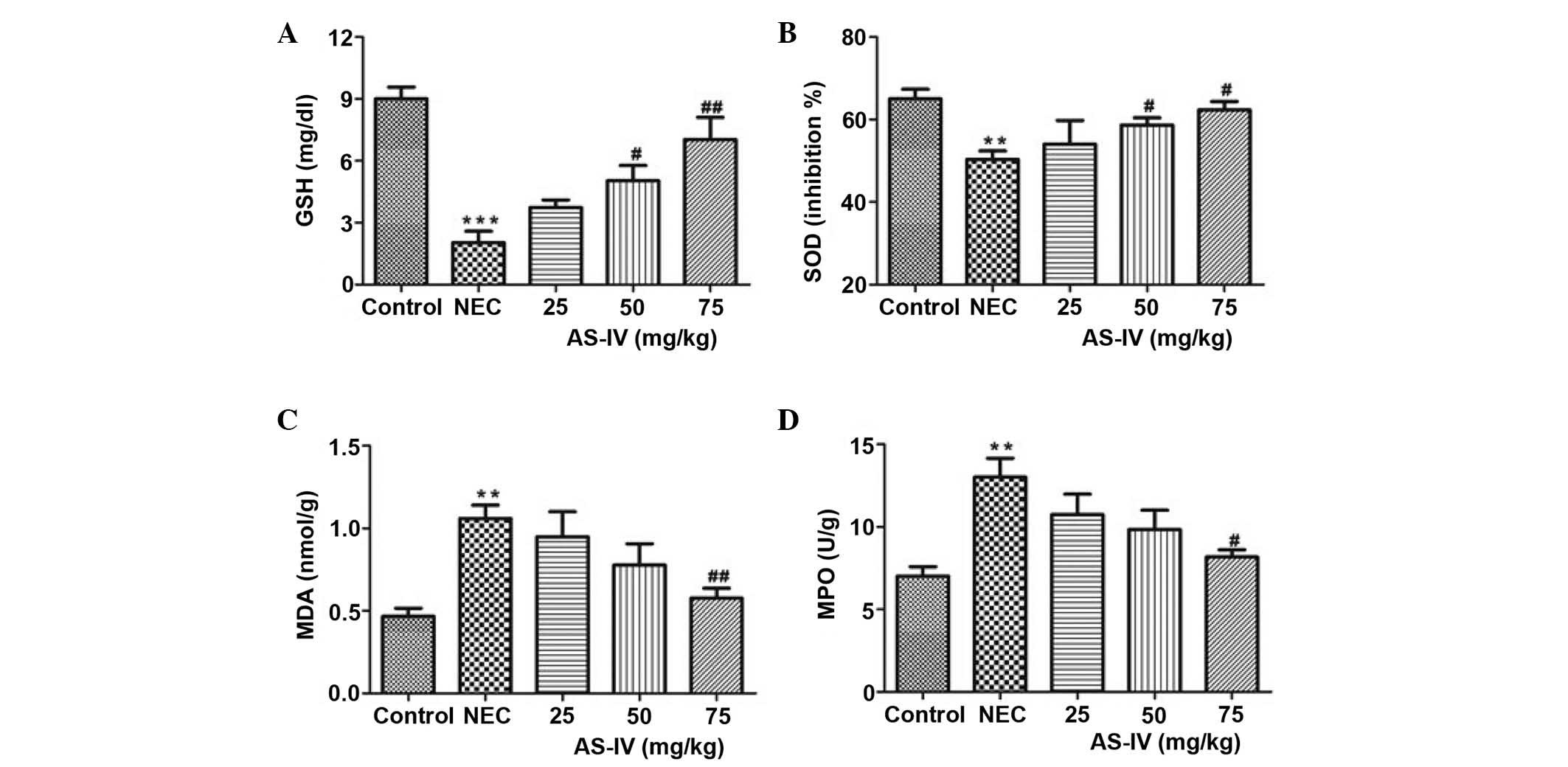 | Figure 2.(A) GSH, (B) SOD, (C) MDA and (D) MPO
concentrations in the distal ileum of rats after 4 days of
treatment. Data are presented as the mean ± standard error of the
mean in each group (n=10), **P<0.01, ***P<0.001, vs. the
control group; #P<0.05, ##P<0.01, vs.
