Introduction
Osteoarthritis (OA) is the most common type of
arthritis in adults worldwide, severely impairing patients' quality
of life (1). OA is characterized by
progressive degeneration of articular cartilage and bony changes,
including increased turnover of the subchondral bone, thinning of
the trabecular structure, osteophytes, bone marrow lesions and
sclerosis of the subchondral plate (2). Previous experimental and clinical
studies have suggested that the structural integrity of articular
cartilage is dependent on normal subchondral bone turnover, intact
chondrocyte function and appropriate biomechanical stress (3,4). Bone
and cartilage health appear to be closely associated, and various
studies have reported secondary positive effects on cartilage
health when bone resorption is suppressed, or deterioration of
cartilage when resorption is enhanced (5,6).
As members of the tumor necrosis factor superfamily,
the molecular triad of osteoprotegerin (OPG)/receptor activator of
nuclear factor κβ (RANK)/receptor activator of nuclear factor κβ
ligand (RANKL) represents a key cytokine system for regulating bone
metabolism (7,8). In OA, remodeling of the subchondral
bone is reported to be RANKL-dependent, and osteoblasts express
RANKL in subchondral bone (4,9).
Furthermore, previous studies suggests that OPG is associated with
the regulation of cartilage metabolism, as OPG-deficient mice
exhibit thinned articular cartilage layers, severe destruction of
growth plate cartilage and enhanced cartilage degradation with
aging (10,11). Structural integrity of articular
cartilage is influenced by changes in subchondral bone as denser
bone is detected below OA cartilage (2). RANKL and OPG are synthesized and
expressed by articular chondrocytes in a position adjacent to
subchondral bone (12,13); therefore, these cytokines may affect
bone turnover and alter bone density.
Despite the lack of detailed insight into the
etiology and pathology of OA, it is well-documented that the
degradation and destruction of type II collagen caused by matrix
metalloproteinase-13 (MMP-13) has a key role in the occurrence and
development of OA (14–16). Therefore, MMP-13 represents a target
for the prevention of the onset or retardation of OA
progression.
It has been demonstrated that the RANKL-OPG system
is associated with the pathogenesis of OA (10,17).
MMP-13 is a crucial collagenase that mediates type II collagen
degradation, which is an important component of cartilage (18–20).
Based on these findings of previous studies (21,22), in
the present study SW1353 human chondrosarcoma cells stimulated by
interleukin (IL)-1β were used as a cell model of OA to investigate
the effects of RAKNL/OPG at various ratios on the MMP-13 mRNA and
protein expression levels of SW1353 chondrosarcoma cells.
Materials and methods
Reagents and cell lines
Recombinant human OPG and RANKL were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany), dissolved in
double-distilled water, diluted to 100 µg/ml using Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), and subsequently stored at −20°C. IL-1β
was stored at −20°C at a concentration of 100 ng/ml. The required
concentrations of OPG, RANKL, and IL-1β used in the following
experiments were prepared by further dilution with DMEM.
SW1353 human chondrosarcoma cells (Cell Applications
Inc., San Diego, CA, USA) were cultivated in DMEM supplemented with
10% (v/v) fetal bovine serum and 100 U/ml penicillin-streptomycin
solution at 37°C in a humidified atmosphere containing 5%
CO2. Prior to the addition of experimental components,
SW1353 cells were seeded in a 6-well culture flask at a density of
~105 cells/cm2/well in serum-free DMEM
supplemented with 100 U/ml penicillin-streptomycin to starve the
cells for 24 h.
MTT assay
Cytotoxicity of various ratios of OPG and RANKL/OPG
(1:160, 1:80, 1:40, 1:20, 1:10, 1:5 and 1:2.5) to SW1353 cells was
evaluated using the MTT assay. Final concentrations of OPG and
RANKL were 200 ng/ml and 1.25, 2.5, 5, 10, 20, 40 and 80 ng/ml.
