Introduction
There is mounting evidence that a history of
clinical chronic prostatitis can be a risk factor for prostate
cancer (1–7). As a consequence, aggressive therapy,
aimed at eradicating or neutralizing the etiological agents of
bacterial or abacterial forms of chronic prostatitis, is of great
importance for the prompt resolution of the severe symptoms of
these conditions, but may also have implications in cancer
prevention.
National Institutes of Health (NIH) category II
chronic bacterial prostatitis (CBP) and category III chronic
prostatitis/chronic pelvic pain syndrome are the two main chronic
symptomatic conditions of the prostate (8). While the latter is often treated with a
variety of agents in an attempt to target its manifold and often
uncertain etiological determinants, the former is usually managed
with antibacterial agents, administered at high doses and for
extended periods of time (weeks, or in some cases, months).
Fluoroquinolones such as ciprofloxacin or
levofloxacin have been first-line agents for the treatment of CBP
for many years, due to their broad antibacterial spectrum and
efficient distribution to the prostate tissue and glandular ducts
(9). However, fluoroquinolone-based
therapy is not always feasible, due to specific contraindications
(for example, a history of tendonitis or long QT syndrome) or to
resistance of causative uropathogens to these agents (10,11). In
this regard, the resistance rates of Enterobacteriaceae and other
pathogens causing community-acquired or healthcare-associated
urogenital infections are increasing markedly. They exceed 50% in
many parts of the world, particularly in Asia, but similar figures
have been reported in other continents. For example,
fluoroquinolone resistance rates in Europe and North America range
from ~10% in rural areas to >30% in established sexual networks
(12).
Although national and international implementation
of targeted stewardship may eventually decrease the deleterious
impact of bacterial chemoresistance (13), new therapeutic options are urgently
required as alternatives to fluoroquinolones. Regretfully, the
armamentarium for the outpatient treatment of CBP appears to be
quite limited, as most β-lactams distribute very poorly to the
prostatic tissue, and trimethoprim therapy is in the majority of
cases unfeasible, since worldwide resistance rates to cotrimoxazole
are equivalent to or higher than those observed for
fluoroquinolones, and cases of fluoroquinolone-cotrimoxazole
cross-resistance are frequently reported (14).
Aminoglycosides are among the recommended agents for
the treatment of category I acute bacterial prostatitis (ABP)
(15). A retrospective multi-center
analysis conducted in 2008 showed that an aminoglycoside is
administered in 80% of cases of ABP (16). However, to the best of our knowledge,
only case reports or small case series describing the successful
administration of these agents to patients affected by CBP are
available (17). Hence, new evidence
is required to support the usage of aminoglycosides for the
treatment of CBP. The present study aimed to analyze the efficacy
and safety of systemic administration of aminoglycoside antibiotics
to a cohort of 78 patients affected by fluoroquinolone-resistant
CBP, or excluded from fluoroquinolone therapy due to various
contraindications.
Patients and methods
The Ethics Committee of the Principal Investigator's
Hospital was notified of the present study, according to Italian
bylaws. This was a subordinate study of a larger observational
investigation, recorded in the Italian Medicines Agency AIFA
clinical trial register (no. 276). For an observational
retrospective study, approval is not required, and a simple
notification accompanying the study protocol is sufficient
(Determinazione AIFA 20/3/2008, GU 76, 31/3/2008). Patients
provided written consent for processing and anonymous publication
of their clinical data. This manuscript was prepared in adherence
to the Strengthening the Reporting of Observational Studies in
Epidemiology Statement (STROBE) guidelines for good reporting of
observational studies (18).
Inclusion criteria
The present study was based on the retrospective
analysis of a database of >1,700 patients with chronic
prostatitis who were diagnosed and treated on an outpatient basis
in Milan, Italy. Patients consecutively diagnosed with CBP from
year 2001 onwards were considered.
Patients between 20 and 65 years of age were
included in this study in the presence of a clinical indication for
treatment with aminoglycoside antibiotics of category II CBP,
diagnosed according to NIH criteria, defined in 1995 at the
National Institute of Diabetes and Digestive and Kidney Diseases
Chronic Prostatitis Workshop (Bethesda, MD, USA).
Patients were excluded if they presented any of the
following conditions: Category I acute bacterial prostatitis,
therapy with antibacterial agents or any medication effective at
the prostatic level within a 90-day period prior to aminoglycoside
treatment, renal/hepatic/cardiac insufficiency, elevated creatinine
plasma levels, a family history or audiometric evidence of hearing
impairment, indwelling catheters, cystostomy or ureterostomy,
previous prostatic surgery or radiotherapy, incomplete compliance
with antibacterial therapy assessed by interviewing patients at
test-of-cure visits, any condition that might represent a major
confounder in the evaluation of the patients with the NIH Chronic
Prostatitis Symptom Index (NIH-CPSI) for example, chronic
consumption of drugs such as antidepressants or tramadol.
Diagnostic procedures and symptom
evaluation
CBP was diagnosed as previously described in detail
(19). Briefly, the patient
evaluation was based on careful collection of clinical history, a
urological visit including a digitorectal examination, transrectal
ultrasound, urine flowmetry [assessment of peak urinary flow rate
(Qmax)], and on microbiological analysis and antibiogram
of lower urinary tract specimens obtained with the Meares &
Stamey ‘4-glass’ test (20). The
total ejaculate was collected thereafter, and analyzed for
pathogens and leukocytes, as previously described (21).
For the diagnosis of prostatic bacterial infection,
colony counts in prostatic specimens (expressed prostatic
secretions or post-massage urine) were required to be ≥10-fold
greater than those assessed in first-voided and pre-massage
midstream urine. Inflammatory leukocytes were counted in expressed
prostatic secretions, post-massage urine and semen. In cases where
infection by more than one pathogen was detected, microbiological
tests were repeated to exclude accidental specimen contamination.
