Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common cancer worldwide, and accounts for >40% of head and
neck malignancies (1). Despite
advances in treatment and diagnostics, the incidence and mortality
rates associated with OSCC are increasing (2). Metastasis and recurrence of OSCC are
significant factors (3,4). As with other cancers, the accumulation
of genetic and epigenetic changes is involved in the development
and progression of OSCC (5).
Therefore, there is an urgent requirement to study these genetic
changes in order to deliver identify diagnostic or biomarkers, and
therapeutic targets for early detection and prognosis.
MicroRNAs (miRNAs) are endogenous non-coding RNAs of
~22 nucleotides, and are evolutionarily conserved (6). They regulate post-transcriptional gene
expression through binding to specific regions of target mRNA in a
sequence-specific manner (6). It is
now clear that miRNAs play crucial roles in various biological
processes such as differentiation, proliferation, invasion and
apoptosis (7). Moreover, increasing
evidence has indicated that miRNAs are involved in the pathogenesis
of various human cancers by serving as either oncogenes or tumor
suppressor genes (8,9). Several miRNAs, such as miR-31 and
miR-99a, have been shown to be dysregulated in OSCC and contribute
to the development and progression of OSCC (10,11).
miR-448 is reportedly associated with various human malignancies.
It is upregulated in breast cancer and early cerebral aneurysm
(12,13), indicating regulatory effects of
miR-448 in various conditions.
In the present study, the expression levels of
miR-448 were investigated in OSCC cancer cell lines and OSCC cancer
tissues from patients. Moreover, an miR-448 inhibitor was
transfected into an OSCC cell line, and the effects of miR-448 on
cancer-related growth, migration and anti-apoptosis were analyzed.
In addition, the involvement of the predicted target of miR-488,
metallophosphoesterase domain containing 2 (MPPED2), in miR-448
activity in OSCC cells was investigated. The results of this study
may provide further insights into the mechanisms responsible for
OSCC tumorigenesis, and indicate whether miR-448 is a potential
therapeutic target in OSCC treatment.
Materials and methods
Clinical specimens and cell
culture
Fresh cancer tissues and matched adjacent
non-cancerous tissues were obtained from 15 patients with OSCC at
Nanjing Stomatological Hospital (Nanjing, China) between November
2013 and February 2014. The samples were snap-frozen in liquid
nitrogen in the operating room and stored at −80°C until extraction
of total RNA. Written informed consent was obtained from all
patients and the study was approved by the Institutional Review
Board of the Affiliated Hospital of Stomatology, Nanjing Medical
University. Human OSCC cell lines SCC-9 and Cal-27 were cultured
with complete medium containing 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) and Dulbecco's modified
Eagle's medium (DMEM)-F12 (Hyclone; GE Healthcare Life Sciences) at
37°C with 5% CO2. The medium was changed every 2 days.
Cell lines were provided by Shanghai Ninth People's Hospital
Affiliated Shanghai Jiaotong University School of Medicine
(Shanghai, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from OSCC cancer tissues and
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
The RNA was digested with RQ1 RNase-Free DNase (M6101; Promega
Corporation, Madison, WI, USA) for 30 min to remove genomic
contaminants. Subsequently, DNase was removed with an RNeasy Mini
kit (Qiagen GmbH, Hilden, Germany). RNA was quantified using a
NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). The RNA was then transcribed into cDNA using
a Mir-X™ miRNA First-Strand Synthesis kit (cat. no. 638313; Takara
Bio, Inc., Otsu, Japan), following the manufacturer's protocol.
Briefly, the transcription was performed in a 10-µl reaction
mixture, containing 5 µl mRQ Buffer (2X), 3 µl RNA sample and 1.0
µl mRQ Enzyme. Reaction was conducted for 1 h at 37°C, and then
terminated by heating at 85°C for 5 min to inactivate the enzyme.