SW1353 cells were cultured in 96-well plates (5,000 cells/well)
with OPG and RANKL/OPG and incubated for 24 h. Subsequently, MTT
regent (Sigma-Aldrich; Merck Millipore) was added to each well and
the cells were incubated for a further 4 h. Supernatants were
removed, and DMSO (Sigma-Aldrich; Merck Millipore) was added to the
wells to dissolve the formazan crystals. Optical absorbance values
of each well were recorded at 450 nm using an enzyme-labeled meter
(Thermo Fisher Scientific, Inc.). The same procedure was repeated
three times.
ELISA
SW1353 cells were pre-treated with OPG and RANKL/OPG
for 1 h, which was followed by stimulation with IL-1β (5 ng/ml) for
24 h or no treatment at all. The effect of IL-1β, OPG and/or
RANKL/OPG on the protein levels of MMP-13 secreted by SW1353 cells
in the culture supernatant was evaluated by ELISA kits (ab100605;
Abcam, Cambridge, UK), according to the manufacturer's
instructions. All ELISA experiments were performed in
triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted using TRIzol
reagent from SW1353 cells (Dingguochangsheng Biotechnology, Co.,
Beijing, China) according to the manufacturer's instructions.
Extracted RNA was subsequently dissolved in
diethylpyrocarbonate-treated water and stored at −80°C prior to
use. First-strand cDNA was synthesized using 1 µg total RNA treated
with DNase to remove genomic DNA with a PrimeScript-RT reagent kit
(Tiangen Biotech, Beijing, China). PCR amplification was performed
using specifically designed primers (Table I), the SYBR Premix Ex Taq
(Takara Biotechnology Co., Ltd., Dalian, China) and the StepOne
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR reaction contained the following: 4 µl total RNA, 4
µl 5X buffer, 1 µl dNTPs (10 mM), 1 µl oligo(dT)18 (50 µM), 0.5 µl
random primer (100 µM), 1 µl MMLV-RT (200 U/µl) and 8.5 µl DEPC
H2O in a total volume of 20 µl. qPCR reaction volumes
are presented in Table II. Typical
thermal conditions were used as follows: Denaturalization at 95°C
for 30 sec; annealing for 40 cycles at 60°C for 32 sec; and
extension at 95°C for 15 sec. GADPH mRNA expression was used as an
endogenous control. MMP-13 mRNA levels were normalized to those of
GAPDH. All experiments were repeated three times and analyzed using
the 2−ΔΔCq method (23).
 | Table I.Primers used for polymerase chain
reaction analysis. |
Table I.
Primers used for polymerase chain
reaction analysis.
| Target | Primer sequence |
|---|
| MMP-13 |
|
|
Forward |
5′-CAGAACATCATCCCTGCCTCT-3′ |
|
Reverse |
5′-GCCCATCAAATGGGTAGAAG-3′ |
| GAPDH |
|
|
Forward |
5′-CAGAACATCATCCCTGCCTCT-3′ |
|
Reverse |
5′-GCTTGACAAAGTGGTCGTTGAG-3′ |
 | Table II.Quantitative polymerase chain reaction
components. |
Table II.
Quantitative polymerase chain reaction
components.
