Introduction
Gastric cancer is a common malignancy and ranks the
first among all gastrointestinal cancers in terms of incidence
(1). Therapies for gastric cancer
include surgery, radiotherapy and chemotherapy (2,3). Due to
absence of the specific symptoms, the majority of cases are
diagnosed at middle to late disease stage. For these patients,
systemic chemotherapy is usually used to improve life quality and
increase overall survival in certain patients (4). However, only a limited effect is
achieved by chemotherapy, with toxic and side effects (5,6).
Constant efforts are now made to investigate novel therapies
against late-stage gastric cancer.
Cell apoptosis is programmed cell death mediated by
various signal transduction pathways under certain physiological or
pathological conditions, in which multiple genes are involved. As a
major pathway to clear abnormally proliferating cells, cell
apoptosis may be intentionally induced to treat cancers (7,8).
Chemotherapy medications combined with traditional Chinese medicine
preparations may greatly enhance the therapeutic effect and prevent
postoperative relapse and metastasis (9,10).
Traditional Chinese medicine preparations are recommended for those
at the late stage without surgical indications to prolong the
survival (10).
Signal transducer and activator of transcription 3
(STAT3) is a latent transcriptional factor in the cytoplasm that is
regulated by Janus kinase (JAK) phosphorylation. STAT3 is
persistently unregulated in various tumor cell lines, including
hematologic cancers (11),
epithelial malignancies (12),
typical breast cancer (13), head
and neck cancer (14), ovarian
cancer (15), prostate cancer
(16) and gastric cancer (17). STAT3 upregulates genes associated
with the proliferation and survival of tumor cells, either directly
or indirectly. JAK2/STAT3 is an important intracellular signal
transduction pathway in gastric cancer which regulates the growth,
differentiation and apoptosis of gastric cancer cells (18). Persistent STAT3 activation has been
shown to be correlated with proliferation stimulation and apoptosis
inhibition of gastric cancer cells (18).
Cucurbitacin B is a tetracyclic triterpenoid
isolated from plants of Cucurbitaceae family and proved to
possess anti-tumor, anti-chemocarcinogenic and anti-inflammatory
effects (19,20). In vivo and in vitro
experiments have shown that cucurbitacin B inhibits the
proliferation of lung cancer cells (21) and pancreatic cancer cells, and
induces apoptosis (22). Although
cucurbitacin B strongly inhibits the growth of numerous tumor cell
types, its effect on MKN-45 cells is unknown. In the present study,
cucurbitacin B was used to treat MKN-45 cells reaching the
logarithmic phase of growth, and its effect on proliferation and
apoptosis of MKN-45 cells was observed. The mechanism was also
discussed.
Materials and methods
Materials
Cucurbitacin B (6199-67-3; 98% purity determined by
high-performance liquid chromatography analysis) was ordered from
Shanghai Winherb Medical Technology Co., Ltd. (Shanghai, China). A
Cell Counting Kit-8 (CCK-8; ER612) assay was obtained from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). Dimethyl sulfoxide
(DMSO) was ordered from Sigma-Aldrich (D2650; Merck KGaA,
Darmstadt, Germany). RPMI 1640 medium, trypsin-EDTA (1316929) and
fetal bovine serum (FBS) were obtained from Gibco (10099; Thermo
Fisher Scientific, Inc., Grand Island, NY, USA). A Cell Cycle and
Apoptosis Analysis Kit (C1052) was purchased from Beyotime
Institute of Biotechnology (Haimen, China). TRIzol®
(15596–026) was purchased from (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA). The antibodies used to
recognize the total and phosphorylated forms of p-STAT3 (4113),
STAT3 (12640S), p-JAK2 (8082S), JAK2 (3230) and GAPDH (2118) were
ordered from Cell Signaling Technology, Inc. (Danvers, MA, USA).
For the in vitro study, cucurbitacin B was dissolved in
DMSO.
Cell culture
Gastric cancer MKN-45 cells (CBP60488) were ordered
from CoBioer Biosciences Co., Ltd. (Nanjing, China) and cultured in
RPMI 1640 medium containing 10% FBS. After 24–48 h incubation at
37°C with 5% CO2, logarithmic growth phase cells were
digested using 0.25% trypsin. After calculating the cell number,
the cells were seeded into plates at a density of 1×105
cells per well. The cells used in this study were collected between
passages 4 and 10.
Measurement of cell proliferation
Cell proliferation was determined using a CCK-8
assay according to the manufacturer's instructions. After the
MKN-45 cells were grown to 80% confluency in 96-well plates, they
were subsequently incubated with 0.1, 1 or 10 µM cucurbitacin B for
12, 24 and 48 h. After the treatment, 10 µl CCK-8 solutions were
added to each well, then the plate was incubated in a 37°C
incubator for 2.5 h. Cell proliferation was determined by measuring
the optical density at 450 nm using a plate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Cell cycle progression assays
Cell cycle progression was determined using a cell
cycle and apoptosis analysis kit in accordance with the
manufacturer's instructions and fluorescence-activated cell sorting
using BD FACSVerse (BD Bioscience, San Jose, CA, USA). Upon
reaching 70–80% confluency in the six-well plates, the MKN-45 cells
were incubated with cucurbitacin B (10 µM) for 24 h prior to
analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from MKN-45 cells using
TRIzol reagent, and its yield and purity were
spectrophotometrically estimated by the A260/A280 ratio, which was
determined using a NanoDrop 2000c (Thermo Fisher Scientific, Inc.).