Validated Italian versions of the NIH-CPSI and International
Prostate Symptom Score (IPSS) tests were used to score the severity
of the clinical symptoms (22).
The baseline workup of each candidate to
aminoglycoside treatment included audiometric testing and
measurement of serum creatinine.
Study outline
After completing clinical and microbiological
evaluations at the baseline visit (visit time-point 0, ‘V0’),
patients were treated with netilmicin (4.5 mg/kg body weight,
once-daily, intramuscular), combined or not with a β-lactam
antibiotic. In Italy, treatment of prostatitis is an approved
indication for aminoglycosides such as netilmicin; hence,
administration of these agents was on-label, non-experimental and
part of routine practice. Patients remained from time-point V0 to
time-point V6 on continuous treatment with the α-adrenoceptor
blocker alfuzosin (10 mg/day), combined with a supplement
containing a Serenoa repens extract (640 mg/day), lycopene
(5 mg/day) and selenium (50 mg/day) in a single formulation
(Profluss®, Konpharma, Rome, Italy) (23). Ketoprofen (160 mg, twice daily) was
administered rectally to a group (n=11) of mildly febrile patients
until normalization of the temperature was achieved.
At 4 weeks after the end of the antimicrobial
treatment all patients were subjected to a complete diagnostic
protocol including microbiological and clinical evaluations. This
time-point was designated as visit for assessment of pathogen
eradication (VERAD).
At the V6 and V12 follow-up visits (6 and 12 months
after VERAD, respectively), patients were subjected to complete
clinical evaluations.
Microbiological response
evaluation
The definitions of Naber et al were used to
report the microbiological response to antibacterial therapy: i)
Eradication: baseline pathogen was eradicated (<103
CFU/ml); ii) eradication with superinfection: baseline pathogen was
eradicated (<103 CFU/ml) with the appearance of a new
pathogen (≥103 CFU ml); iii) persistence: baseline
pathogen was not eradicated (≥103 CFU/ml); iv)
persistence with superinfection: baseline pathogen was persistent
(≥103 CFU/ml) with the appearance of a new pathogen
(≥103 CFU/ml) (24).
Aminoglycoside ototoxicity risk
assessment
There were 38 patients who gave written consent for
genetic analysis limited to the 1555A>G, 1494C>T and
1556C>T mutations in the mitochondrial DNA, which predispose an
individual to irreversible sensorineural hearing loss following
exposure to aminoglycosides. A specimen of cells was obtained from
each patient by gently scraping the inner cheek mucosa with a
sterile mucosal brush. Mitochondrial DNA was extracted, using the
Quick-DNA™ Universal kit and the DNA Clean & Concentration kit
(both Zymo Research Corp., Irvine, CA, USA) according to the
manufacturer's protocol, and used as a template for the
identification of ototoxicity-predisposing mutations by two
different techniques: Polymerase chain reaction-restriction
fragment length polymorphism (PCR-RFLP) (25) and multiplex PCR (26). The full sequences of the primers, the
final concentration of all reagents in the reaction, and the
cycling conditions were identical to those described by the
developers of the two analytical techniques (25,26). All
personnel analyzing the genetic material and the outcome assessor
were blinded to the identity of the patients. The genetic material
was destroyed immediately following the release of the anonymized
results of the mutation analysis. The entire procedure (handling of
genetic material and data) was legitimated by the General
Authorization No. 8/2014 for the Processing of Genetic Data, of the
Italian Data Protection Authority.
Data handling and statistical
analysis
To analyze pre- vs. post-therapy paired differences
in NIH-CPSI and IPSS scores the Wilcoxon signed-rank test was used;
intergroup differences (eradicated vs. persistent cases) were
analyzed with the Mann-Whitney-Wilcoxon rank sum test. The measure
of central tendency for NIH-CPSI and IPSS ordinal scores was the
median, and the interquartile range (IQR) was used to indicate
statistical data dispersion. For continuous variables [urinary peak
flow rate, serum prostate-specific antigen (PSA) concentration and
prostate volume], paired or unpaired t-tests were used to analyze
differences between means. An α error of <5% was set as the
significance level for each comparison. Comparison between paired
patient proportions was made using the McNemar's test for
correlated proportions.
The VassarStats on-line statistics platform
(vassarstats.net) and the JASP software
(jasp-stats.org/) were used for the statistical
analysis of data. Post-hoc computation of achieved power was
performed using the G*Power 3.1 program (27).
Results
Clinical presentation of patients and
treatment protocols
From the clinical database, the data of 93 patients
affected by category II CBP, for whom therapy with aminoglycoside
antibiotics was initially considered, were retrieved. The main
reasons for aminoglycoside indication were resistance of causative
pathogens to fluoroquinolones, intolerance to fluoroquinolones (for
example, a history of drug-induced tendonitis or severe
gastrointestinal disturbances) or ineffectiveness of previous
cycles of therapy with fluoroquinolones (Table I).
 | Table I.Rationale for aminoglycoside
therapy. |
Table I.
Rationale for aminoglycoside
therapy.
|
| Adverse effects to
first-line agents |
|
|
|---|
|
|
|
|
|
|---|
| Persistence after
first-line agents |
Gastrointestinal | Tendonitis | Resistance of
causative pathogens to first-line agents | Patients, n |
|---|
| Yes |
|
|
| 3 |
|
| Yes |
|
| 11 |
|
|
| Yes |
| 7 |
|
|
|
| Yes | 15 |
| Yes |
| Yes |
| 2 |
|
| Yes |
| Yes | 5 |
|
|
| Yes | Yes | 6 |
| Yes | Yes | Yes |
| 6 |
|
| Yes | Yes |
| 14 |
|
| Yes | Yes | Yes | 9 |
Fifteen patients refused treatment or were excluded
from therapy following preliminary otolaryngological tests, kidney
function evaluations, or careful scrutiny of personal and family
history (Table II), and 78 cases
(median age, 43 years; IQR, 22 years) initiated treatment with
aminoglycosides, combined or not with other antibiotics
(β-lactams).
 | Table II.Rationale for rejection or
contraindication of treatment following initial general indication
of aminoglycoside therapy. |
Table II.