| Content | Volume (µl) | Concentration |
|---|
| cDNA | 1 | – |
| Forward primer | 0.5 | 20 pmol/µl |
| Reverse primer | 0.5 | 20 pmol/µl |
| 2X Mix | 12.5 | 1X |
| 10X SYBR Green I | 1 | 0.4X |
| ddH2O | 9.5 | – |
| Total volume | 25 | – |
Western blotting
Total protein was extracted using
radio-immunoprecipitation assay buffer supplemented with 1%
protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN,
USA). Phosphorylated protein was obtained using a phosphorylated
protein extraction kit (Roche Diagnostics). Protein samples (40 µg)
were separated by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinylidene difluoride
membranes. Membranes were blocked with 5% non-fat milk for 2 h,
which was dissolved in 1% Tris-buffered saline with Tween 20
(TBS-T) buffer and incubated with primary antibodies against MMP-13
(sc-31811; 1:500; polyclonal goat IgG) and with monoclonal mouse
IgG1 β-actin (sc-130301; 1:5,000; both Santa Cruz Biotechnology,
Dallas, USA) as the internal reference. Both incubations were for 1
h. Membranes in the buffer were gently shaken at 4°C overnight, and
then washed with 1% TBS-T for 3 min thrice, followed by incubation
with the appropriate horseradish peroxidase-labeled secondary
antibodies for 1 h at room temperature. Proteins were visualized
using enhanced chemiluminescence reagent (Beyotime Institute of
Biotechnology, Haimen, China). A ChemiDoc XRS+ (Bio-Rad
Laboratories, Hercules, CA, USA) system was used to analyze the
protein bands. Data from three independent experiments were
obtained and calculated as the ratio of the gray value of MMP-13
protein divided by that of β-actin.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical differences were evaluated using one-way
analysis of variance and P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of OPG and RANKL/OPG on SW1353
cell viability
The cytotoxic effect of OPG (200 ng/ml) and
RANKL/OPG on SW1353 cells at various ratios (1:160, 1:80, 1:40,
1:20, 1:10, 1:5 and 1:2.5; OPG, 200 ng/ml; RANKL, 1.25–80 ng/ml)
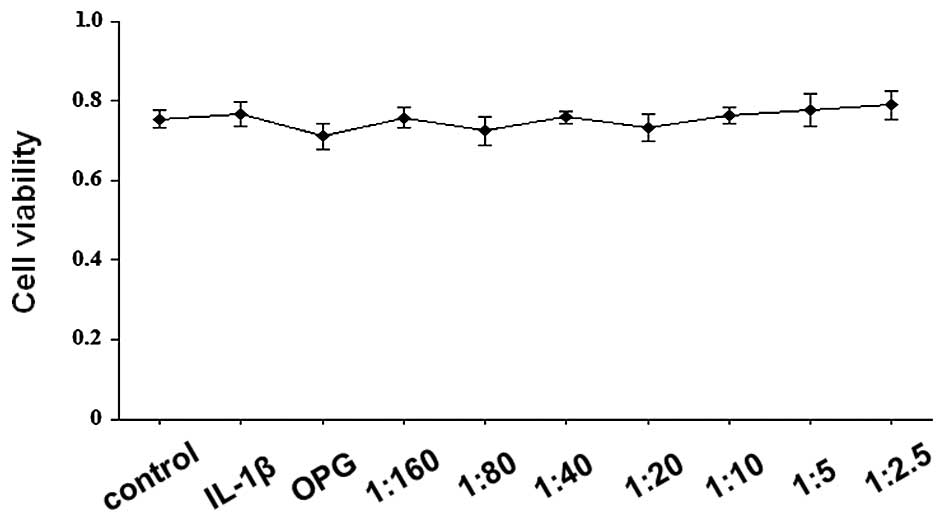
was assessed using an MTT assay. As shown in Fig. 1, cell viability values were
consistently >70%, indicating that OPG and RANKL/OPG did not
exhibit significant cytotoxic effects. The RANKL/OPG mixture at
these ratios was used in the subsequent experiments.