Addition of 90 µl ddH2O then increased the total volume
to 100 µl.
qPCR was then performed on a 7500 Real-Time-PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) using a
Mir-X™ miRNA qRT-PCR SYBR® kit (Takara Bio, Inc.). The
experiment was performed in a 25-µl reaction mixture, containing 9
µl DDH20, 12.5 µl SYBR Advantage Premix (2X), 0.5 µl ROX
Dye (50X), 0.5 µl miRNA-specific Primer (10 µM), 0.5 µl mRQ 3′
Primer 0.5 and 2.0 µl cDNA. PCR was then performed with the
following cycling profile: 95°C for 5 sec, followed by 30 cycles of
60°C for 30 sec. Data were normalized to the internal control, U6,
to obtain ΔCt. The final value of the gene of interest relative to
the untreated cells was converted by the 2−ΔΔCT method
(14). Primers used were as follows:
miR-448 forward: 5′-TTGCATATGTAGGATGTCCCAT-3′ and reverse:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGATGGGACA-3′; U6 forward:
5′-CGCTTCGGCAGCACATATAC-3′ and reverse: 5′-ACGAATTTGCGTGTCATCCT-
3′. The mRQ 3′ Primer was provided with the Mir-X™ miRNA qRT-PCR
SYBR® kit. All samples were analyzed in triplicate.
Western blot analysis
Total protein was extracted from cells with lysis
buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The lysates
were analyzed using a BCA protein assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). Proteins (20 µl) were subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis with 10%
polyacrylamide gels and transferred to polyvinylidene fluoride
membranes. These were blocked with 5% non-fat milk for 2 h at room
temperature, prior to incubation with primary antibodies against
MPPED2 (1:500; ab87077; Abcam, Cambridge, UK) at 4°C overnight. The
membranes were washed three times and incubated with horseradish
peroxidase (HRP)-conjugated IgG secondary antibody (1,1000; BA1055;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) at room
temperature for 2 h. Subsequently, protein bands were detected by
enhanced chemiluminescence (Immobilon™ Western Chemiluminescent HRP
Substrate; WBKLS0100; EMD Millipore, Billerica, MA, USA) and
visualized using a n ImageQuantLAS 4000 mini imaging system (GE
Healthcare Life Sciences, Chicago, IL, USA). Protein levels were
normalized to β-actin, using a rabbit monoclonal anti-β-actin
antibody (1:100; BM0627; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China).
Plasmids and transfection
The miR-448 inhibitor (miR-448-in) and negative
control (NC-in) were designed and synthesized by Shanghai
GenePharma Co., Ltd. (Shanghai, China). OSCC cells were grown in
6-well plates to 60% confluence prior to transfection. The
transfection procedure was performed using Lipofectamine 2000 in
accordance with the manufacturer's protocol (Invitrogen; Thermo
Fisher Scientific, Inc.). Total RNA was extracted at 48 h
post-transfection and used for RT-qPCR analysis.
Cell proliferation assay
Cell proliferation activity was examined with the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. At 6 h after transfection, cells in three groups [blank
(untransfected), miR-448-in and NC-in] were digested and seeded
into a 96-well microtiter plate for 0, 24, 48 and 72 h. MTT
solution (10 µl) was added to each well at the above time points.
The optical density (OD) values were measured at 570 nm using a
microplate reader (Labsystems, Santa Fe, NM, USA). Each
experimental condition was tested three times and average results
were calculated.
Wound healing assay
Cell migration was evaluated using a scratched wound
healing assay conducted with plastic plate wells. When the cell
confluence reached ~80% (at ~48 h post-transfection), scratch
wounds were made by scraping the cell layer across each culture
plate using the tip of a 10-µl pipette. After wounding, the debris
was removed by washing the cells with phosphate-buffered saline
(PBS). Three wounds were made for each sample, and photographic
images of the wound were captured at 0 h and subsequent time points
(24, 36 and 48 h). Cell migration was evaluated by measuring the
width of the wound at the same position. The experiments were
performed in triplicate.
Measurement of apoptosis by flow
cytometry
OSCC cancer cells were grown and transfected as
above. For apoptotic analysis, the transfection group and control
group were seeded in a 6-well culture plate. After 48 h of culture,
cells including those suspended in the medium were collected,
washed with PBS and resuspended in 1X binding buffer at a
concentration of 1×105 cells/ml.
Annexin-V-allophycocyanin (APC) (5 µl) and 7-aminoactinomycin D
(7-AAD; 5 µl) were added to the cell suspension and the suspension
was incubated for 15 min in the dark. Cell apoptosis was assessed
by flow cytometry (Becton Dickinson, San Jose, CA, USA).
Target prediction
Three online programs, miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http:www.targetscan.org), and TarBase (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=tarbase/index)
were used in combination with previous reports, for predicting the
target gene of miR-448.