the model group. GSH, glutathione; SOD, superoxide dismutase; MDA,
malondialdehyde; MPO, myeloperoxidase; NEC, necrotizing
enterocolitis; AS-IV, astragaloside IV. |
In the NEC group, the tissue SOD activity was
significantly increased compared with the normal group (P=0.0088;
Fig. 2B). In comparison with the
model rats, AS-IV supplementation in NEC rats prevented an increase
in SOD levels, and the values in the AS-IV-treated NEC rats were
significantly reduced compared with the model rats in a
dose-dependent manner.
As shown in Fig. 2C,
in the model group MDA levels were significantly increased, and
this effect was gradually recovered by AS-IV treatment; the AS-IV
group presented the lowest MDA levels compared with the dose
treatments in the 75 mg/kg dose group (P=0.0087). Furthermore, MPO
levels were elevated by >85% (P=0.0097; Fig. 2D) in the NEC group compared to the
age-matched normal control rats.
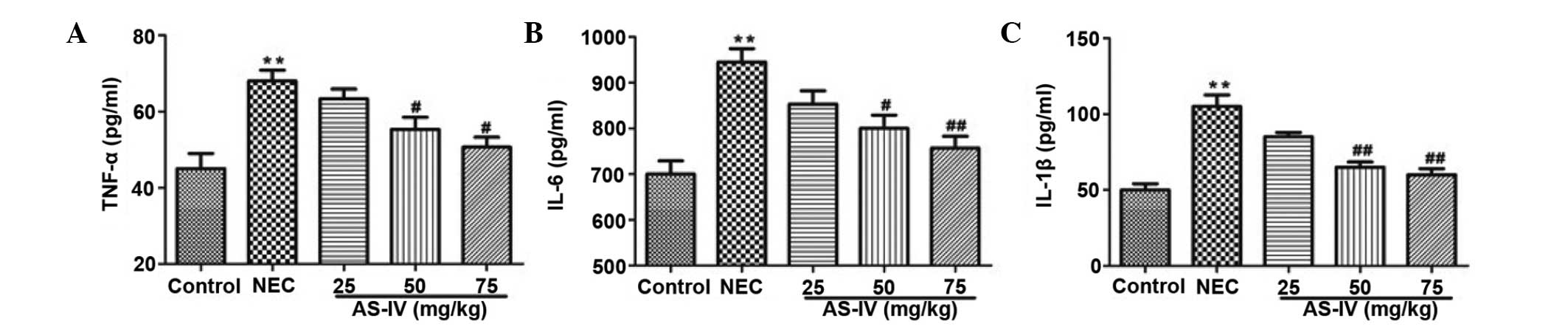
Inflammatory parameters
As illustrated in Fig.
3, TNF-α (P=0.0098), IL-1β (P=0.0031) and IL-6 (P=0.004) levels
were observed to be significantly increased in the NEC group
compared with the normal group. AS-IV (50, and 75 mg/kg)
pretreatment efficiently reduced the production of TNF-α (P=0.042