MMP-13 mRNA expression levels in
IL-1β-stimulated SW1353 cells treated with OPG and RANKL/OPG
SW1353 cells were treated with OPG (200 ng/ml) and
RANKL/OPG at various ratios (1:160, 1:80, 1:40, 1:20, 1:10, 1:5 and
1:2.5; OPG, 200 ng/ml; RANKL, 1.25–80 ng/ml) for 1 h, and were
subsequently stimulated by 5 ng/ml IL-1β for 24 h. Total RNA and
cell extracts were collected after 48 h and the mRNA expression
levels of MMP-13 were detected in the cell extracts by RT-qPCR. As
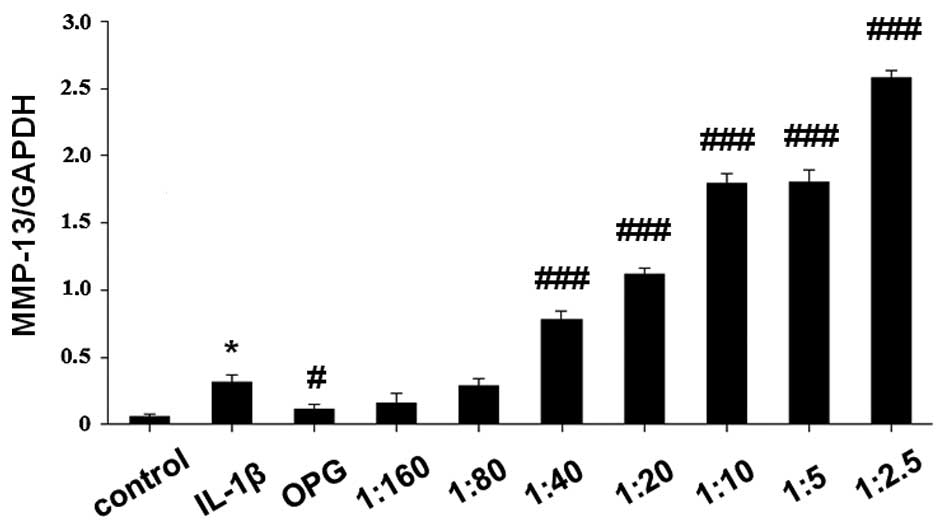
demonstrated in Fig. 2, OPG
significantly inhibited the expression levels of MMP-13 mRNA
(P<0.05 vs. IL-1β-stimulated cells). MMP-13 mRNA expression
levels were significantly elevated by the increasing RANKL/OPG
ratio in a concentration-dependent manner (P<0.001 vs.
IL-1β-stimulated cells).
MMP-13 protein expression levels in
IL-1β-stimulated SW1353 cells treated with OPG and RANKL/OPG
To investigate the effect of OPG and RANKL/OPG on
MMP-13 protein expression levels, SW1353 cells were pretreated with
OPG (200 ng/ml) and RANKL/OPG at various ratios (1:160, 1:80, 1:40,
1:20, 1:10, 1:5, 1:2.5; OPG, 200 ng/ml; RANKL, 1.25–80 ng/ml) for 1
h, followed by co-incubation with IL-1β (5 ng/ml) for 24 h. MMP-13
protein was extracted and analyzed by western blot analysis, and
the supernatant was collected and analyzed using an ELISA kit
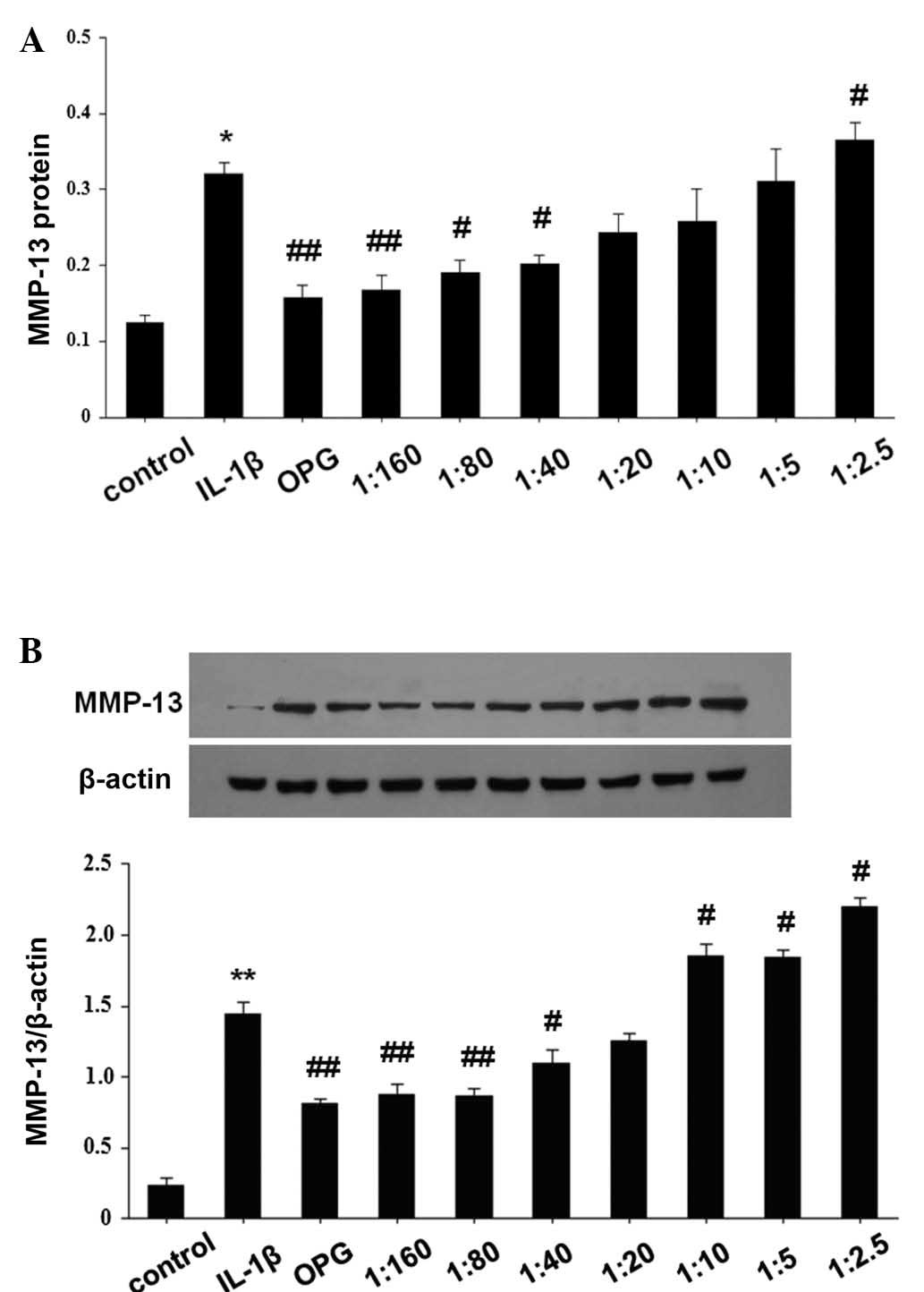
(Fig. 3). The results of protein
expression analysis were consistent with that of RT-qPCR. As shown
in Fig. 3, OPG treatment
significantly reduced MMP-13 protein expression levels (P<0.01
vs. IL-1β-stimulated cells); however, as the RANKL/OPG ratio
increased, MMP-13 protein expression was significantly enhanced in
IL-1β-stimulated SW1353 cells (P<0.05 vs. IL-1β-stimulated
cells).