Vector construction and luciferase
reporter assay
The 3′ untranslated region (3′-UTR) of the human
MPPED2 sequence (the predicted target) was amplified by PCR from
human genomic DNA using the following primers: Forward (F),
5′-CTTGACTCGAGAGCTCTAAATGCCCTATTGG-3′ and reverse (R),
5′-ATTGCGGCCGCGTGGGTAATAAAAATTTATTG-3′. Mutant 3′-UTRs of MPPED2
were obtained by overlapping PCR using the following primers: m1-F,
5′-GCCTTTATATtaAAATAAAATTGC-3′; m1-R
5′-GCAATTTTATTTtaATATAAAGGC-3′; m2-F
5′-GATTTATTCATATtaAACATCAGTA-3′; m2-R,
5′-TACTGATGTTtaATATGAATAAATC-3′ (mutations are indicated by lower
case letters). Each sequence was cloned into the pmiR-RB-REPORT™
vector (Promega Corporation, Madison, WI, USA) and the construct
was verified by sequencing. A luciferase assay was conducted using
a Dual-Luciferase Reporter Assay System (ZZE1980; Promega
Corporation) according to the manufacturer's instructions.
Renilla luciferase was cotransfected into the cells as a
control for normalization.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation from at least three
separate experiments. Differences between groups were analyzed
using a Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-448 in OSCC tissues
and cells
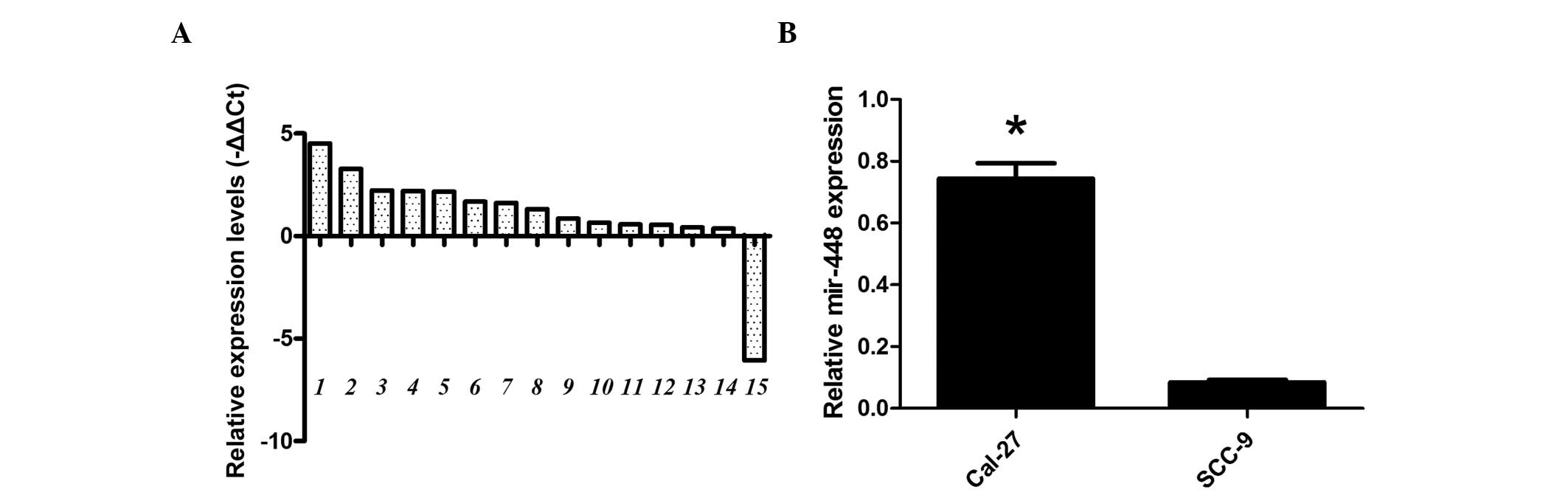
The expression patterns of miR-448 were determined
by RT-qPCR analysis in 15 pairs of OSCC and matched adjacent
non-cancerous oral tissues. As shown in Fig. 1A, the expression levels of miR-448
were clearly upregulated in OSCC tissues compared with the levels
in non-cancerous oral tissues. The expression of miR-448 was also
detected in the OSCC cell lines Cal-27 and SCC-9. RT-qPCR indicated
that the expression level of miR-448 was higher in the Cal-27 cell
line than in the SCC-9 cell line (Fig.
1B). These results suggest that miR-448 might be involved in
the development of OSCC in humans. The difference between the
miR-488 levels in the Cal-27 and SCC-9 cell lines may be associated
with differences in the aggressiveness of these tumors.