and P=0.0112, respectively), IL-1β (P=0.0086 and P=0.0065,
respectively) and IL-6 (P=0.0243 and P=0.0083, respectively) in a
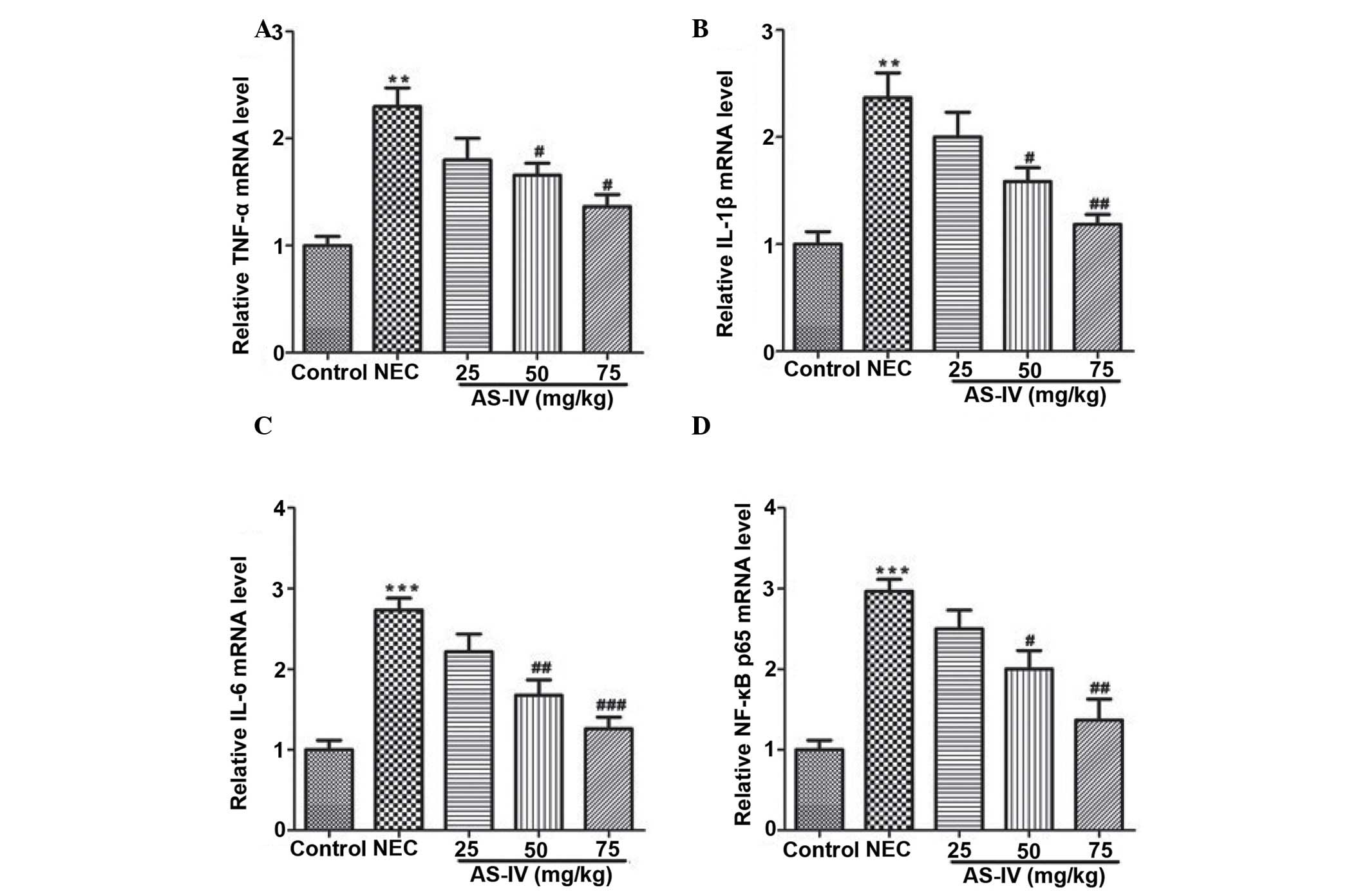
dose-dependent manner. At the mRNA level, the markedly increased
transcripts of TNF-α, IL-1β, IL-6 and NF-κB were detected in the
distal ileum from the NEC model rats, whereas AS-IV administration
significantly reversed these effects. Furthermore, the TNF-α value
in the distal ileum of NEC rats was increased ~2-fold higher
compared with the untreated normal distal ileum of the control
group (P=0.0026) as well as the IL-1β (P=0.0073; Fig. 4A and B). In addition, IL-6 was
elevated by >2.7 fold in the distal ileum that was obtained from
NEC rats (P=0.00069; Fig. 4C). In
addition, the results demonstrated that NF-κB values (Fig. 4D) in the AS-IV-treated rats were
significantly lower compared with the untreated NEC. Differences
were significant between the 25 and 75 mg/kg treated groups
(P=0.0312).
Effect of AS-IV on the VDUP1/NF-κB
signaling pathway
In order to investigate the change in VDUP1/NF-κB
pathway protein expression in the distal ileum following AS-IV
treatment, the proteins involved were examined by western blotting.
