Introduction
The exposure of animals to heat stress has
previously been associated with increased morbidity and mortality,
which in turn have led to substantial economic losses to animal
product industries (1). Typically,
the increased mortality rate associated with heat stress is a
result of organ failure, particularly heart failure preceded by
cardiovascular damage (2).
Heat shock proteins (HSPs), which are ubiquitously
expressed and highly conserved in prokaryotes and eukaryotes
(3) are classified into families
according to their molecular size, including small HSPs, HSP27,
HSP40, HSP60, HSP70, HSP90 and HSP110 (4). HSPs are molecular chaperones that have
been shown to perform important functions in the folding, unfolding
(5) and translocation (6,7) of
proteins, in addition to the assembly and disassembly of protein
complexes (8,9).
HSP60 and HSP10 are two important chaperones that
interact in a two-step folding mechanism in the mitochondria of
prokaryotic and eukaryotic cells (10). HSP10 has been reported to be a
cofactor involved in HSP60-mediated protein folding and sorting
(11). Previous studies
investigating HSP60 and HSP10 have focused on their roles in
prokaryotic (12) and tumor cells
(13,14). In addition, in eukaryotic cells,
there have been reports that HSP60 may be involved in
angiocardiopathy (15,16). Furthermore, a previous study reported
significantly increased levels of HSP60 in the heart tissue, but
not in the liver and kidney tissues, of heat-stressed chickens and
pigs that have been transported for a long period of time (17). In a previous study, the abnormal
trafficking of HSP60 to the cell surface was suggested to be an
early trigger for myocyte loss and the progression of heart failure
(18). Previous studies have
associated HSP10 with numerous cellular processes, including
cellular differentiation (19,20),
cell proliferation (21,22), cell apoptosis (23,24) and
cytoprotection (25,26). Although there is an increasing
awareness regarding the protective functions of HSPs, and their
importance in numerous regulatory pathways, little is known
regarding the expression levels of HSP60 and HSP10 in the heart
tissue of mammals under conditions of heat stress. Therefore, the
present study aimed to investigate the dynamic expression levels of
HSP60 and HSP10, and their associations, in the heart tissue of
heat-stressed rats in vivo.
Materials and methods
Animals and experimental design
A total of 40 adult Sprague-Dawley (SD) rats (20
female and 20 male rats; weight, ~220 g) were purchased from
Qinglong Mountain Animal Breeding Ground of the Nanjing Jiangning
District (Nanjing, China) for use in the present study. The rats
were randomly divided into four groups (n=10 per group). The rats
were maintained under standard conditions for 7 days to allow them
to acclimatize to their new surroundings and to recover from
environmental stress. During this period, the humidity of the
chamber was maintained at 60±10% and the room temperature was
25±1°C. On day 8, all rats were transferred to a controlled climate
chamber (RX8-500D; New Jiangnan, Co., Ltd., Ningbo, China) and
exposed to 42±1°C for 0 (control), 20, 80 and 100 min. During the
heat stress period, the rats received ad libitum access to
feed-stuff and water. The rats were sacrificed by decapitation,
after which blood was collected to prepare serum, manually
eviscerated and the hearts were rapidly dissected. Half of the
heart tissue samples were fixed in 10% neutral-buffered formalin (G
fan Biological Technology Co., Ltd., M003, Shanghai, China) for
histopathological analyses and the other half was stored in liquid
nitrogen (−196°C) for biological analyses.
The present study was conducted in accordance with
the recommendations outlined in the Guide for the Care and Use of
Laboratory Animals of the Nanjing Agricultural University (Nanjing,
China), and the guidelines of the Animal Ethics Committee of
Jiangsu Province (Nanjing, China). The protocol was approved by the
Committee on the Ethics of Animal Experiments of Nanjing
Agricultural University (permit no. SYXK (su) 2011-0036).
Determination of enzyme activity
The activities of serum lactate dehydrogenase (LDH;
A020-2) and creatine kinase isoenzyme MB (CKMB; H197) were
determined using commercial kits (Nanjing Jiancheng Biochemical
Reagent Co., Nanjing, China) and a clinical autoanalyzer (Vital
Scientific NV, Dieren, Netherlands), according to the
manufacturer's protocols.
Histopathological analysis
Paraffin-embedded heart tissues that had been fixed
in 10% neutral-buffered formalin were serially sliced into 4-µm
sections. One of the sections was stained with hematoxylin and
eosin (HE; Liansuo Biological Technology Co., Ltd., Shanghai,
China), and examined under a light microscope (Axioskop 2 plus;
Zeiss GmbH, Jena, Germany).