Silencing of miR-448 inhibits cell
growth in vitro
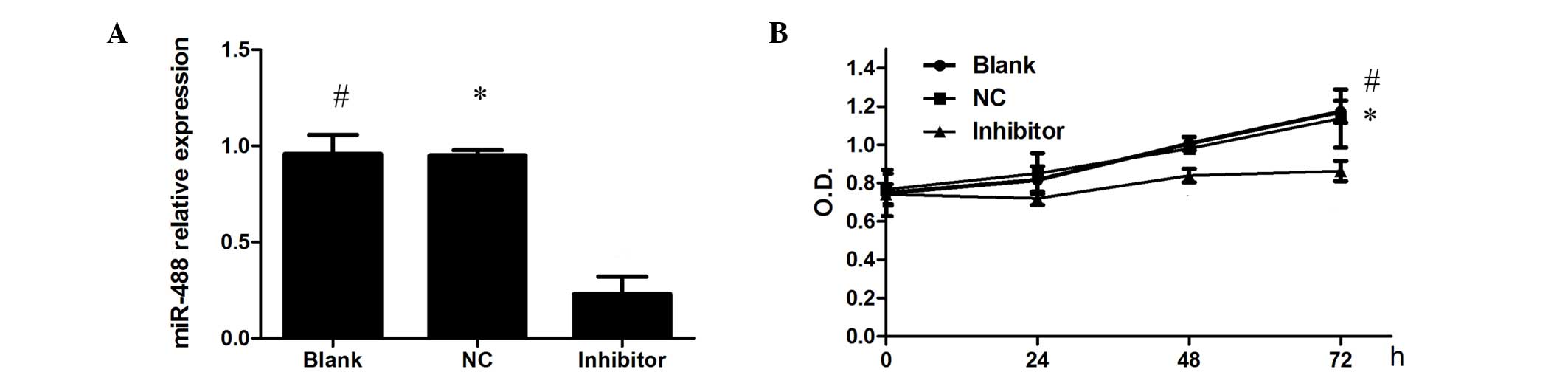
To assess the effect of miR-448 on the biological
properties of OSCC cancer cells, miR-448 inhibitor (miR-448-in) or
negative control (NC-in) was transfected into Cal-27 cells. The
knock-down of the expression level of miR-448 was verified by
RT-qPCR (Fig. 2A). Subsequently, the
effect of miR-448 on the proliferation of OSCC cells was examined
using an MTT assay. It was observed that the viability of the cells
was reduced by the inhibition of miR-448, suggesting that miR-448
promotes the proliferation of Cal-27 cells (Fig. 2B). These results suggest a
growth-promoting role of miR-448 in OSCC.
miR-448 inhibition significantly
suppresses the migration of Cal-27 cells in vitro
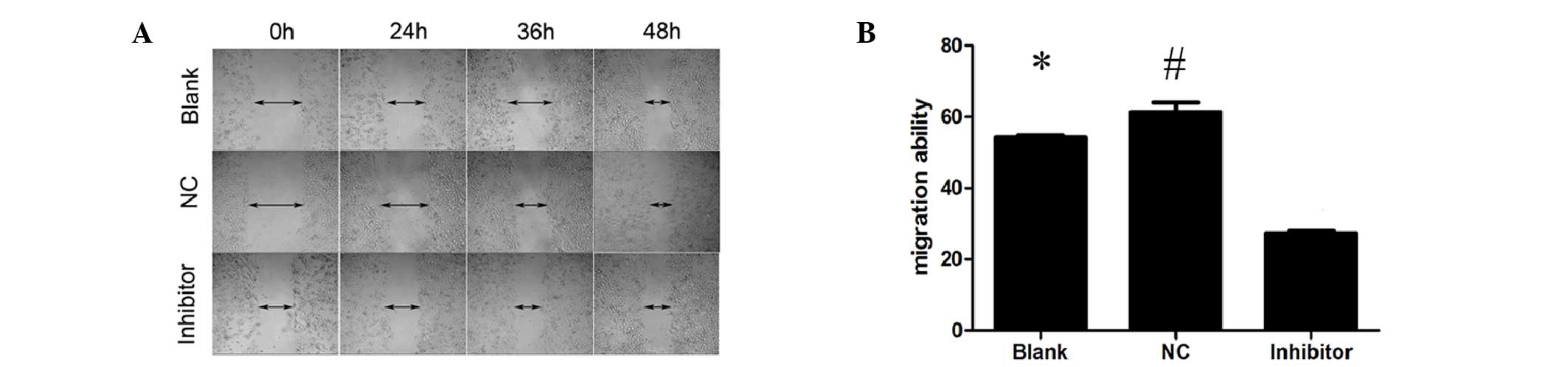
A wound healing assay was used to observe changes in
tumor migration ability. The results showed that following
transfection into Cal-27 cells, the miR-448 inhibitor reduced the
ability of the cells to migrate (Fig.