Discussion
Despite current treatment methods, including total
joint arthroplasty, OA remains a troublesome disease that affects
numerous elderly people (24,25). In
the present study, MMP-13 mRNA and protein expression levels were
elevated in IL-1β-stimulated SW1353 human chondrosarcoma cells
treated with an increased RANKL/OPG ratio. To the best of our
knowledge, the present study was the first report to investigate
the association between RANKL/OPG at various ratios and MMP-13,
which indicates that RANKL/OPG may have an important role in the
progression of OA.
OA development is an irreversible bone disorder
caused by cartilage destruction due to the degradation of type II
collagen (26). Previous studies
have demonstrated that aberrant expression of MMPs has a pivotal
role in the destruction of articular cartilage (14,27).
MMPs, as a family of collagenolytic enzymes, regulate various
functions in articular cartilage, including turnover, catabolism
and the degradation of the extracellular matrix. Among all MMPs,
MMP-13 is the primary collagenase in OA, with an activity on type
II collagen that is much higher than the other MMPs (28). MMP-13 is predominantly localized in
the deeper layers of cartilage (29). In a study performed by Upton et
al (30), increased RANKL mRNA
expression levels were observed in grade II OA cartilage,
particularly in the deep layer of cartilage. Various previous
studies have reported that RANKL is expressed by chondrocytes in
normal and OA cartilage (12,31).
However, the role of RANKL in OA is yet to be fully elucidated, and
the association between RANKL/OPG and MMP-13 may aid understanding
of this mechanism. The findings of the present study showed that an
elevated ratio of RANKL/OPG increased the expression of MMP-13.