Rationale for rejection or
contraindication of treatment following initial general indication
of aminoglycoside therapy.
| Rationale | Patients, n |
|---|
| Professional
musician | 1 |
| Semiprofessional
involvment in music or professional activity requiring fine
hearing | 5 |
| Referred history of
reversible hearing impairment upon treatment with
aminoglycosides | 2 |
| High frequency
(4,000 Hz) hearing impairment assessed during audiometry
performed |
| prior to
aminoglycoside therapy initiation | 2 |
| Bilateral hearing
impairment in family with matrilinear hearing loss | 2 |
| Elevated serum
creatinine assessed during previous exposure to amikacin | 1 |
| Unilateral hearing
impairment referred during anamnestic interview prior to
aminoglycoside |
|
| therapy
initiation | 2 |
| Referred tinnitus
plus recurrent otitis | 1 |
| Total | 15 |
Five different antibacterial protocols were adopted.
Table III summarizes these
protocols, together with the rationale for use of the listed
agents. All protocols contained the aminoglycoside netilmicin (4.5
mg/kg, intramuscular), administered once-daily.
 | Table III.Antibacterial protocols administered
to CBP patients and rationale for drug selection. |
Table III.
Antibacterial protocols administered
to CBP patients and rationale for drug selection.
| Therapeutic
protocol | Drugs and
dosages | Rationale |
|---|
| 1 | Netilmicin,
intramuscular, 4.5 mg/kg, once daily for 2 weeks. Cefuroxime
axetil, oral, 250 mg, twice daily for 2 weeks | Cefuroxime was
administered to exploit the synergic activity of combined
aminoglycosides and β-lactams; this 2nd generation cephalosporin
showssufficient prostate penetration (43). Pathogen sensitivity was assessed by
antibiogram |
| 2 | Netilmicin,
intramuscular, 4.5 mg/kg, once daily for 2 weeks. Cefoperazone,
intramuscular, 1 g, twice daily for 2 weeks | Cefoperazone was
administered to exploit the synergic activity of combined
aminoglycosides and β-lactams; this 3rd generation cephalosporin
shows sufficient prostate penetration (43). Pathogen sensitivity was assessed by
antibiogram |
| 3 | Netilmicin,
intramuscular, 4.5 mg/kg, once daily for 2 weeks. Piperacillin, 2
g, plus tazobactam, 250 mg, intramuscular, once daily for 2
weeks | Piperacillin was
administered to exploit the synergic activity of combined
aminoglycosides and β-lactams in the presence of resistance to
cephalosporins (pathogen sensitivity assessed by antibiogram), or
upon cephalosporin contra-indication. Piperacillin shows adequate
prostate penetration (44) |
| 4 | Netilmicin,
intramuscular, 4.5 mg/kg, once daily for 2 weeks. Co-amoxiclav 875
mg + 125 mg, oral, twice daily for 2 weeks | Co-amoxiclav can be
recovered in the prostate tissue (44), and was administered to exploit the
synergic activity of combined aminoglycosides and β-lactams in the
presence of resistance to cephalosporins (pathogen sensitivity
assessed by antibiogram), or of contraindication to cephalosporins
or piperacillin |
| 5 | Netilmicin,
intramuscular, 4.5 mg/kg, once daily for 2 weeks | Netilmicin was
administered as single agent when causative pathogens showed
resistance to β-lactams. |
Eight patients were noncompliant with therapy or
dropped-out during treatment and were excluded from the present
per-protocol efficacy analysis.
There were 11 patients who presented with low-grade
pyrexia (<38.3°C/101°F), but did not show the array of signs and
symptoms typical of acute bacterial prostatitis. In all cases,
fever resolved within 72–96 h after the first dose of antibiotic.
As prostate massage is contraindicated in febrile patients until
normalization of the temperature occurs, the initial diagnosis of
prostatitis was based on sonography, clinical signs and symptoms
and a midstream urine culture, eventually followed by the ‘4-glass’
test.
Safety of aminoglycoside therapy
Therapy was in general well tolerated. Adverse
effects possibly linked to aminoglycoside exposure were reported in
two cases. In 1 patient the serum creatinine level increased from
1.1 mg/dl at time-point V0 to 2.3 mg/dl at time-point VERAD. This
effect was reversible, and the serum creatinine level dropped to
1.2 mg/dl 8 weeks later. One patient complained of tinnitus by the
end of treatment, and audiometry confirmed hearing impairment, with
a notch at 8 KHz. The examining otolaryngologist suggested that
therapy might have induced the worsening of a pre-existing
high-frequency hearing impairment.
Microbiological results
Eradication of the causative pathogen at time-point
VERAD was observed in 55 out of 70 per-protocol patients (78.6%)
and 15 patients exhibited microbiological persistence. No cases of
superinfection were reported.
There were 45 and 22 patients that presented with a
single-pathogen infection caused by a gram-negative or
gram-positive pathogen, respectively, whereas 3 patients had mixed
gram-positive/gram-negative infections (Table IV). Escherichia coli and
Enterococcus faecalis were the most prevalent pathogens (35
and 19 isolates, respectively). Single-pathogen E.faecalis
and E.coli infections were eradicated in 56 and 85% of
cases, respectively (Table IV).
Cases of infection recurrence were not reported in the cohort of 70
treated patients.
 | Table IV.Microbiological eradication of each
pathogen, assessed at time-point VERAD in the per-protocol study
cohort of a total of 70 patients. |
Table IV.