3).
miR-448 reduces the apoptosis of OSCC
cells
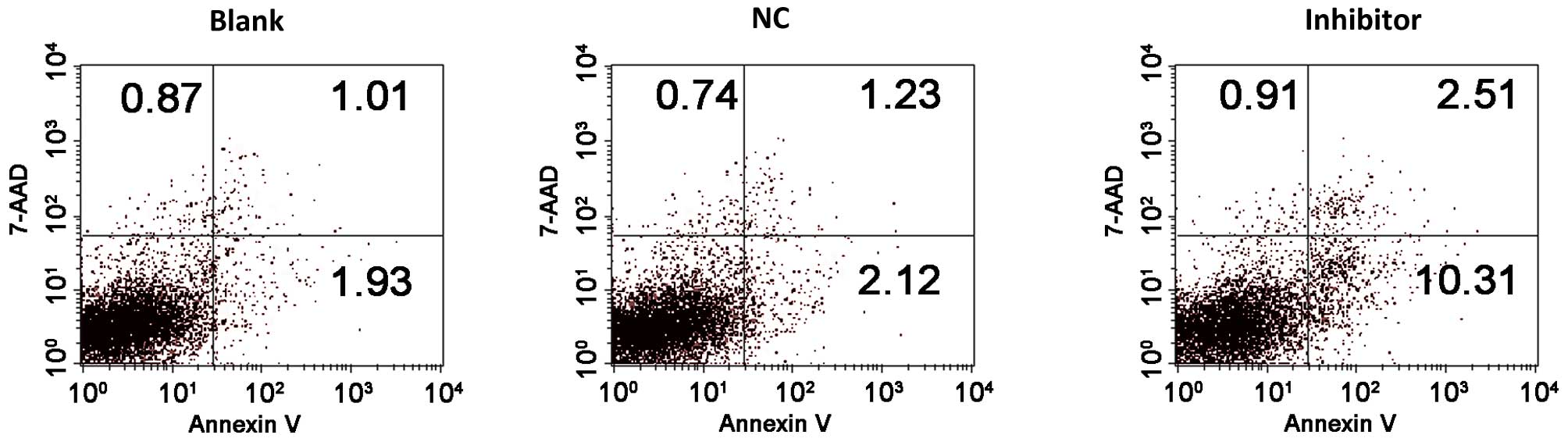
To evaluate the effect of miR-448 on OSCC cell
apoptosis, apoptosis was measured at 48 h after NC or miR-448
inhibitor transfection by flow cytometry. Annexin V-APC+
apoptotic cells were markedly increased in the miR-448
inhibitor-transfected group compared with the NC or blank control
groups. The percentage of apoptotic cells in the group transfected
with miR-448 inhibitor was higher than that of the control groups
(Fig. 4). The findings indicate an
anti-apoptotic role for miR-448 in OSCC cells.