Immunohistochemical analysis
The heart tissue sections from the heat-stressed and
control groups were examined using the
streptavidin-biotin-peroxidase complex procedure (85–6643;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
sections were dewaxed and rehydrated, then incubated with 3%
hydrogen peroxide in methanol for 10 min in order to inhibit
endogenous peroxidases. Subsequently, the tissue sections were
placed in 10 mM citric acid buffer (pH 6.0), then heated in a
microwave oven at 800 W for 3 min and 400 W for 10 min. The
sections were incubated with antibodies against HSP60 and HSP10
[1:200 in phosphate-buffered saline (PBS; Wuhan Boster Biological
Technology, Ltd., Wuhan, China); polyclonal rabbit HSP10
(ADI-SPA-110; Enzo Life Sciences, Inc., Farmingdale, NY, USA);
mouse monoclonal HSP60 (ab5478; Abcam, Cambridge, UK)] overnight at
4°C. For the negative control, PBS was run instead of the primary
antibody. The slices were incubated with a biotinylated secondary
mouse antibody from a Histostain-Plus IHC Kit (85–6643; Thermo
Fisher Scientific, Inc.) for 20 min at 37°C in a humidified
chamber. Subsequently, the tissue sections were washed three times
with PBS, incubated for 20 min in horseradish
peroxidase-streptavidin (85–6643; Thermo Fisher Scientific, Inc.),
then washed three times with PBS for 5 min each. Antibody complexes
were visualized by incubating the tissue sections with
3–3′-diaminobenzidine (00–2014; Thermo Fisher Scientific, Inc.),
after which the tissue sections were incubated with hematoxylin for
30 sec for nuclear counterstaining, followed by mounting. The
corresponding negative control sections were prepared by omitting
the antibodies.
Western blot analysis
Total protein was extracted from the heart tissues
of the rats in the control and heat-stressed groups using
ultrasonication (JY99-IIDN; Ningbo New Cheese Instrument Co., Ltd.,
Ningbo, China) and Radio Immunoprecipitation Assay lysis buffer
(Beyotime Institute of Biotechnology, Nanjing, China), and protein
concentrations were determined using the bicinchoninic acid assay
(232235; Micro BCA™ Protein Assay kit; Thermo Fisher Scientific,
Inc.). Heart protein extract (80 µg) was electrophoresed using a 5%
sodium dodecyl sulfate (SDS) polyacrylamide spacer gel (60 V; 30
min) and a 12% SDS separation gel (100 V; 1.5 h; both from Tiandz,
Inc., Beijing, China), then transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA) by
electrotransfer (200 mA; 1 h). The membranes were washed four times
in washing buffer [20 mM Tris base, pH 7.6; 12.5 mM NaCl; and 0.5%
Tween-20 (TBST buffer); Beijing Donglinchangsheng Biotechnology
Co., Ltd., Beijing, China] then blocked with 5% non-fat milk in
Tris-buffered saline (20 mM Tris-HCl, pH 7.6; 137 mM NaCl)
containing 0.1% Tween-20 (TBST) for 1 h at room temperature.
Subsequently, the membranes were incubated with anti-rat monoclonal
antibodies against HSP10 (1:2,000), HSP60 (1:20,000; ab13532;
Abcam), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
1:1,000; ab8224; Abcam) for 1 h at 37°C. After washing with TBST,
the membranes were incubated with peroxidase-conjugated secondary
antibody (1:1,000; BA1038; Boster Systems, Inc., Pleasanton, CA,
USA) at room temperature for 1 h. Western blotting luminal reagent
(Thermo Fisher Scientific, Inc.) was used to detect the
antibody-antigen complexes. Bands on the developed film were
quantified using Quantity One software, version 4.6.2 (Bio-Rad
Laboratories, Inc.). The densities of the HSP60 and HSP10 protein
bands were normalized against GAPDH.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the heart tissues of
the rats using the RNAiso Plus reagent (D9108A; Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol. The optical density (OD) at 260 nm
(OD260)/OD280 value of all the RNA samples was between 1.8 and 2.0,
and the concentration of each RNA sample exceeded 1,000 ng/µl. RNA
samples were reverse transcribed into cDNA using the PrimeScript RT
Master Mix (DRR036A; Takara Biotechnology, Co., Ltd.), according to
the manufacturer's protocol, and the reaction products were stored
at −80°C until further experimentation. cDNA samples (2 µl) were
suspended in a qPCR reaction system containing 10 µl 2X SYBR Premix
Ex Taq (DRR041S; Takara Biotechnology, Co., Ltd.), 0.6 µl each of
the forward and reverse primers, and double-distilled water to a
total volume of 20 µl. PCR primers were designed according to
target mRNAs using Primer Premier software, version 5.0 (Premier
Biosoft International, Palo Alto, CA, USA). The accession numbers
of the mRNA sequences obtained from the GenBank database
(www.ncbi.nlm.nih.gov/genbank/) were
NM_012966.1, NM_022229.2 and NM_031144.3 for HSP10, HSP60
and β-actin, respectively. The primer sequences were as
follows: HSP10 (147 bp) forward, 5′-GAGTATTGGTTGAAAGGAGTG-3′
and reverse, 5′-TGACAGGCTGAATCTCTCC-3′; HSP60 (128 bp)
forward, 5′-CCGCCCCGCAGAAATGCTTCGA-3′ and reverse,
5′-AGGCTCGAGCATCCGCACCAA-3′; and β-actin (110 bp) forward,
5′-TGCGCAAGTTAGGTTTTGTCA-3′ and reverse,
5′-GCAGGAGTACGATGAGTCCG-3′. PCR was conducted using the Bio-Rad iQ5
Real-Time PCR Thermocycler (Bio-Rad Laboratories, Inc.), according
to the manufacturer's protocol. Briefly, enzyme activation was
performed at 95°C for 3 min, followed by 40 cycles of denaturation
at 95°C for 20 sec, annealing at 60°C for 30 sec, and elongation at
72°C for 30 sec. For each run, a negative control without cDNA was
analyzed along with the experimental samples to ensure that there
was no contaminating genomic DNA. A fourfold multiproportion
dilution series of the cDNA was used in the qPCR reactions to
obtain standard curves as follows: HSP10 mRNA slope=−3.39 and
r2=0.995; HSP60 mRNA slope=−3.43 and
r2=0.998; and β-actin mRNA slope=−3.49 and
r2=0.998. The amplification efficiencies of the target
and reference genes were approximately equal. Therefore, the HSP60
and HSP10 mRNA levels were normalized against β-actin mRNA
levels using the 2−ΔΔCq method (27).