As shown in Fig. 5, the
phosphorylation of IκBα and NF-κB was significantly upregulated in
the model group (P=0.0056 and P=0.0073, respectively). In the AS-IV
groups, the protein expression levels of p-IκBα, NF-κB p65 and
p-NF-κB were significantly reduced in comparison with the model
group. In the AS-IV groups, the protein expression levels of VDUP1
and IκBα were significantly increased compared with the model
group, particularly the high dose group (P=0.049 and P=0.0039,
respectively).
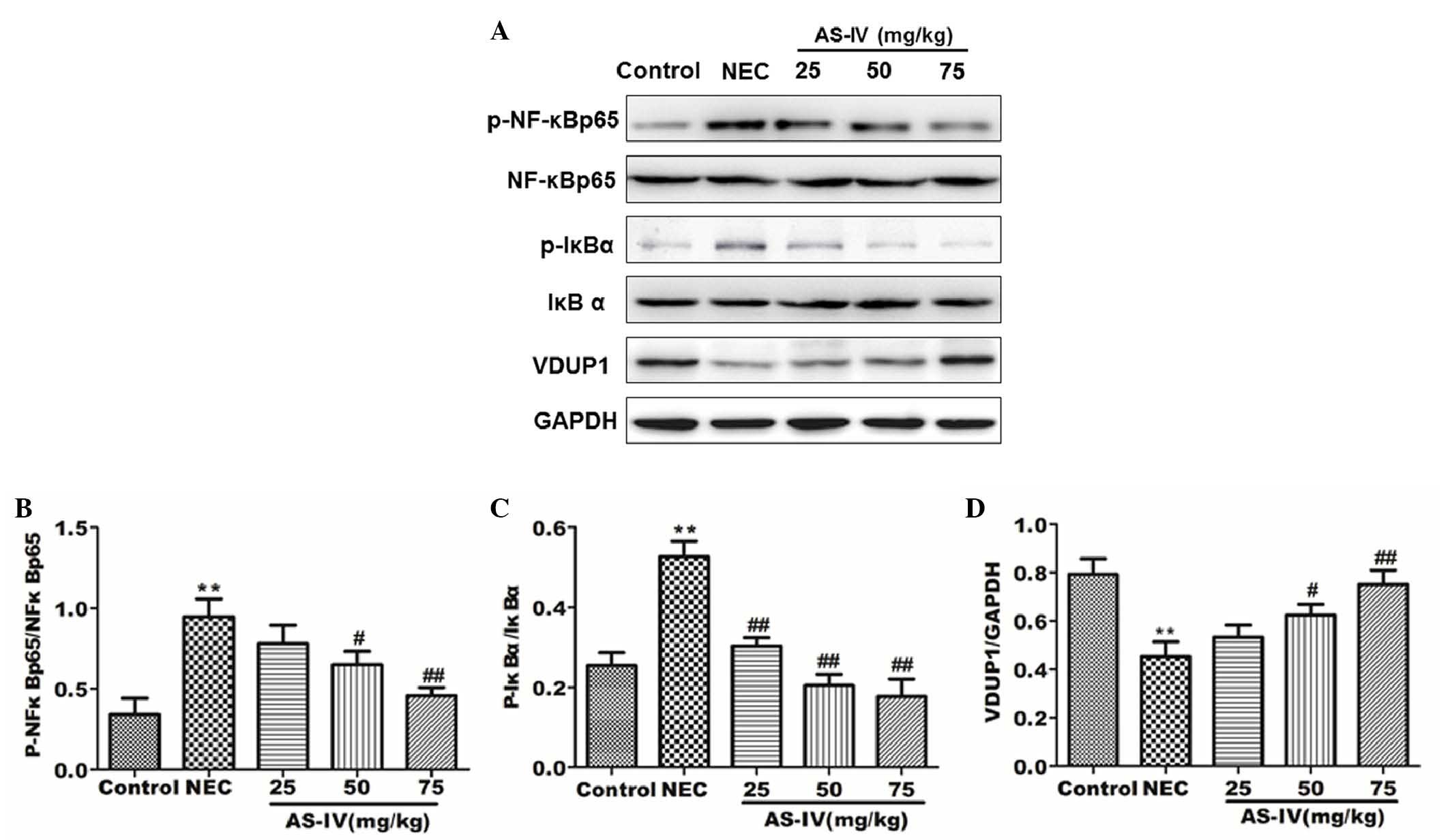 | Figure 5.Protein expression levels in rat
distal ileum after 4 days treatment were detected by (A) western
blot analysis. The relative expressions of (B) p-NF-κB/NF-κB, (C)
p-IκBα/IκBα and (D) VDUP1 were normalized to GAPDH. Data are
presented as the means ± standard error of the mean in each group
(n=3), **P<0.01, vs. the control group; #P<0.05
and ##P<0.01, vs. the model group. NF-κB, nuclear
factor-κB; VDUP1, vitamin D3-upregulated protein 1; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; NEC, necrotizing
enterocolitis; AS-IV, astragaloside IV; p, phosphorylated. |
Discussion
To the best of our knowledge, the present study is
the first report demonstrating that AS-IV has beneficial effects on
an NEC animal rat model, including on oxidative stress and
inflammation, which are considered to be important in the
intestines during NEC development. The present study illustrated
the potential protective effects of AS-IV on NEC rats by
attenuating oxidative stress and suppressing inflammation. Its
mechanism of action may be via the regulation of the VDUP1/NF-κB
signaling pathway.
Oxidative stress, which has a negative effect and is
caused by free radicals produced in vivo, is the keystone in
multiple lines of evidence converging on the development and
genesis of alimentary system disorders. It can cause profound
damage to the intestines through dysregulation of the intracellular
physiology leading to disorders. Clinical evidence suggests a
systemic activation of the inflammatory response (24). Furthermore, recent studies have
demonstrated samples from patients with NEC demonstrated elevated
levels of various inflammatory mediators compared with gestational
age-matched controls (25,26). In addition, the antioxidant capacity
of AS-IV has been considered to be confirm via its beneficial
effects on the antioxidant defense system. The results of the