Microbiological eradication of each
pathogen, assessed at time-point VERAD in the per-protocol study
cohort of a total of 70 patients.
| Pathogen | Eradicated, n
(%) | Persistent, n
(%) | Total cases |
|---|
| Escherichia
coli | 29 (85.29) | 5 (14.71) | 34 |
| Enterococcus
faecalis | 9
(56.25) | 7 (43.75) | 16 |
| Staphylococcus
aureus | 2 (100.00) | – | 2 |
| Proteus
mirabilis | 2 (100.00) | – | 2 |
| Klebsiella
oxytoca | 2 (66.67) | 1 (33.33) | 3 |
| Pseudomonas
aeruginosa | 1 (100.00) | – | 1 |
| Enterobacter
cloacae | – | 1 (100.00) | 1 |
| Morganella
morganii | 1 (50.00) | 1 (50.00) | 2 |
| Haemophylus
spp. | 1 (100.00) | – | 1 |
| Citrobacter
spp. | 1 (100.00) | – | 1 |
|
Streptococcusβ-haemolyticus | 4 (100.00) | – | 4 |
| E. faecalis
plus E. coli | 1 (100.00) | – | 1 |
| E. faecalis
plus P. aeruginosa | 2 (100.00) | – | 2 |
| Total | 55 (78.57) | 15 (21.43) | 70 |
Microbiological eradication at time-point VERAD was
observed in 10 out of 11 febrile patients (90.91%), suggesting that
aminoglycoside therapy might be an effective therapeutic option in
certain complicated cases. Such therapy can usually be managed in
an outpatient setting.
Clinical and laboratory findings
The clinical findings of the total patient
population at enrollment (V0), at test of eradication (VERAD) and 6
or 12 months thereafter (time-points V6 and V12, respectively) are
summarized in Fig. 1 and Table V.
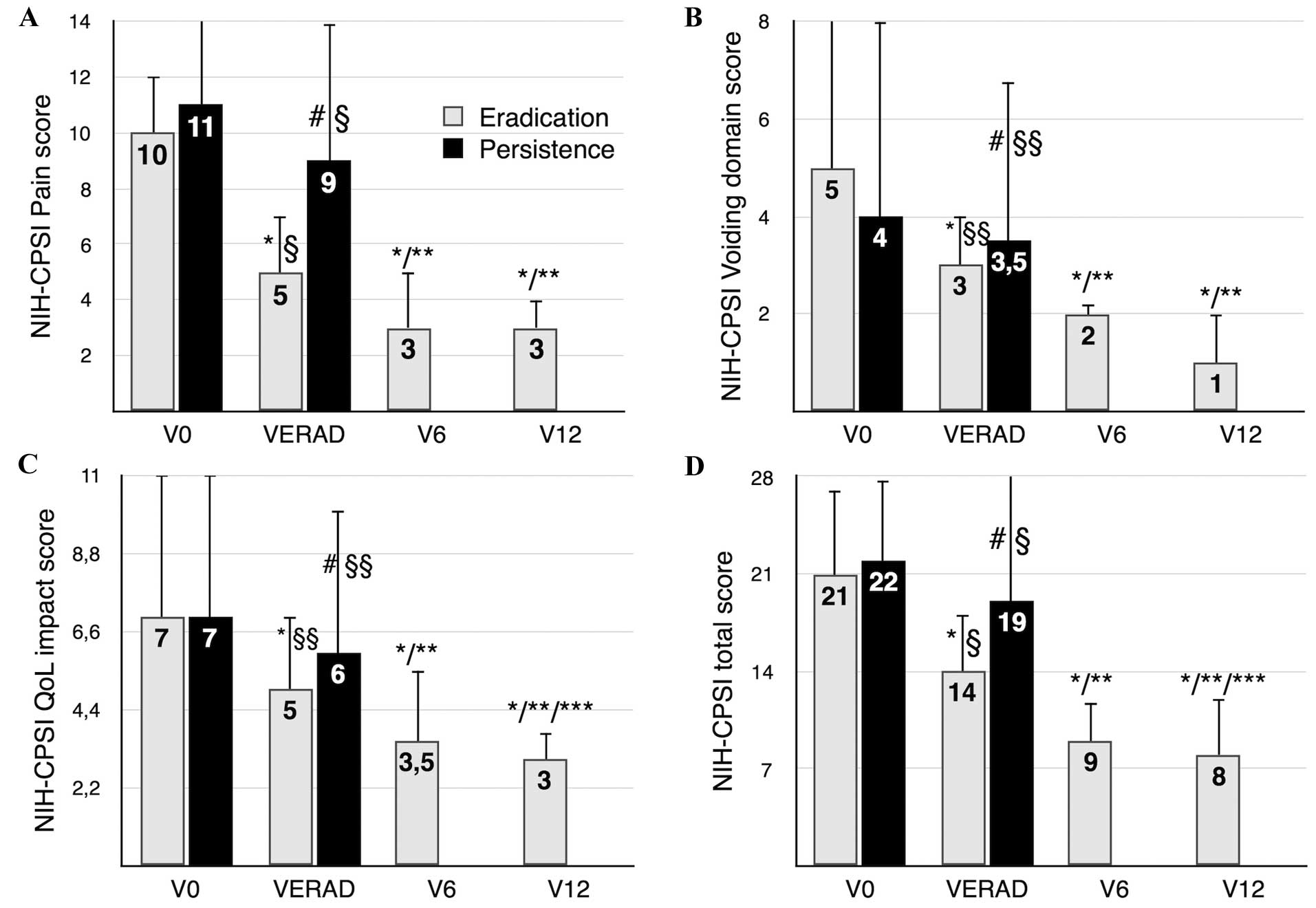 | Figure 1.NIH-CPSI scores, assessed at baseline
(time-point V0), at test of microbiological eradication (VERAD),
and at follow-up (V6 or V12, 6 or 12 months after VERAD,
respectively). Results are stratified according to the
microbiological outcome of therapy (eradication vs. persistence of
infection). Median values of NIH-CPSI scores are indicated, and
interquartile range bars are shown. (A) Pain domain, (B) voiding
symptoms domain, (C) impact of the disease on patients' QoL and (D)
total NIH-CPSI scores. Due to the retrospective nature of the
study, the follow-up data of patients showing microbiological
persistence are missing, as these patients were routed towards a
third-level diagnostic and therapeutic protocol. *P<0.001 vs.