Statistical analysis
Differences between two groups were compared using
one-way analysis of variance, followed by Fisher's Least
Significant Difference and Duncan's new multiple range test,
conducted using SPSS software, version 17.0 for Windows (SPSS,
Inc., Chicago, IL, USA). Data are presented as the mean ± standard
deviation of at least three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
Mortality of the rats during the heat
stress period
After 20 min heat stress the rats began to exhibit
signs of polypnea and nervousness (identified by signs of
agitation). After 40 min heat stress, the rats were sweating and
exhibited signs of thirst (identified by a high frequency of
drinking water). After 60 min heat stress, a few of the rats were
pronated, while after 100 min heat stress all the rats were
pronated and appeared comatose, such that the experiment was
terminated.
Enzyme activities and clinical
symptoms of heat-stressed rats
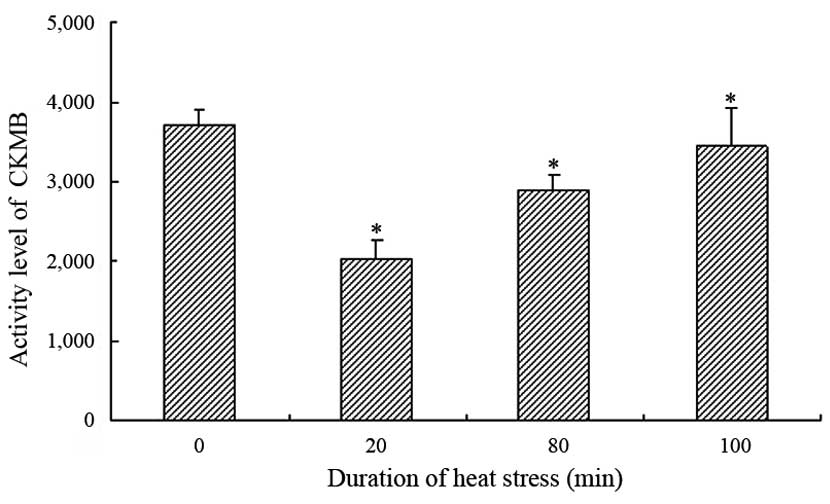
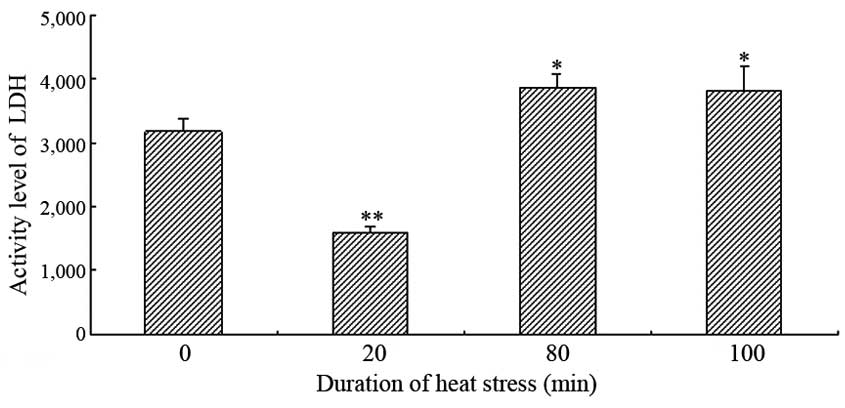
The serum activities of CKMB and LDH exhibited
similar patterns in the heat-stressed rats exposed to heat for
various time periods (Figs. 1 and
2). The activities of LDH and CKMB
were significantly decreased following 20 min heat stress, as
compared with the control group (P<0.05); however, they showed
an overall increasing trend with exposure time. The serum LDH
activity was significantly increased after 80 min heat stress in
the heat-stressed rats, as compared with the control group
(P<0.05). Conversely, although the serum activity of CKMB showed
the same increasing trend, the CKMB activity was significantly
lower at 80 and 100 min heat stress, as compared with the control
group.