Although the exact underlying mechanism remains unclear, these
results indicate that RANKL overexpression may exacerbate cartilage
destruction by increasing the expression of MMP-13. A previous
biochemical analysis of the circulating levels of macromolecules
released from cartilage and bone in humans revealed a convergence
of the pathological processes in cartilage and subchondral bone in
OA at each stage (6). Furthermore, a
previous study demonstrated that RANKL secreted by chondrocytes
diffuse across the thin layer of calcified cartilage into
subchondral bone, resulting in morphological changes to subchondral
bone, which is an important factor in OA pathophysiology (30). Combined with the results of the
present study, we hypothesize that RANKL overexpression in
subchondral bone may diffuse into cartilage and elevate MMP-13
expression levels, which subsequently accelerates cartilage
degradation.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that an increased
RAKNL/OPG ratio induces MMP-13 mRNA and protein expression. These
finding may indicate a potential strategy for OA treatment.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (grant no. 31171672). The authors would
also would like to thank the Beijing Key Laboratory of Translation
Medicine in Liver Cirrhosis (Beijing, China) and the National
Clinical Research Center of Digestive Diseases (Beijing, China) for
assistance.
References
|
1
|
Moyer RF, Ratneswaran A, Beier F and
Birmingham TB: Osteoarthritis year in review 2014: Mechanics -
basic and clinical studies in osteoarthritis. Osteoarthritis
Cartilage. 22:1989–2002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayami T, Pickarski M, Wesolowski GA,
McLane J, Bone A, Destefano J, Rodan GA and Duong LT: The role of
subchondral bone remodeling in osteoarthritis: Reduction of
cartilage degeneration and prevention of osteophyte formation by
alendronate in the rat anterior cruciate ligament transection
model. Arthritis Rheum. 50:1193–1206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaspiris A, Mikelis C, Heroult M, Khaldi
L, Grivas TB, Kouvaras I, Dangas S, Vasiliadis E, Lioté F, Courty J
and Papadimitrou E: Expression of the growth factor pleiotrophin
and its receptor protein tyrosine phosphatase beta/zeta in the
serum, cartilage and subchondral bone of patients with
osteoarthritis. Joint Bone Spine. 80:407–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwan Tat S, Pelletier JP, Lajeunesse D,
Fahmi H, Lavigne M and Martel-Pelletier J: The differential
expression of osteoprotegerin (OPG) and receptor activator of
nuclear factor kappaB ligand (RANKL) in human osteoarthritic
subchondral bone osteoblasts is an indicator of the metabolic state
of these disease cells. Clin Exp Rheumatol. 26:295–304.
2008.PubMed/NCBI
|
|
5
|
Hayami T, Pickarski M, Zhuo Y, Wesolowski
GA, Rodan GA and Duong LT: Characterization of articular cartilage
and subchondral bone changes in the rat anterior cruciate ligament
transection and meniscectomized models of osteoarthritis. Bone.
38:234–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petersson IF, Boegård T, Svensson B,
Heinegård D and Saxne T: Changes in cartilage and bone metabolism
identified by serum markers in early osteoarthritis of the knee
joint. Br J Rheumatol. 37:46–50. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin TJ: Historically significant events
in the discovery of RANK/RANKL/OPG. World J Orthop. 4:186–197.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walsh MC and Choi Y: Biology of the
RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front Immunol.
5:5112014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones DH, Kong YY and Penninger JM: Role
of RANKL and RANK in bone loss and arthritis. Ann Rheum Dis. 61
Suppl 2:ii32–ii39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu S, Asou Y, Itoh S, Chung UI,
Kawaguchi H, Shinomiya K and Muneta T: Prevention of cartilage
destruction with intraarticular osteoclastogenesis inhibitory
factor/osteoprotegerin in a murine model of osteoarthritis.