present study indicated that after 4 days administration of 25, 50
and 75 mg/kg AS-IV, the levels of MDA and MPO were significantly
decreased, with a more evident decrease following the
administration of 75 mg/kg AS-IV. In addition, various doses of
AS-IV could improve the levels of GSH and SOD in NEC rats,
particularly at high doses. This suggests that AS-IV has the
potential to inhibit the overall oxidative damage undergone by the
intestines in NEC. In conclusion, the data of the present study
suggest that AS-IV therapy may protect the intestine against NEC by
modulating the oxidative/antioxidative status.
Inflammation is a common event that drives the
development of various intestinal changes in patients with NEC. The
pathophysiology of NEC involves a complex interaction of
inflammatory mediators. The results of previous studies
demonstrated the importance of inflammation in intestinal apoptosis
and in metabolic memory, and pinpoints the importance of the
duration of the reversal in its outcome (27,28). NEC
has been demonstrated to upregulate various pro-inflammatory
mediators during pathogenesis in the intestines, including IL-1β,
IL-6, TNF-α, NF-κB and localized inflammatory processes that are
considered to be important in the development of NEC (25). In the present study the levels of
TNF-α, IL-1β and IL-6 in the serum were increased compared with the
control group. In addition, the mRNA expression levels of TNF-α,
IL-1β, IL-6 and NF-κB were determined in the tissue of the
intestine. However, the AS-IV-treated distal ileum revealed
significantly lower levels of cytokines compared with the
non-treated distal ileum.
AS-IV has been widely studied for its
anti-inflammatory properties in rats (29). In the present study, three doses of
AS-IV (25, 50 and 75 mg/kg body weight) were used. All the doses
demonstrated potential protective effects against NEC. However, the
75 mg/kg dose exhibited a marked response against the anti-oxidant
and anti-inflammatory parameters, though effects were comparable
with the 25 mg/kg body weight-treated group on the remaining
parameters. However, the effects were comparable with those of the
25 mg/kg dose in terms of the IL-1β, IL-6, TNF-α and NF-κB
expression levels. Furthermore, since a lower dose may induce less
potential adverse events, more studies are required to investigate
this low dose of AS-IV.