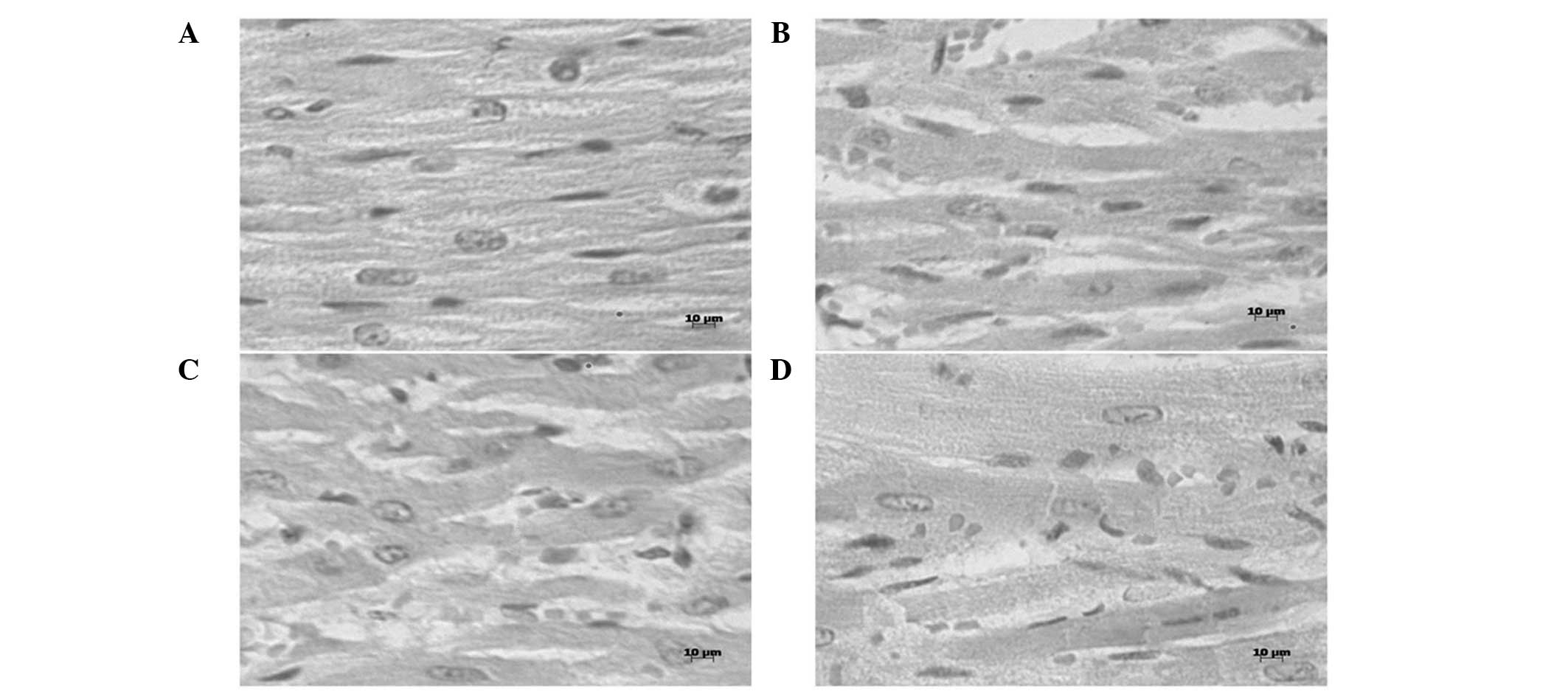
Histopathological analyses
Heat stress-induced acute degeneration in the heart
tissue of the heat-stressed rats was detected by histopathological
analyses (Fig. 3). After 20 min heat
stress, edema, which was characterized by increased interstitial
spaces between the muscle fibers, a cloudy cytoplasm in swollen
myocardial fibers and light hyperemia, was observed. After 80 min
heat stress, granular degeneration, which was characterized by an
enlarged cell size, a cloudy cytoplasm in myocardial fibers and
obvious hyperemia in blood capillaries, was observed. Furthermore,
after 100 min heat stress, necrosis, which was identified by
karyolysis in the myocardial fibers, and obvious edema, were
observed. Throughout the heat stress period, necrosis was
occasionally observed.
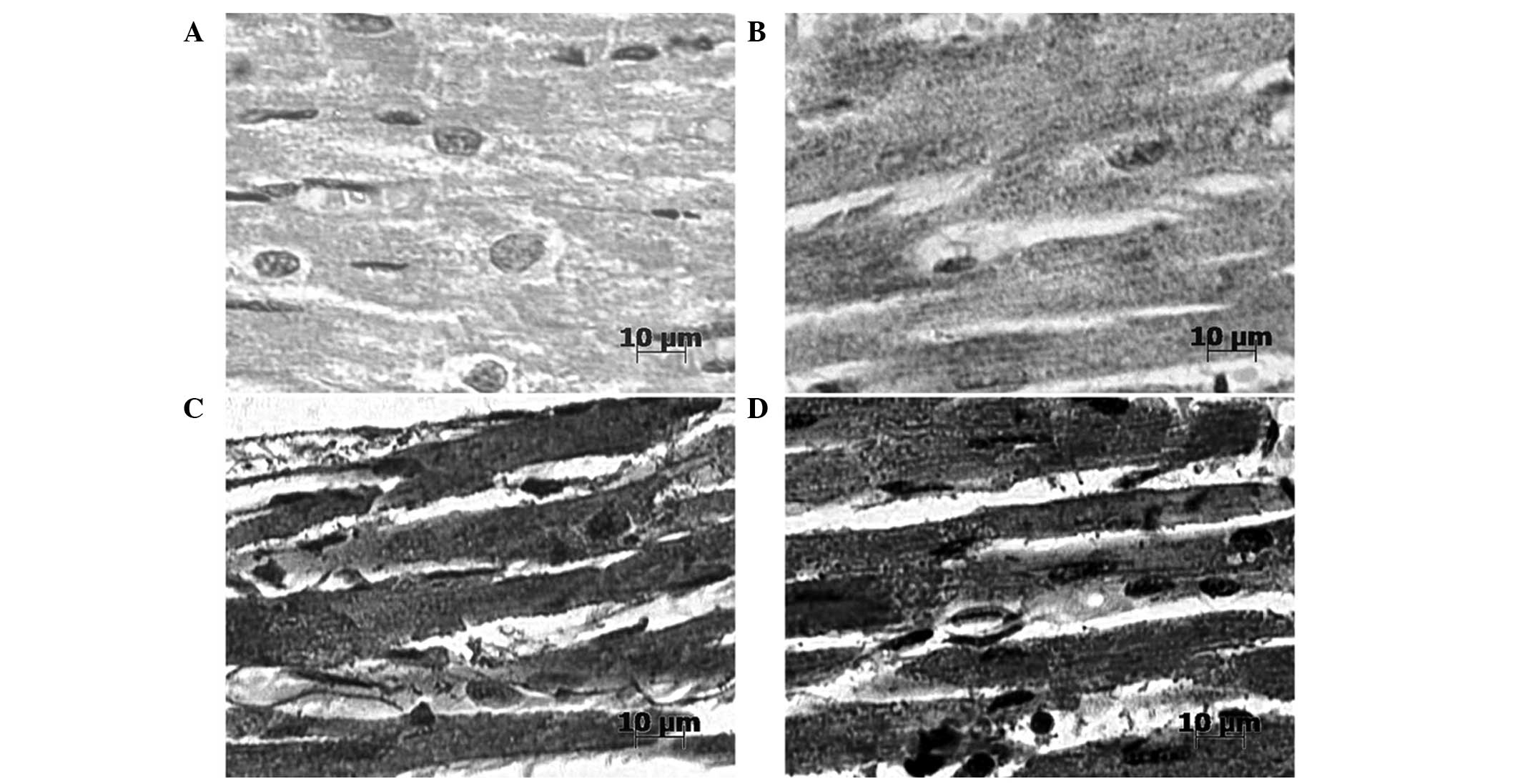
Immunohistochemical analyses
Immunohistochemical analyses demonstrated that
HSP60- and HSP10-positive signals were predominantly located in the
cytoplasm of myocardial cells (Fig.
4). Prior to heat stress, HSP60 was not clearly detectable in
the heart tissue of rats. However, following 100 min heat stress,
markedly stronger positive signals of HSP60, showing a punctiform
distribution, were detected in the cytoplasm of the myocardial
cells. Prior to heat stress, HSP10 staining was strongly positive
and was predominantly located in the cytoplasm of myocardial cells.