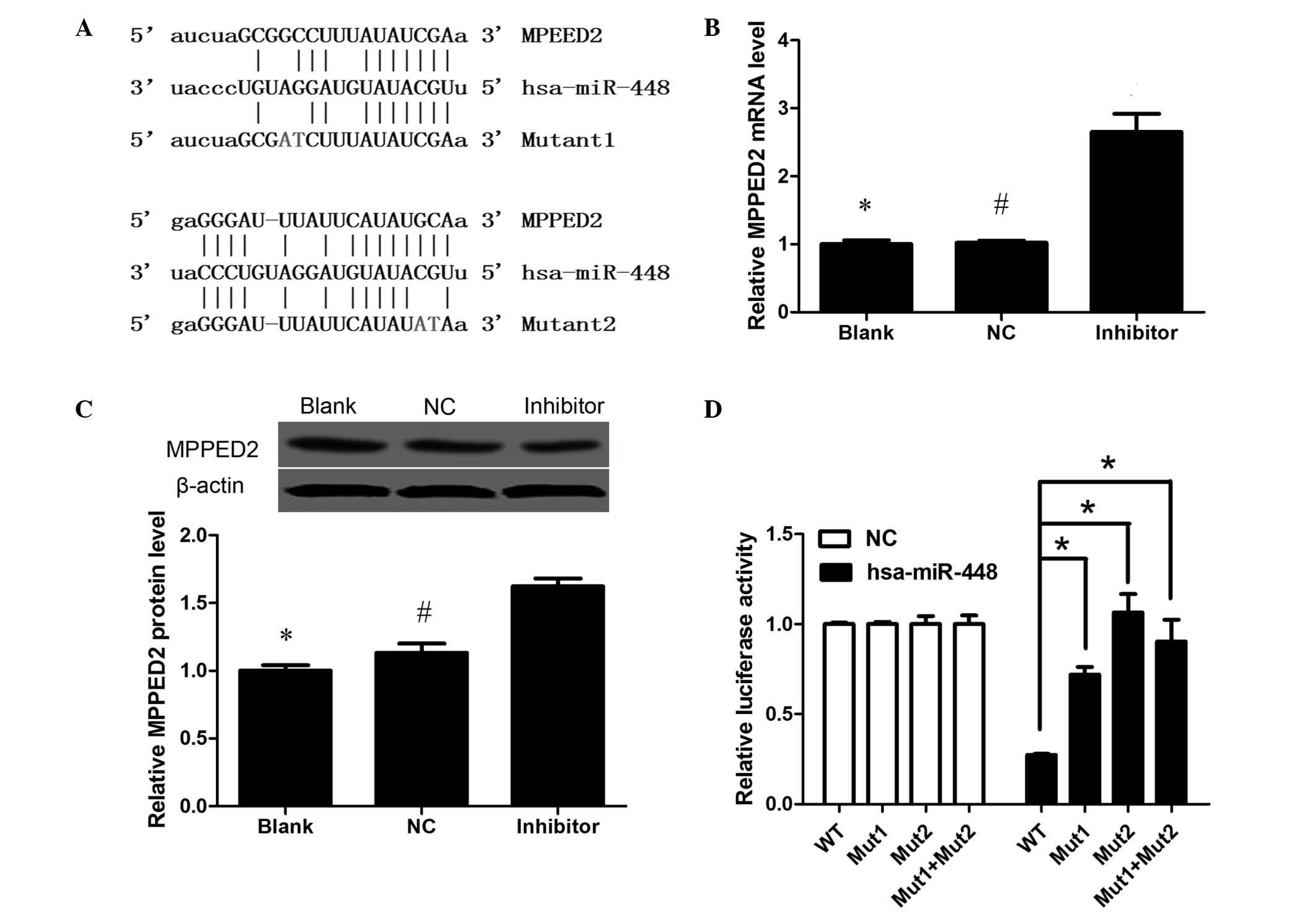
miR-448 directly inhibits the
expression of MPPED2 by binding to the 3′-UTR
Bioinformatics analysis identified that MPPED2 is
target of miR-448 having a close association with miR-448. The
predicted binding sites between miR-448 and the 3′-UTR of MPPED2
are illustrated in Fig. 5A. To
explore the association between MPPED2 and miR-448, qPCR and
western blot analysis was used to measure the change of MPPED2
expression that occurred when miR-488 was inhibited. The results
showed that the miR-448 inhibitor increased MPPED2 expression at
the mRNA and protein levels, indicating that miR-488 reduces MPPED2
expression (Fig. 5B and C). To
determine if the suppressive effects of miR-448 on MPPED2 were
achieved via direct action, fragments containing the miR-448
binding sites of wild-type and mutant 3′-UTRs of MPPED2 were
subcloned into a luciferase reporter vector. As shown in Fig. 5D, miR-448 suppressed MPPED2
luciferase activities in Cal-27 cells, and this suppression of
activity was abrogated by mutations in the miR-448 binding sites,
suggesting that miR-448 directly targets MPPED2.
Discussion
OSCC is a health problem worldwide with increasing
incidence and mortality rates. Approximately 300,000 patients are
estimated to have oral cancer worldwide annually (15,16).
Previous studies have identified that the occurrence and
development of OSCC correlates with various molecules (17,18).