To further characterize the mechanism underlying the
AS-IV protective effect on NEC rats, the effects of AS-IV on the
activation of the VDUP1/NF-κB signaling pathways were examined. As
observed in the results, the level of VDUP1 was nearly recovered to
the normal level following AS-IV treatment in the 75 mg/kg dose
group. Furthermore, the levels of phosphorylation of NF-κBp65 and
IκBα were evidently increased in the model group, and
administration of AS-IV impaired phosphorylation of these molecules
in a dose-dependent manner. Furthermore, the present results
demonstrated that AS-IV was able to significantly regulate the
VDUP1/NF-κB signaling pathway in order to protect NEC rats.
In conclusion, AS-IV has beneficial effects in
experimental studies of the NEC rats, which are characterized by
increased oxidative stress and inflammatory reactions, supporting
its clinical use. To the best of our knowledge, the present study
is the first to demonstrate that AS-IV therapy has beneficial
effects on rat NEC-like injuries by reducing the levels of
inflammatory cytokines and improving antioxidant defense. The
present results also demonstrated that AS-IV significantly
regulated the VDUP1/NF-κB signaling pathway. Thus, AS-IV appears to
be a useful adjunct therapy to possibly inhibit the
development/progression of NEC, and the lethal complication faced
by patients with NEC.
Acknowledgements
The present study was supported in part by grants
from the Southeast University of China. The authors gratefully
acknowledge associate Professor Su Ning for her assistance in the
histopathological analysis.
References
|
1
|
Chen CC and Allan WW: Probiotics and the
mechanism of necrotizing enterocolitis. Semin Pediatr Surg.
22:94–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nanthakumar N, Meng D, Goldstein AM, Zhu
W, Lu L, Uauy R, Llanos A, Claud EC and Walker WA: The mechanism of
excessive intestinal inflammation in necrotizing enterocolitis: An
immature innate immune response. PLoS One. 6:e177762011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schnabl KL, Van Aerde JE, Thomson AB and
Clandinin MT: Necrotizing enterocolitis: A multifactorial disease
with no cure. World J Gastroenterol. 14:2142–2161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chandler JC and Hebra A: Necrotizing
enterocolitis in infants with very low birth weight. Semin Pediatr
Surg. 9:63–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung JW, Jeon JH, Yoon SR and Choi I:
Vitamin D3 upregulated protein 1 (VDUP1) is a regulator for redox
signaling and stress-mediated diseases. J Dermatol. 33:662–669.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pan CL and Dong ZW: Antiasthmatic effects
of eugenol in a mouse model of allergic asthma by regulation of
vitamin D3 upregulated protein 1/NF-κB pathway. Inflammation.
38:1385–1393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SY, Suh HY, Chuang JW, Yoon SR and
Choi I: Diverse functions of VDUP1 in cell proliferation,
differentiation and diseases. Cell Mol Immunol. 4:345–351.
2007.PubMed/NCBI
|
|
8
|
Kutuk O and Basaga H: Inflammation meets
oxidation: NF-κB as a mediator of initial lesion development in
atherosclerosis. Trends Mol Med. 9:549–557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanner SM, Berryhill TF, Ellenburg JL,
Jilling T, Cleveland DS, Lorenz RG and Martin CA: Pathogenesis of
necrotizing enterocolitis: Modeling the innate immune response. Am
J Pathol. 185:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shilan M and Mohammad A: A review on the
role of oxidative stress and inflammation in necrotizing
enterocolitis and benefits of the phosphodiesterase inhibitor
pentoxifylline. International Journal of Pharmacology. 9:245–250.