Arthritis Rheum. 56:3358–3365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amizuka N, Shimomura J, Li M, Seki Y, Oda
K, Henderson JE, Mizuno A, Ozawa H and Maeda T: Defective bone
remodelling in osteoprotegerin-deficient mice. J Electron Microsc
(Tokyo). 52:503–513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komuro H, Olee T, Kühn K, Quach J, Brinson
DC, Shikhman A, Valbracht J, Creighton-Achermann L and Lotz M: The
osteoprotegerin/receptor activator of nuclear factor
kappaB/receptor activator of nuclear factor kappaB ligand system in
cartilage. Arthritis Rheum. 44:2768–2776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Troeberg L and Nagase H: Proteases
involved in cartilage matrix degradation in osteoarthritis. Biochim
Biophys Acta. 1824:133–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takaishi H, Kimura T, Dalal S, Okada Y and
D'Armiento J: Joint diseases and matrix metalloproteinases: A role
for MMP-13. Curr Pharm Biotechnol. 9:47–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haynes DR, Barg E, Crotti TN, Holding C,
Weedon H, Atkins GJ, Zannetino A, Ahern MJ, Coleman M,
Roberts-Thomson PJ, et al: Osteoprotegerin expression in synovial
tissue from patients with rheumatoid arthritis,
spondyloarthropathies and osteoarthritis and normal controls.
Rheumatology (Oxford). 42:123–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Y and Tuan RS: Origin and function
of cartilage stem/progenitor cells in osteoarthritis. Nat Rev
Rheumatol. 11:206–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silverwood V, Blagojevic-Bucknall M, Jinks
C, Jordan JL, Protheroe J and Jordan KP: Current evidence on risk
factors for knee osteoarthritis in older adults: A systematic
review and meta-analysis. Osteoarthritis Cartilage. 23:507–515.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blagojevic M, Jinks C, Jeffery A and
Jordan KP: Risk factors for onset of osteoarthritis of the knee in
older adults: UA systematic review and meta-analysis.
Osteoarthritis Cartilage. 18:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tetsunaga T, Nishida K, Furumatsu T,
Naruse K, Hirohata S, Yoshida A, Saito T and Ozaki T: Regulation of
mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2
transcriptional factor in SW1353 chondrocyte-like cells.
Osteoarthritis Cartilage. 19:222–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gebauer M, Saas J, Sohler F, Haag J, Söder
S, Pieper M, Bartnik E, Beninga J, Zimmer R and Aigner T:
Comparison of the chondrosarcoma cell line SW1353 with primary
human adult articular chondrocytes with regard to their gene
expression profile and reactivity to IL-1beta. Osteoarthritis
Cartilage. 13:697–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang HY, Blunt L, Jiang XQ, Brown L,
Barrans S and Zhao Y: Femoral stem wear in cemented total hip
replacement. Proc Inst Mech Eng H. 222:583–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang HY, Brown L, Barrans S, Blunt L and
Jiang XQ: Investigation of relative micromotion at the stem-cement
interface in total hip replacement. Proc Inst Mech Eng H.
223:955–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sulzbacher I: Osteoarthritis: Histology
and pathogenesis. Wien Med Wochenschr. 163:212–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: An overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Konttinen YT, Ainola M, Valleala H, Ma J,
Ida H, Mandelin J, Kinne RW, Santavirta S, Sorsa T, López-Otín C
and Takag M: Analysis of 16 different matrix metalloproteinases
(MMP-1 to MMP-20) in the synovial membrane: Different profiles in
trauma and rheumatoid arthritis. Ann Rheum Dis. 58:691–697. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fernandes JC, Martel-Pelletier J,
Lascau-Coman V, Moldovan F, Jovanovic D, Raynauld JP and Pelletier
JP: Collagenase-1 and collagenase-3 synthesis in normal and early
experimental osteoarthritic canine cartilage: An
immunohistochemical study. J Rheumatol. 25:1585–1594.
1998.PubMed/NCBI
|
|
30
|
Upton AR, Holding CA, Dharmapatni AA and
Haynes DR: The expression of RANKL and OPG in the various grades of
osteoarthritic cartilage. Rheumatol Int. 32:535–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Tuyl LH, Voskuyl AE, Boers M, Geusens
P, Landewé RB, Dijkmans BA and Lems WF: Baseline RANKL:OPG ratio
and markers of bone and cartilage degradation predict annual
radiological progression over 11 years in rheumatoid arthritis. Ann
Rheum Dis. 69:1623–1628. 2010. View Article : Google Scholar : PubMed/NCBI
|