After 100 min heat stress, immunoreactive HSP10 was present at
markedly higher levels in the cytoplasm of the myocardial
cells.
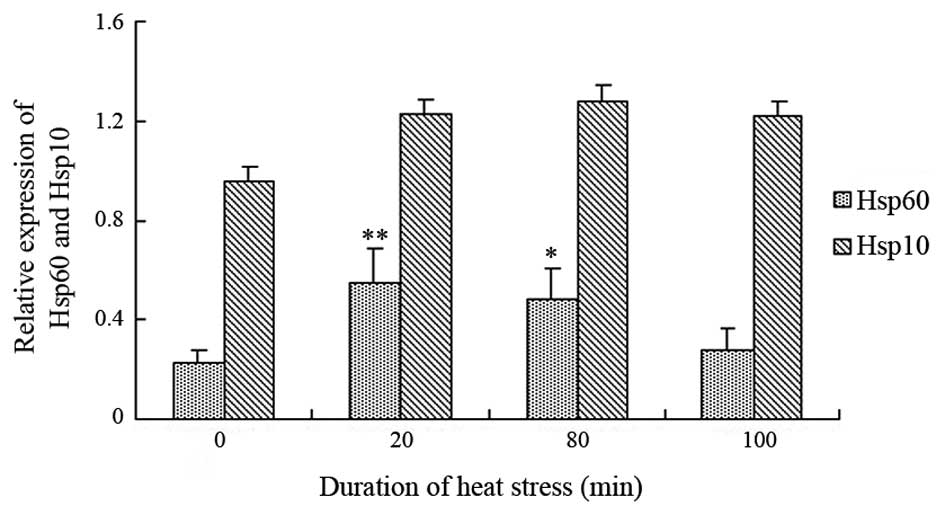
HSP60 and HSP10 protein expression
levels
The protein expression levels of HSP60 and HSP10
were detected in rat heart tissues and were normalized against
GAPDH (Fig. 5). After 20 min heat
stress, the protein expression levels of HSP60 were significantly
increased (P<0.01), and remained constant until 80 min heat
stress (P<0.05), as compared with the control group. However,
the protein expression levels of HSP60 returned to normal at 100
min heat stress. The protein expression levels of HSP10 exhibited a
similar trend, although HSP10 was constitutively expressed under
normal conditions and heat stress. The protein expression levels of
HSP10 did not significantly alter in the heat-stressed rats;
however, there was a slight increasing trend (P>0.05) from the
beginning of heat stress.
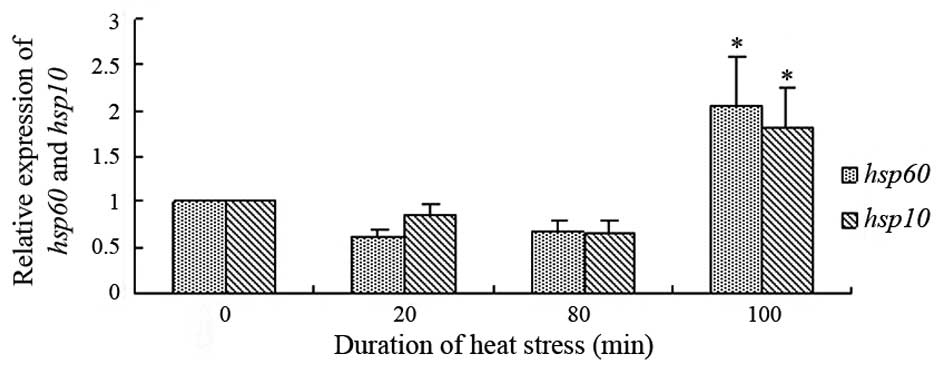
HSP60 and HSP10 mRNA expression levels
in the rat heart tissues
Fig. 6 presents the
mRNA expression levels of HSP60 and HSP10, normalized against
β-actin mRNA levels, in the heat-stressed heart tissues. Following
100 min heat stress, there were significant increases in the mRNA
expression levels of HSP60 and HSP10, as compared with the control
group (P<0.05). The mRNA expression levels of HSP10 in the heart
tissue of the heat-stressed rats showed a similar trend to
HSP60.