V0, paired comparison (Wilcoxon signed rank test). **P<0.001 vs.
VERAD, paired comparison (Wilcoxon signed rank test). ***P<0.05
vs. V6, paired comparison (Wilcoxon signed rank test).
#P≥0.05 vs. V0, paired comparison (Wilcoxon signed rank
test). §P<0.01, intergroup comparison at VERAD
(eradication vs. infection persistence groups),
Mann-Whitney-Wilcoxon rank sum test. §§P≥0.05,
intergroup comparison at VERAD (eradication vs. infection
persistence groups), Mann-Whitney-Wilcoxon rank sum test. NIH-CPSI,
National Institutes of Health Chronic Prostatitis Symptom Index;
VERAD, visit for assessment of pathogen eradication; QoL, quality
of life. |
 | Table V.Serum PSA, prostate volume, Qmax, %
voided bladder and IPSS at various time points. |
Table V.
Serum PSA, prostate volume, Qmax, %
voided bladder and IPSS at various time points.
|
| Study time
point |
|---|
|
|
|
|---|
| Variable | V0 | VERAD | V6 | V12 |
|---|
| PSA (ng/ml) |
|
|
|
|
|
Eradicated |
3.32±2.89 |
1.89±1.37a | NA | NA |
|
Persistent |
3.31±3.73 |
2.29±1.98b, c | NA | NA |
| Prostate volume
(ml) |
|
|
|
|
|
Eradicated |
30.0±15.44 |
24.6±12.17a | NA | NA |
|
Persistent |
25.5±9.21 |
25.27±14.50c, d | NA | NA |
| Qmax (ml/sec) |
|
|
|
|
|
Eradicated |
17.93±7.25 |
18.97±5.62b |
19.32±5.48b |
18.66±4.06b |
|
Persistent |
18.21±5.60 |
19.93±5.73b, c | NA | NA |
| % voided
bladder |
|
|
|
|
|
Eradicated |
88.95±17.25 |
97.75±9.18b |
97.90±8.00b |
96.75±12.04b |
|
Persistent |
93.85±11.37 |
97.66±9.03c, d | NA | NA |
| IPSS score |
|
|
|
|
|
Eradicated | 12
(9) | 8.5
(5.5)e | 7
(4.25)e | 7 (5)e |
|
Persistent | 10.25 (3) | 9
(3)f, g | NA | NA |
Whereas the symptoms of prostatitis did not improve
in the group of patients exhibiting pathogen persistence, the
median total NIH-CPSI score, as well as the pain, voiding symptom
and quality of life (QoL) subdomain scores of the NIH-CPSI
decreased significantly in patients showing microbiological
eradication at time-point VERAD, and showed further significant
attenuation during follow-up. A caveat is that the statistical
power and robustness of unpaired comparisons between groups showing
microbiological eradication versus persistence are questionable, as
cohorts show considerably different sizes (n=55 vs. n=15,
respectively), and baseline imbalances are present (for example,
baseline prostate volume).
NIH-CPSI total score
In patients showing pathogen eradication, the
NIH-CPSI total score decreased significantly from a baseline median
value of 21 to 14 at time-point VERAD, and to 9 and 8 at
time-points V6 and V12, respectively (P<0.001 for all paired
comparisons vs. V0, Wilcoxon signed rank test, n=55). The
differences between VERAD and V6/V12, and between V12 and V6 are
also significant (P<0.001; Wilcoxon, n=55).
In patients showing persistence of infection, the
score at VERAD was not significantly different from baseline
values.
A clinically appreciable reduction (28) of ≥6 points of the total score of the
NIH-CPSI questionnaire was achieved in 32 out of 55 (58%) patients
in which infection was eradicated, and in 4 out of 15 (26%)
patients with persistent infection (P<0.05, two-tailed
probability associated with the z-ratio, n=55).
NIH-CPSI pain domain
In the patients in which eradication of infection
was successful, the NIH-CPSI pain score decreased significantly at
VERAD, declined further at time-point V6, and remained unchanged at
V12 (Fig. 1). In patients showing
persistence of infection, the median pain score at VERAD was not
significantly different from baseline values.
The disease severity was categorized using the full
pain domain score of the NIH-CPSI, according to Wagenlehner's
criteria (29). At time-point V0, 27
patients in the eradicated group showed mild pain (0–7 points), 22
patients moderate pain (8–13 points) and 6 severe pain (14–21
points). At the end of therapy 49, 6 and 0 patients showed mild,
moderate and severe pain, respectively [P<0.01, two-tailed
McNemar's test for correlated proportions, dichotomized data (mild
vs. moderate/severe), n=55].
Voiding signs and symptoms
Voiding symptom scores decreased significantly at
VERAD in the eradicated group, and were sustained at time-points V6
and V12 (Fig. 1). This finding was
also evident when the results of the IPSS test were analyzed
(Table V). In patients showing
persistence of infection, the voiding symptom scores at VERAD were
not significantly different from baseline values.