MicroRNAs have been found to be differentially altered in various
tumors and might contribute to the activation and upregulation of
key molecules in cancers (19–21). It
may assist us to identify predictive marker of prognosis and
potential treatment target to investigate the effective molecular
expression in OSCC (22,23). Despite evidence indicating the
regulatory role of microRNAs in cancer progression and invasion,
their regulatory role in oral cancer metastasis remains poorly
understood.
miR-448 has been reported to be associated with
various types of cancer. It is upregulated in breast cancer
(24) and early cerebral aneurysm
(12), and downregulated in
hepatocellular carcinoma (25). It
has also been reported that miR-448 is the most downregulated
microRNA following chemotherapy in breast cancer (13), suggesting that it may act as an
oncogene or anti-oncogene in different cancers. The results of the
present study suggest that the expression of miR-448 in OSCC
tissues was significantly higher than that in matched
paraneoplastic normal tissues. The molecular mechanisms underlying
the upregulation in OSCC remain unclear. It may be speculated that
miR-448 has an association with the occurrence and development of
OSCC. The results of the present study demonstrated that miR-448
was upregulated in OSCC tissues and cell lines. Moreover, they
indicate that miR-448 promotes the proliferation and migration of
OSCC cells in vitro, suggesting an oncogenic role in
OSCC..
The molecular mechanisms by which miR-448 promotes
the proliferation and migration of OSCC cells were further
investigated in the present study. Publicly available bioinformatic
algorithms predicted MPPED2 as a theoretical target gene of
miR-448. MPPED2 has been recognized to have a role in brain
development and tumorigenesis (26).
Also MPPED2 has been found to be significantly associated with
caries via meta-analysis (27).
MPPED2 is downregulated in oral epithelial cells in response to
oral pathogens (28). These
observations encourage further research into MPPED2. The results of
the present study indicated that MPPED2 expression was suppressed
by miR-448 in OSCC cells, while inhibition of miR-448 rescued the
expression of MPPED2. Luciferase reporter assays revealed that
miR-448 directly targeted the 3′-UTR of MPPED2 mRNA. These results
suggest that miRNA-448 promotes the proliferation of OSCC cells by
negatively regulating the expression of MPPED2 via directly
targeting the 3′-UTR of this gene. Although the detailed molecular
mechanisms of miR-448 in OSCC have not been clearly clarified,
these results imply that miR-448, as a tumor promoting microRNA,
could regulate cancer cell proliferation and metastasis in OSCC.
These results might help to elucidate the cause and effects of
altered expression of miR-448 in the initiation and progression of
OSCC in the future.
In summary, the present study has demonstrated that
miR-448 expression is significantly upregulated in OSCC tissues and
cells. It also confirmed that miR-448 negatively regulates MPPED2
transcription and translation through directly binding to the
3′-UTR of this gene. Furthermore, the study demonstrated that the
repression of miR-448 reduced the proliferation and metastasis of
OSCC cells, and thereby indicated that miR-448 induces the
proliferation and metastasis of these cells. These results suggest
that miR-448 is potentially useful as a biomarker and therapeutic
target in OSCC.
Acknowledgements
This study was supported by a project funded by the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD, 2014-37).
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carvalho AL, Singh B, Spiro RH, Kowalski
LP and Shah JP: Cancer of the oral cavity: A comparison between
institutions in a developing and a developed nation. Head Neck.
26:31–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grimm M, Alexander D, Munz A, Hoffmann J
and Reinert S: Increased LDH5 expression is associated with lymph
node metastasis and outcome in oral squamous cell carcinoma. Clin
Exp Metastasis. 30:529–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olasz L, Orsi E, Markó T and Szalma J:
Induction chemotherapy response and recurrence rates in correlation
with N0 or N+ stage in oral squamous cell cancer (OSCC). Cancer
Metastasis Rev. 29:607–611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang MK and Park NH: Conversion of normal
to malignant phenotype: Telomere shortening, telomerase activation
and genomic instability during immortalization of human oral
keratinocytes. Crit Rev Oral Biol Med. 12:38–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao WT, Li TT, Wang ZG, Wang SY, He MR,
Ye YP, Qi L, Cui YM, Wu P, Jiao HL, et al: MicroRNA-224 promotes
cell proliferation and tumor growth in human colorectal cancer by
repressing PHLPP1 and PHLPP2. Clin Cancer Res. 19:4662–4672. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hung PS, Tu HF, Kao SY, Yang CC, Liu CJ,
Huang TY, Chang KW and Lin SC: MiR-31 is upregulated in oral
premalignant epithelium and contributes to the immortalization of
normal oral keratinocytes. Carcinogenesis. 35:1162–1171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yen YC, Shiah SG, Chu HC, Hsu YM, Hsiao
JR, Chang JY, Hung WC, Liao CT, Cheng AJ, Lu YC and Chen YW:
Reciprocal regulation of microRNA-99a and insulin-like growth
factor I receptor signaling in oral squamous cell carcinoma cells.