Discussion
The results of serum enzyme (CKMB and LDH) assays
are typically used as indexes of myocardial injury (28). A previous study demonstrated that the
diversity of aspartate aminotransferase, LDH and CK often
foreshadow heat shock-induced changes in cellular structure
(29), and the activity of these
enzymes in intercellular fluid has been associated with heart
disease (30). Furthermore, these
enzymes serve as molecular predictors of damage to cardiac muscle
cells during heat stress (31). In
the present study, the serum activities of LDH and CKMB were
detected as indicators of heart damage under heat stress
conditions, and an increasing trend with exposure to heat stress
was observed. An elevation of plasma CKMB levels, which has been
shown to be indicative of heart muscle damage, results from the
disruption of the function and permeability of the muscle cell
membrane (sarcolemma) (32). The
activity of LDH has previously been evaluated as an indicator of
stress during transportation, and a previous study reported acute
cellular lesions in the hearts of transported pigs (33). This is consistent with the present
study in which obvious lesions, characterized by granular and
vascular degeneration and even necrosis, were observed in the heart
tissue of heat-stressed rats using histopathological analyses.
Furthermore, the activities of enzymes associated with myocardial
cell damage were gradually increased with the duration of heat
stress and reached statistical significance at 100 min.
Histopathological analyses revealed acute degeneration, including
granular and vascular degeneration of myocardial cells; however,
there was no obvious and extensive myocardial cell necrosis. These
results suggested that acute degeneration may be sufficient to
cause sudden death in animals during heat stress by disrupting the
function and permeability of the myocardial cell membrane.
The distribution of HSPs may be associated with
their protective function (34). In
the present study, a higher density of HSP60-positive signals were
detected in the heat-stressed groups, as compared with the control
group, and strong positive signals of HSP10 that exhibited a
punctiform distribution were detected in the cytoplasm of the
heat-stressed myocardial cells. These results suggested that HSP60
and HSP10 were synthesized in response to heat stress. In addition,
HSP60 and HSP10 exhibited a punctiform distribution in the
mitochondria of heat-stressed cells. This was consistent with the
results of a previous study, in which HSP60 was reported to be
predominantly located in the cytoplasm and mitochondria of muscle
fibers in humans (35). Furthermore,
another study demonstrated that mammalian HSP60 was rapidly
transported into the mitochondria following dehydration (36). Therefore, these molecules may be
transported into the mitochondria under conditions of heat stress;
however, further studies are required in order to confirm this.
In the present study, western blotting demonstrated
that the protein expression levels of HSP60 were significantly
increased following 20 and 80 min heat stress; thus suggesting that
the elevation of HSPs in the heart may confer protection against
stress-induced myocardial injury (37,38). The
protein expression levels of HSP60 were decreased following 100 min
heat stress; however, the serum levels of HSP60 were high and
histopathological analysis of the heat-stressed tissue revealed
obvious lesions. In a previous study, HSP60 expression in the
cytoplasm of myocardial cells was more prominent in intact areas
than in degenerated areas (39).
Concordantly, in the present study, HSP60 staining was markedly
reduced in the cytoplasm of granular degenerated areas in
myocardial cells. These results suggested that HSP60 may be
considered a potential biomarker of heat stress-induced injuries of
the heart. In the present study, the presence and localization of
HSP60 and HSP10 in rat heart tissue in response to heat stress were
evaluated. Although HSP60 and HSP10 should be functionally
correlated, HSP10 was present in a higher number of specimens and
had a higher expression level, as compared with HSP60; thus
suggesting that HSP10 may have a different role in the heart tissue
of rats. Similar results were reported in a recent study in which
the HSP60 and HSP10 expression levels were investigated in a series
of normal human bone marrows and within the cytoplasm of tumor
cells (40). HSP10 was more
obviously and constitutively expressed in unstressed myocardial
cells under normal conditions, as compared with HSP60 in
heat-stressed rats.
A previous study demonstrated that the expression
levels of HSP60 and HSP10 were regulated
simultaneously during carcinogenesis, since these genes are
localized head-to-head on the chromosome (41). However, the quantitative relationship
between the mRNA and protein expression levels of a gene has yet to
be completely recognized. In the present study, the mRNA expression
levels of HSP60 and HSP10 were gradually and
significantly increased when the duration of heat stress was
increased to 100 min (P<0.05). The HSP60 protein expression
levels were significantly increased following 20 min heat stress,
but returned to normal at 100 min, whereas HSP10 was constitutively
expressed. These results suggested that HSP60 in myocardial tissue
may be more susceptive to the effects of heat stress, as compared
with HSP10 (42). HSP60 and HSP10
have previously been shown to form mitochondrial chaperone
complexes that are believed to have a role in maintaining normal
mitochondrial function (43).