The reduction of voiding symptoms was confirmed by
assessment of the peak urinary flow and of the bladder voiding
efficiency: Mean Qmax increased from 17.93 ml/sec at V0 to 18.97
ml/sec at VERAD, and the percentage bladder voided volume (%BVV;
89% of total bladder content at V0) was also significantly
increased at VERAD (98%) (P<0.05 for both comparisons, paired,
two-tailed t-test, n=55; Table V);
these two signs remained sustainedly improved throughout the entire
follow-up period.
In patients showing pathogen persistence, a
significant increase of the peak urinary flow (from 18.2 ml/sec at
V0 to 19.9 ml/sec at VERAD) was observed. In this cohort, as well
as in the larger cohort of patients in which infection was
eradicated, the administration of alfuzosin up to time-point V6 may
have acted as a major confounder, and no conclusion can be drawn
from data concerning short-term improvement of voiding symptoms.
However, Fig. 1 shows that the
improvement of voiding symptoms was sustained at the 1-year
follow-up time-point (V12), when patients had been off alfuzosin
therapy for 6 months.
NIH-CPSI QoL domain
Pain and voiding symptom relief, assessed with the
NIH-CPSI test, resulted in significant and sustained attenuation of
the impact of the disease on QoL in patients with eradiation of
infection, but not in patients showing persistent infection
(Fig. 1).
Sonography and laboratory
assessments
A significant reduction of the prostatic volume was
identified in patients showing pathogen eradication at time-point
VERAD (mean difference, 5.4 ml lower; P<0.0001, paired t-test,
n=55), but not in patients not responding to the antibacterial
therapy (25.27 ml at VERAD vs. 25.5 ml at V0; P≥0.05, n=15).
The mean PSA serum level decreased significantly in
the eradicated group (mean difference, 0.23 ng/ml lower;
P<0.0001, n=55). However, PSA also decreased significantly in
patients showing pathogen persistence (2.29 at VERAD vs. 3.31 at
V0; P<0.05, n=15).
Study power
Post-hoc computation of achieved power of the
analyses was based on an α error probability of 0.05, a calculated
correlation between paired datasets equal to 0.7 and a sample size
of 55 patients with eradicated infection. The estimated power for
the pre- and post-therapy paired comparison of the total score of
the NIH-CPSI test was >0.9. Sensitivity analysis, given a sample
size of 55, a normal distribution (two-tailed), an α error
probability of 0.05, and an imputed power of 0.99, resulted in a
required effect size of 1.007 (the effect size for the above
comparison calculated in the present study was 1.2).
Discussion
Few drugs are able to reach the prostatic ducts or
acini via the systemic circulation, due to the existence of a
blood-prostate barrier activity preventing the distribution of a
number of chemicals, including antibacterial agents, to the various
cells and regions within the gland (30).
Goto et al authored one of the few studies
investigating the distribution of aminoglycosides and other four
different antibacterials to prostatic fluid/secretions (31). It was shown that the aminoglycoside
amikacin (200 mg, intramuscular) reached the highest concentrations
in prostatic fluids (3.2 µg/ml), compared with β-lactams
(piperacillin, 0.3 µg/ml), tetracyclines (minocycline, 0.62 µg/ml)
or fluoroquinolones (ofloxacin, 1.3 µg/ml), although the latter
showed the highest prostatic fluid-to-serum ratio (ofloxacin: 0.9;
amikacin: 0.25) (31). Notably,
patients enrolled in the study by Goto et al were not affected by
acute prostatitis, known to facilitate the distribution of any drug
to the prostate (31). Thus,
positive evidence is available about the distribution of certain
aminoglycosides to the sites of chronic infection in the
prostate.
Cure of CBP achieved with aminoglycosides was
reported as early as 1976 and 1991 by Pfau and Sacks in isolated
cases or a small series of patients treated with parenteral
kanamycin (1 g twice daily for 3 days, and 500 mg twice daily for
11 days; n=13) or streptomicin (1 g/day for 12 days; 1 case)
(32,33), but to the best of our knowledge the
overall evidence in this respect is scant.
Within the limits of an observational study, the
data in the present study show that microbiological eradication and
sustained clinical symptom remission was achieved in 79% of
patients (or 71%, if a intent-to-treat setting is considered,
including 8 noncompliant/dropped-out patients) subjected to a
course of treatment with an aminoglycoside, administered alone or
in combination with a β-lactam antibiotic. Notably, similar
pathogen eradication rates, ranging between 70 and 80%, have been
reported in the past in CBP patients treated with various
fluoroquinolones (19,24,34,35). In
summary, the results of the present study suggest that
aminoglycosides may be administered to selected CBP patients,
provided that candidates to therapy are at low risk for the serious
adverse effects of these agents.
Therapy with aminoglycosides exposes patients to the
risk of hearing impairment. In particular, the carriers of the 1555
A>G point-mutation within the mitochondrial DNA locus of the 12S
ribosomal RNA develop profound and irreversible non-syndromic
sensorineural hearing loss even after short-term exposure to
aminoglycosides (36). One in 520
(0.19%) European children, 6 in 865 Chinese newborns (0.7%), and 1
in 500 adults of European descent (0.2%) are estimated to be
carriers of this mutation, and are at risk for such severe toxic
effects (37–39). Other important
ototoxicity-predisposing mutations have been discovered, for
example the 1494 C>T and 1556 C>T substitutions in the same
12S rRNA (40,41). In our routine clinical practice,
consenting patient candidates for aminoglycoside therapy are
screened for a small panel of mutations predisposing to severe,
irreversible hearing loss. This preliminary analysis can be
performed in few hours prior to the first dose of the antibiotic,
and allows safer administration of aminoglycosides. However, a
negative pharmacogenetic examination result does not exempt
patients from less severe forms of hearing impairment, possibly
caused by reactive oxygen species. Administration of aspirin has
been shown to partly protect patients from these forms of
ototoxicity (42).