2013. View Article : Google Scholar
|
|
11
|
Frost BL, Jilling T and Caplan MS: The
importance of pro-inflammatory signaling in neonatal necrotizing
enterocolitis. Semin Perinatol. 32:100–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cevik M, Karadag CA, Sakiz DE, Tander B
and Embleton DD: The role of nitric oxide in an experimental
necrotising enterocolitis model. Afr J Paediatr Surg. 10:24–28.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Y, Du M, Gao Y, Liu H, Wang H, Wu X and
Wang Z: Astragaloside IV attenuates experimental autoimmune
encephalomyelitis of mice by counteracting oxidative stress at
multiple levels. PLoS One. 8:e764952013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai PK, Chan JY, Cheng L, Lau CP, Han SQ,
Leung PC, Fung KP and Lau CB: Isolation of anti-inflammatory
fractions and compounds from the root of Astragalus membranaceus.
Phytother Res. 27:581–587. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Z, Cai Y, Zhang W, Liu X and Liu S:
Astragaloside IV inhibits platelet-derived growth
factor-BB-stimulated proliferation and migration of vascular smooth
muscle cells via the inhibition of p38 MAPK signaling. Exp Ther
Med. 8:1253–1258. 2014.PubMed/NCBI
|
|
17
|
Yazıcı S, Akşit H, Korkut O, Sunay B and
Çelik T: Effects of boric acid and 2-aminoethoxydiphenyl borate on
necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 58:61–67.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Gu T, Fu X and Zhao R: Effect of
salvianolic acid A and C compatibility on inflammatory cytokines in
rats with unilateral ureteral obstruction. J Tradit Chin Med.
35:564–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nadler EP, Dickinson E, Knisely A, Zhang
XR, Boyle P, Beer-Stolz D, Watkins SC and Ford HR: Expression of
inducible nitric oxide synthase and interleukin-12 in experimental
necrotizing enterocolitis. J Surg Res. 92:71–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hillegass LM, Griswold DE, Brickson B and
Albrightson-Winslow C: Assessment of myeloperoxidase activity in
whole rat kidney. J Pharmacol Methods. 24:285–295. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu HE, Chen Y, Sun XB, Tong B and Fan XH:
Effects of luteolin on retinal oxidative stress and inflammation in
diabetes. RSC Advances. 5:4898–4904. 2015. View Article : Google Scholar
|
|
22
|
Williams CM and Coleman JW: Induced
expression of mRNA for IL-5, IL-6, TNF-alpha, MIP-2 and IFN-gamma
in immunologically activated rat peritoneal mast cells: Inhibition
by dexamethasone and cyclosporin A. Immunology. 86:244–249.
1995.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lasanianos NG, Kanakaris NK, Dimitriou R,
Pape HC and Giannoudis PV: Second hit phenomenon: Existing evidence
of clinical implications. Injury. 42:617–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi W, Shen Q, Zhang L, Han LP and Wang S:
Study on the inflammatory intervention of erythropoietin on NEC.
Exp Ther Med Jun. 11:2221–2224. 2016.
|
|
26
|
Heida FH, Hulscher JB, Schurink M, van
Vliet MJ, Kooi EM, Kasper DC, Pones M, Bos AF and Benkoe TM:
Bloodstream infections during the onset of necrotizing
enterocolitis and their relation with the pro-inflammatory
response, gut wall integrity and severity of disease in NEC. J
Pediatr Surg. 50:1837–1841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chung CY, Park YL, Kim N, Oh HH, Myung DS,
Kim JS, Cho SB, Lee WS, Kim HS, Ahn BW and Joo YE: Rice prolamin
extract ameliorates acute murine colitis by inhibiting nuclear
factor-kappaB and modulating intestinal apoptosis and cell
proliferation. Clin Exp Immunol. 178:537–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Everard A, Geurts L, Caesar R, Van Hul M,
Matamoros S, Duparc T, Denis RG, Cochez P, Pierard F, Castel J, et
al: Intestinal epithelial MyD88 is a sensor switching host
metabolism towards obesity according to nutritional status. Nat
Commun. 5:56482014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu M, Tang F, Zhang J, Luan A, Mei M, Xu
C, Zhang S, Wang H and Maslov LN: Astragaloside IV attenuates
injury caused by myocardial ischemia/reperfusion in rats via
regulation of toll-like receptor 4/nuclear factor-κB signaling
pathway. Phytother Res. 29:599–606. 2015. View Article : Google Scholar : PubMed/NCBI
|