However, the detailed functions of these two HSPs in myocardial
cells are yet to be fully elucidated.
Acknowledgements
The present study was supported by the National Key
Basic Research program of China (973 program; grant no.
2014cB138502), the National Natural Science Foundation of China
(grant no. 31372403), the National Department Public Benefit
Research Foundation (agriculture; grant no. 201003060-11), the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD), Jiangsu Province Plans to Graduate Research
and Innovation Projects and the Sino-German Agricultural
Cooperation Project of the Federal Ministry of Food, the
Agriculture and Consumer Production.
References
|
1
|
Chen H, Adam A, Cheng Y, Tang S, Hartung J
and Bao E: Localization and expression of heat shock protein 70
with rat myocardial cell damage induced by heat stress in vitro and
in vivo. Mol Med Rep. 11:2276–2284. 2015.PubMed/NCBI
|
|
2
|
Lee WC, Lin KY, Chiu YT, Lin JH, Cheng HC,
Huang HC, Yang PC, Liu SK and Mao SJ: Substantial decrease of heat
shock protein 90 in ventricular tissues of two sudden-death pigs
with hypertrophic cardiomyopathy. FASEB J. 10:1198–1204.
1996.PubMed/NCBI
|
|
3
|
Feder ME and Hofmann GE: Heat-shock
proteins, molecular chaperones and the stress response:
Evolutionary and ecological physiology. Annu Rev Physiol.
61:243–282. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tissiéres A, Mitchell HK and Tracy UM:
Protein synthesis in salivary glands of Drosophila melanogaster:
Relation to chromosome puffs. J Mol Biol. 84:389–398. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartl FU: Molecular chaperones in cellular
protein folding. Nature. 381:571–579. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryan MT and Pfanner N: Hsp70 proteins in
protein translocation. Adv Protein Chem. 59:223–242. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Zhou R, Li X, Ursano RJ and Li H:
Stress-induced change of mitochondria membrane potential regulated
by genomic and non-genomic GR signaling: A possible mechanism for
hippocampus atrophy in PTSD. Med Hypotheses. 66:1205–1208. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hightower LE: Heat shock, stress proteins,
chaperones and proteotoxicity. Cell. 66:191–197. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glover JR and Lindquist S: Hsp104, Hsp70
and Hsp40: A novel chaperone system that rescues previously
aggregated proteins. Cell. 94:73–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Richardson A, Schwager F, Landry SJ and
Georgopoulos C: The importance of a mobile loop in regulating
chaperonin/co-chaperonin interaction humans versus Escherichia
coli. J Biol Chem. 276:4981–4987. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hansen JJ, Bross P, Westergaard M, Nielsen
MN, Eiberg H, Børglum AD, Mogensen J, Kristiansen K, Bolund L and
Gregersen N: Genomic structure of the human mitochondrial
chaperonin genes: HSP60 and HSP10 are localised head to head on
chromosome 2 separated by a bidirectional promoter. Hum Genet.
112:71–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Damien P, Hemsley S and Canfield PJ:
Association of uterine and salpingeal fibrosis with chlamydial
hsp60 and hsp10 antigen-specific antibodies in chlamydia infected
koalas. Clin Diagn Lab Immunol. 12:632–639. 2005.PubMed/NCBI
|
|
13
|
Cappello F, Czarnecka AM, La Rocca G, Di
Stefano A, Zummo G and Macario AJ: Hsp60 and Hsp10 as antitumor
molecular agents. Cancer Biol Ther. 6:42007. View Article : Google Scholar
|
|
14
|
Cappello F, David S, Rappa F, Bucchieri F,
Marasà L, Bartolotta TE, Farina F and Zummo G: The expression of
HSP60 and HSP10 in large bowel carcinomas with lymph node
metastase. BMC Cancer. 5:1392005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pockley AG, Wu R, Lemne C, Kiessling R, de
Faire U and Frostegård J: Circulating heat shock protein 60 is
associated with early cardiovascular disease. Hypertension.