In conclusion, administration of aminoglycosides
eradicated causative pathogens in 79% of patients and significantly
decreased the symptoms of the disease, as assessed with the
NIH-CPSI and IPSS questionnaires. The impact of CBP on the QoL of
patients was also significantly attenuated. Therapy was also
effective in significantly decreasing the prostate volume, probably
by acting on inflammatory edema. The therapy was in general well
tolerated, and genetic testing for mitochondrial mutations
predisposing to sensorineural deafness allowed safer administration
of the antibiotic in a considerable fraction of patients. Thus, the
data presented in this paper indicate that aminoglycosides may be
an interesting option for the treatment of CBP in specific,
carefully selected cases. These findings should be confirmed and
further validated in a randomized-controlled setting.
References
|
1
|
Dennis LK, Lynch CF and Torner JC:
Epidemiologic association between prostatitis and prostate cancer.
Urology. 60:78–83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts RO, Bergstralh EJ, Bass SE, Lieber
MM and Jacobsen SJ: Prostatitis as a risk factor for prostate
cancer. Epidemiology. 15:93–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rybicki BA, Kryvenko ON, Wang Y, Jankowski
M, Trudeau S, Chitale DA, Gupta NS, Rundle A and Tang D: Racial
differences in the relationship between clinical prostatitis,
presence of inflammation in benign prostate and subsequent risk of
prostate cancer. Prostate Cancer Prostatic Dis. 19:145–150. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boehm K, Valdivieso R, Meskawi M, Larcher
A, Schiffmann J, Sun M, Graefen M, Saad F, Parent MÉ and
Karakiewicz PI: Prostatitis, other genitourinary infections and
prostate cancer: Results from a population-based case-control
study. World J Urol. 34:425–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang J, Li J, Yunxia Z, Zhu H, Liu J and
Pumill C: The role of prostatitis in prostate cancer:
Meta-analysis. PLoS One. 8:e851792013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung SD, Keller JJ and Lin HC: A
case-control study of chronic prostatitis/chronic pelvic pain
syndrome and colorectal cancer. BJU Int. 110:550–554. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng I, Witte JS, Jacobsen SJ, Haque R,
Quinn VP, Quesenberry CP, Caan BJ and Van Den Eeden SK:
Prostatitis, sexually transmitted diseases, and prostate cancer:
The California Men's Health Study. PLoS One. 5:e87362010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krieger JN, Nyberg L Jr and Nickel JC: NIH
consensus definition and classification of prostatitis. JAMA.
282:236–237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perletti G, Marras E, Wagenlehner FM and
Magri V: Antimicrobial therapy for chronic bacterial prostatitis.
Cochrane Database Syst Rev. 8:CD0090712013.PubMed/NCBI
|
|
10
|
Noel GJ, Natarajan J, Chien S, Hunt TL,
Goodman DB and Abels R: Effects of three fluoroquinolones on QT
interval in healthy adults after single doses. Clin Pharmacol Ther.
73:292–303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arabyat RM, Raisch DW, McKoy JM and
Bennett CL: Fluoroquinolone-associated tendon-rupture: A summary of
reports in the Food and Drug Administration's adverse event
reporting system. Expert Opin Drug Saf. 14:1653–1660. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalhoff A: Global fluoroquinolone
resistance epidemiology and implictions for clinical use.
Interdiscip Perspect Infect Dis. 2012:9762732012.PubMed/NCBI
|
|
13
|
Sarma JB, Marshall B, Cleeve V, Tate D,
Oswald T and Woolfrey S: Effects of fluoroquinolone restriction
(from 2007 to 2012) on resistance in Enterobacteriaceae:
Interrupted time-series analysis. J Hosp Infect. 91:68–73. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borgmann S, Jakobiak T, Gruber H, Schröder
H and Sagel U: Ciprofloxacin treatment of urinary infections
results in increased resistance of urinary E. coli to ciprofloxacin
and co-trimoxazole. Pol J Microbiol. 58:371–373. 2009.PubMed/NCBI
|
|
15
|
Brede CM and Shoskes DA: The etiology and
management of acute prostatitis. Nat Rev Urol. 8:207–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Etienne M, Chavanet P, Sibert L, Michel F,
Levesque H, Lorcerie B, Doucet J, Pfitzenmeyer P and Caron F: Acute
bacterial prostatitis: Heterogeneity in diagnostic criteria and
management. Retrospective multicentric analysis of 37 patients
diagnosed with acute prostatitis. BMC Infect Dis. 8:122008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanus PM and Danziger LH: Treatment of
chronic bacterial prostatitis. Clin Pharm. 3:49–55. 1984.PubMed/NCBI
|
|
18
|
von Elm E, Altman DG, Egger M, Pocock SJ,
Gøtzsche PC and Vandenbroucke JP: STROBE Initiative: The
Strengthening the Reporting of Observational Studies in
Epidemiology (STROBE) statement: Guidelines for reporting
observational studies. Lancet. 370:1453–1457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Magri V, Montanari E, Škerk V, Markotić A,
Marras E, Restelli A, Naber KG and Perletti G:
Fluoroquinolone-macrolide combination therapy for chronic bacterial
prostatitis: Retrospective analysis of pathogen eradication rates,
inflammatory findings and sexual dysfunction. Asian J Androl.
13:819–827. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stamey TA: Prostatitis. J R Soc Med.