Mol Cancer. 13:62014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong SH, Lee HJ, Yi JS, Lee HJ, Lee IW,
Park KC and Yang JH: Identification of microRNAs with altered
expression profiles in a rat model of experimentally induced early
cerebral aneurysms. Korean J Neurotrauma. 9:412013. View Article : Google Scholar
|
|
13
|
Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen
YY, Wang WJ, Chen Q, Tang F, Liu XP and Xu ZD: Involvement of
NF-κB/miR-448 regulatory feedback loop in chemotherapy-induced
epithelial-mesenchymal transition of breast cancer cells. Cell
Death Differ. 18:16–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rautava J, Luukkaa M, Heikinheimo K, Alin
J, Grenman R and Happonen RP: Squamous cell carcinomas arising from
different types of oral epithelia differ in their tumor and patient
characteristics and survival. Oral Oncol. 43:911–919. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehrotra R, Singh MK, Pandya S and Singh
M: The use of an oral brush biopsy without computer-assisted
analysis in the evaluation of oral lesions: A study of 94 patients.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 106:246–253.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng CH, Liao CT, Peng SC, Chen YJ, Cheng
AJ, Juang JL, Tsai CY, Chen TC, Chuang YJ, Tang CY, et al: A novel
molecular signature identified by systems genetics approach
predicts prognosis in oral squamous cell carcinoma. PLoS One.
6:e234522011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen R, Yang K, Zhao NB, Zhao D, Chen D,
Zhao CR and Tang H: Abnormal expression of PER1 circadian-clock
gene in oral squamous cell carcinoma. Onco Targets Ther. 5:403–407.
2012.PubMed/NCBI
|
|
19
|
Kunej T, Godnic I, Ferdin J, Horvat S,
Dovc P and Calin GA: Epigenetic regulation of microRNAs in cancer:
An integrated review of literature. Mutat Res. 717:77–84. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang YW and Chen LA: MicroRNAs as tumor
inhibitors, oncogenes, biomarkers for drug efficacy and outcome
predictors in lung cancer (review). Mol Med Rep. 5:890–894.
2012.PubMed/NCBI
|
|
21
|
Perrotti D and Eiring AM: The new role of
microRNAs in cancer. Future Oncol. 6:1203–1206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lohavanichbutr P, Houck J, Doody DR, Wang
P, Mendez E, Futran N, Upton MP, Holsinger FC, Schwartz SM and Chen
C: Gene expression in uninvolved oral mucosa of OSCC patients
facilitates identification of markers predictive of OSCC outcomes.
PLoS One. 7:e465752012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu DS, Chang SY, Liu CJ, Tzeng CH, Wu KJ,
Kao JY and Yang MH: Identification of increased NBS1 expression as
a prognostic marker of squamous cell carcinoma of the oral cavity.
Cancer Sci. 101:1029–1037. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mak KK, Wu AT, Lee WH, Chang TC, Chiou JF,
Wang LS, Wu CH, Huang CY, Shieh YS, Chao TY, et al: Pterostilbene,
a bioactive component of blueberries, suppresses the generation of
breast cancer stem cells within tumor microenvironment and
metastasis via modulating NF-κB/microRNA 448 circuit. Mol Nutr Food
Res. 57:1123–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katayama Y, Maeda M, Miyaguchi K, Nemoto
S, Yasen M, Tanaka S, Mizushima H, Fukuoka Y, Arii S and Tanaka H:
Identification of pathogenesis-related microRNAs in hepatocellular
carcinoma by expression profiling. Oncol Lett. 4:817–823.
2012.PubMed/NCBI
|
|
26
|
Pattaro C, Köttgen A, Teumer A, Garnaas M,
Böger CA, Fuchsberger C, Olden M, Chen MH, Tin A, Taliun D, et al:
Genome-wide association and functional follow-up reveals new loci
for kidney function. PLoS Genet. 8:e10025842012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shaffer JR, Wang X, Feingold E, Lee M,
Begum F, Weeks DE, Cuenco KT, Barmada MM, Wendell SK, Crosslin DR,
et al: Genome-wide association scan for childhood caries implicates
novel genes. J Dent Res. 90:1457–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stanley BO, Feingold E, Cooper M, Vanyukov
MM, Maher BS, Slayton RL, Willing MC, Reis SE, McNeil DW, Crout RJ,
et al: Genetic association of MPPED2 and ACTN2 with dental caries.
J Dent Res. 93:626–632. 2014. View Article : Google Scholar : PubMed/NCBI
|