36:303–307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rizzo M, Cappello F, Marfil R, Nibali L,
Gammazza AM, Rappa F, Bonaventura G, Galindo-Moreno P, O'Valle F,
Zummo G, et al: Heat-shock protein 60 kDa and atherogenic
dyslipidemia in patients with untreated mild periodontitis: A pilot
study. Cell Stress Chaperones. 17:399–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan J, Bao E and Yu J: Heat shock protein
60 expression in heart, liver and kidney of broilers exposed to
high temperature. Res Vet Sci. 86:533–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin L, Kim SC, Wang Y, Gupta S, Davis B,
Simon SI, Torre-Amione G and Knowlton AA: HSP60 in heart failure:
Abnormal distribution and role in cardiac myocyte apoptosis. Am J
Physiol Heart Circ Physiol. 293:H2238–H2247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cappello F, Tripodo C, Farina F, Franco V
and Zummo G: HSP10 selective preference for myeloid and
megakaryocytic precursors in normal human bone marrow. Eur J
Histochem. 48:261–266. 2004.PubMed/NCBI
|
|
20
|
Corrao S, Campanella C, Anzalone R, Farina
F, Zummo G, de Macario E Conway, Macario AJ, Cappello F and La
Rocca G: Human Hsp10 and early pregnancy factor (EPF) and their
relationship and involvement in cancer and immunity: Current
knowledge and perspectives. Life Sci. 86:145–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cappello F, Bellafiore M, David S,
Anzalone R and Zummo G: Ten kilodalton heat shock protein (HSP10)
is overexpressed during carcinogenesis of large bowel and uterine
exocervix. Cancer Lett. 196:35–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akyol S, Gercel-Taylor C, Reynolds LC and
Taylor DD: HSP-10 in ovarian cancer: Expression and suppression of
T-cell signaling. Gynecol Oncol. 101:481–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan YX, Liu TJ, Su HF, Samsamshariat A,
Mestril R and Wang PH: Hsp10 and Hsp60 modulate Bcl-2 family and
mitochondria apoptosis signaling induced by doxorubicin in cardiac
muscle cells. J Mol Cell Cardiol. 35:1135–1143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Y, Shang X, Sun J, Zhang L, Zhao W,
Tian Y, Cheng H and Zhou R: Gonadal apoptosis during sex reversal
of the rice field eel: Implications for an evolutionarily conserved
role of the molecular chaperone heat shock protein 10. J Exp Zool B
Mol Dev Evol. 314:257–266. 2010.PubMed/NCBI
|
|
25
|
Landry SJ, Steede NK and Maskos K:
Temperature dependence of backbone dynamics in loops of human
mitochondrial heat shock protein 10. Biochemistry. 36:10975–10986.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agnello D, Scanziani E, Di GM, Leoni F,
Modena D, Mascagni P, Introna M, Ghezzi P and Villa P: Preventive
administration of Mycobacterium tuberculosis 10-kDa heat shock
protein (hsp10) suppresses adjuvant arthritis in Lewis rats. Int
Immunopharmacol. 2:463–474. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bakay RA and Ward AA Jr: Enzymatic changes
in serum and cerebrospinal fluid in neurological injury. J
Neurosurg. 58:27–37. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Z, Lv Y, Zhang M, Yue Z, Tang S, Islam
A, Rehana B, Bao E and Hartung J: Hsp110 expression changes in rat
primary myocardial cells exposed to heat stress in vitro. Genet Mol
Res. 11:4728–4738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koelkebeck KW and Odom TW: Laying hen
responses to acute heat stress and carbon dioxide supplementation:
II Changes in plasma enzymes, metabolites and electrolytes. Comp
Biochem Physiol A Physiol. 112:119–122. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haagensen L, Jensen D and Gesser H:
Dependence of myosin-ATPase on structure bound creatine kinase in
cardiac myofibrils from rainbow trout and freshwater turtle. Comp
Biochem Phys A Mol Integr Physiol. 150:404–409. 2008. View Article : Google Scholar
|
|
32
|
Mitchell M and Sandercock D: Creatine
kinase isoenzyme profiles in the plasma of the domestic fowl
(Gallus domesticus): Effects of acute heat stress. Res Vet Sci.
59:30–34. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu L, Bao E, Zhao R and Hartung J:
Expression of heat shock protein 60 in the tissues of transported
piglets. Cell Stress Chaperon. 14:61–69. 2009. View Article : Google Scholar
|
|
34
|
Georgopoulos C and Welch WJ: Role of the
major heat shock proteins as molecular chaperones. Annu Rev Cell
Biol. 9:601–634. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kervinen H, Huittinen T, Vaarala O,
Leinonen M, Saikku P, Manninen V and Mänttäri M: Antibodies to
human heat shock protein 60, hypertension and dyslipidemia. A study
of joint effects on coronary risk. Atherosclerosis. 169:339–344.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Itoh H, Komatsuda A, Ohtani H, Wakui H,
Imai H, Sawada K, Otaka M, Ogura M, Suzuki A and Hamada F:
Mammalian HSP60 is quickly sorted into the mitochondria under
conditions of dehydration. Eur J Biochem. 269:5931–5938. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu J, Bao E, Yan J and Lei L: Expression
and localization of Hsps in the heart and blood vessel of
heat-stressed broilers. Cell Stress Chaperon. 13:327–335. 2008.
View Article : Google Scholar
|
|
38
|
Hutter JJ, Mestril R, Tam EK, Sievers RE,
Dillmann WH and Wolfe CL: Overexpression of heat shock protein 72
in transgenic mice decreases infarct size in vivo. Circulation.
94:1408–1411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kervinen H, Huittinen T, Vaarala O,
Leinonen M, Saikku P, Manninen V and Mänttäri M: Antibodies to
human heat shock protein 60, hypertension and dyslipidemia. A study
of joint effects on coronary risk. Atherosclerosis. 169:339–344.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cappello F, David S, Rappa F, Bucchieri F,
Marasà L, Bartolotta TE, Farina F and Zummo G: The expression of
HSP60 and HSP10 in large bowel carcinomas with lymph node
metastase. BMC Cancer. 5:1392005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Q, Wang J, Levichkin IV,
Stasinopoulos S, Ryan MT and Hoogenraad NJ: A mitochondrial
specific stress response in mammalian cells. EMBO J. 21:4411–4419.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim KK, Kim R and Kim SH: Crystal
structure of a small heat-shock protein. Nature. 394:595–599. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lau S, Patnaik N, Sayen MR and Mestril R:
Simultaneous overexpression of two stress proteins in rat
cardiomyocytes and myogenic cells confers protection against
ischemia-induced injury. Circulation. 96:2287–2294. 1997.
View Article : Google Scholar : PubMed/NCBI
|