74:22–40. 1981.PubMed/NCBI
|
|
21
|
Magri V, Wagenlehner FM, Montanari E,
Marras E, Orlandi V, Restelli A, Torresani E, Naber KG and Perletti
G: Semen analysis in chronic bacterial prostatitis: Diagnostic and
therapeutic implications. Asian J Androl. 11:461–477. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giubilei G, Mondaini N, Crisci A, Raugei
A, Lombardi G, Travaglini F, Del Popolo G and Bartoletti R: The
Italian version of the National Institutes of Health Chronic
Prostatitis Symptom Index. Eur Urol. 47:805–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morgia G, Mucciardi G, Galì A, Madonia M,
Marchese F, Di Benedetto A, Romano G, Bonvissuto G, Castelli T,
Macchione L, et al: Treatment of chronic prostatitis/chronic pelvic
pain syndrome category IIIA with Serenoa repens plus selenium and
lycopene (Profluss) versus S. repens alone: An Italian randomized
multicenter-controlled study. Urol Int. 84:400–406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naber KG: European Lomefloxacin
Prostatitis Study Group: Lomefloxacin versus ciprofloxacin in the
treatment of chronic bacterial prostatitis. Int J Antimicrob
Agents. 20:18–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casano RA, Bykhovskaya Y, Johnson DF,
Hamon M, Torricelli F, Bigozzi M and Fischel-Ghodsian N: Hearing
loss due to the mitochondrial A1555G mutation in Italian families.
Am J Med Genet. 79:388–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scrimshaw BJ, Faed JM, Tate WP and Yun K:
Rapid identification of an A1555G mutation in human mitochondrial
DNA implicated in aminoglycoside-induced ototoxicity. J Hum Genet.
44:388–390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faul F, Erdfelder E, Lang AG and Buchner
A: G*Power 3: A flexible statistical power analysis program for the
social, behavioral, and biomedical sciences. Behav Res Methods.
39:175–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Propert KJ, McNaughton-Collins M, Leiby
BE, O'Leary MP, Kusek JW and Litwin MS: Chronic Prostatitis
Collaborative Research Network: A prospective study of symptoms and
quality of life in men with chronic prostatitis/chronic pelvic pain
syndrome: The National Institutes of Health Chronic Prostatitis
Cohort study. J Urol. 175:619–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wagenlehner FM, van Till JW, Magri V,
Perletti G, Houbiers JG, Weidner W and Nickel JC: National
Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI)
symptom evaluation in multinational cohorts of patients with
chronic prostatitis/chronic pelvic pain syndrome. Eur Urol.
63:953–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang Y, Cui D and Yi S: Opening tight
junctions may be key to opening the blood-prostate barrier. Med Sci
Monit. 20:2504–2507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goto T, Makinose S, Ohi Y, Yamauchi D,
Kayajima T, Nagayama K and Hayami H: Diffusion of piperacillin,
cefotiam, minocycline, amikacin and ofloxacin into the prostate.
Int J Urol. 5:243–246. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pfau A: The treatment of chronic bacterial
prostatitis. Infection. 19(Suppl 3): S160–S164. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pfau A and Sacks T: Chronic bacterial
prostatitis: New therapeutic aspects. Br J Urol. 48:245–253. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bundrick W, Heron SP, Ray P, Schiff WM,
Tennenberg AM, Wiesinger BA, Wright PA, Wu SC, Zadeikis N and Kahn
JB: Levofloxacin versus ciprofloxacin in the treatment of chronic
bacterial prostatitis: A randomized double-blind multicenter study.
Urology. 62:537–541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Magri V, Trinchieri A, Pozzi G, Restelli
A, Garlaschi MC, Torresani E, Zirpoli P, Marras E and Perletti G:
Efficacy of repeated cycles of combination therapy for the
eradication of infecting organisms in chronic bacterial
prostatitis. Int J Antimicrob Agents. 29:549–556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fischel-Ghodsian N, Prezant TR, Chaltraw
WE, Wendt KA, Nelson RA, Arnos KS and Falk RE: Mitochondrial gene
mutation is a significant predisposing factor in aminoglycoside
ototoxicity. Am J Otolaryngol. 18:173–178. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bitner-Glindzicz M, Pembrey M, Duncan A,
Heron J, Ring SM, Hall A and Rahman S: Prevalence of mitochondrial
1555A––>G mutation in European children. N Engl J Med.
360:640–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vandebona H, Mitchell P, Manwaring N,
Griffiths K, Gopinath B, Wang JJ and Sue CM: Prevalence of
mitochondrial 1555A>G mutation in adults of European descent. N
Engl J Med. 360:642–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen G, Wang X and Fu S: Prevalence of
A1555G mitochondrial mutation in Chinese newborns and the
correlation with neonatal hearing screening. Int J Pediatr
Otorhinolaryngol. 75:532–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao H, Li R, Wang Q, Yan Q, Deng JH, Han
D, Bai Y, Young WY and Guan MX: Maternally inherited
aminoglycoside-induced and nonsyndromic deafness is associated with
the novel C1494T mutation in the mitochondrial 12S rRNA gene in a
large Chinese family. Am J Hum Genet. 74:139–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tanimoto H, Nishio H, Matsuo M and Nibu K:
A novel mitochondrial mutation, 1556C––>T, in a Japanese patient
with streptomycin-induced tinnitus. Acta Otolaryngol. 124:258–261.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sha SH, Qiu JH and Schacht J: Aspirin to
prevent gentamicin-induced hearing loss. N Engl J Med.
354:1856–1857. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Charalabopoulos K, Karachalios G,
Baltogiannis D, Charalabopoulos A, Giannakopoulos X and Sofikitis
N: Penetration of antimicrobial agents into the prostate.
Chemotherapy. 49:269–279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kobayashi I, Ikawa K, Nakamura K,
Nishikawa G, Kajikawa K, Yoshizawa T, Watanabe M, Kato Y, Zennami
K, Kanao K, et al: Penetration of piperacillin-tazobactam into
human prostate tissue and dosing considerations for prostatitis
based on site-specific pharmacokinetics and pharmacodynamics. J
Infect Chemother. 21:575–580. 2015. View Article : Google Scholar : PubMed/NCBI
|