RNA (2 µg of each sample) was reverse transcribed into cDNA using
oligo (dT) primers and the Transcriptor First Strand cDNA Synthesis
kit (04896866001; Roche Diagnostics, Basel, Switzerland) according
to the manufacturer's instructions. SYBR Green PCR Master Mix
(04707516001; Roche Diagnostics) was then used to quantify PCR
amplifications using a Light Cycler 480 instrument with designated
software (version 1.5; Roche Diagnostics). Conditions for PCR were
as follows: Initial denaturation at 94°C for 2 min, followed by
25–35 amplification cycles consisting of denaturation at 94°C for
40 sec, annealing at 58°C for 45 sec and elongation at 72°C for 1
min. The 2−ΔΔCq method was used to calculate the
relative mRNA levels of each target gene (23). The target gene mRNA expression was
normalized against the internal control GAPDH and expressed
relative to the control group. The primer sequences used were as
follows: Cyclin-dependent kinase 4 (CDK4), forward
5′-AATGTTGTCCGGCTGATGGA-3′ and reverse 5′-ACTGGCGCATCAGATCCTTG-3′;
CDK2, forward 5′-AAATCCTCCTGGGCTGCAAAT-3′ and reverse
5′-GCGAGTCACCATCTCAGCAA-3′; cyclin D1, forward
5′-GATGCCAACCTCCTCAACGA-3′ and reverse 5′-ACTTCTGTTCCTCGCAGACC-3′;
cyclin E, forward 5′-AGAGGAAGGCAAACGTGACC-3′ and reverse
5′-GAGCCTCTGGATGGTGCAAT-3′; P27, forward 5′-TCCGGCTAACTCTGAGGACA-3′
and reverse 5′-AAGAATCGTCGGTTGCAGGT-3′; B-cell lymphoma 2 (Bcl-2),
forward 5′-GTCATGTGTGTGGAGAGCGT-3′ and reverse
5′-GAAATCAAACAGAGGCCGCA-3′; Bcl-2-associated protein X (Bax),
forward 5′-TCCACCAAGAAGCTGAGCGAG-3′ and reverse
5′-GTCCAGCCCATGATGGTTCT-3′. GAPDH, forward
5′-GTCAAGGCTGAGAACGGGAA-3′ and reverse
5′-TGGACTCCACGACGTACTCA-3′.
Western blot analysis
Cells were lysed in RIPA Lysis Buffer (G2002; Wuhan
Google Biological Technology Co., Ltd., Wuhan, China) containing 50
mM Tris-Hcl, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate
and 0.1% SDS, which were then scraped into 1.5-ml centrifuge tubes.
The cell suspension centrifuged at 4°C for 30 min at 3,362 ×
g, and the protein concentration was determined using a
bicinchoninic acid protein assay kit (23227; Thermo Fisher
Scientific, Inc.). A total of 20 µg protein extract was used for
SDS-PAGE, blotted onto immobilon-FL transfer membranes (EMD
Millipore, Billerica, MA, USA), blocked with 5% non-fat milk for 2,
then probed with the relevant antibodies (dilution, 1:1,000)
overnight with gentle shaking at 4°C. The goat anti-rabbit IRdye
800 CW (cat. no. 926-32211) IgG and goat anti-mouse IRdye 800 CW
(cat. no. 926-32210) secondary antibodies (both purchased from
LI-COR, Lincoln, NE, USA) were used at 1:10,000 dilution and
incuvated at 37°C in Odyssey blocking for 1 h. The protein
expression was quantified using the Odyssey infrared imaging system
(Li-COR Biosciences, Lincoln, NE, USA) and protein expression
levels were normalized against the GAPDH internal control in the
total cell lysate.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL was performed using the in situ Cell
Death Detection kit (11684795910; Roche Diagnostics), according to
the manufacturer's instructions. The cells were grown on cover
slips in a 24-well plate, which were fixed in 4% paraformaldehyde
(30525-89-4; Amresco, LLC, Cleveland, OH, USA) and permeabilized
using 0.1% Triton X-100 (9002-93-1; Amresco, LLC) after treatment.
The cells were incubated in 13 µl (per cover slip) TUNEL reaction
mixture (Roche Diagnostics) at 37°C for 1 h. The nuclei were
labeled with 13 µl (per cover slip) 4′,6-diamidino-2-phenylindole
(DAPI; S36939; Invitrogen; Thermo Fisher Scientific, Inc.) for 30
sec, and DNA fragmentation was quantified using an IX51 microscope
(Olympus Corporation, Tokyo, Japan) at ×200. The percentages of
TUNEL-positive cells relative to the DAPI-positive cells were
counted by an investigator in a blinded-manner. Image analysis was
performed using Image Pro-Plus software, version 6.0 (Media
Cybernetics, Inc., Silver Spring, MD, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Differences between the groups were determined via
one-way analysis of variance followed by a Student-Newman-Keuls
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cucurbitacin B inhibits proliferation
of MKN-45 cells
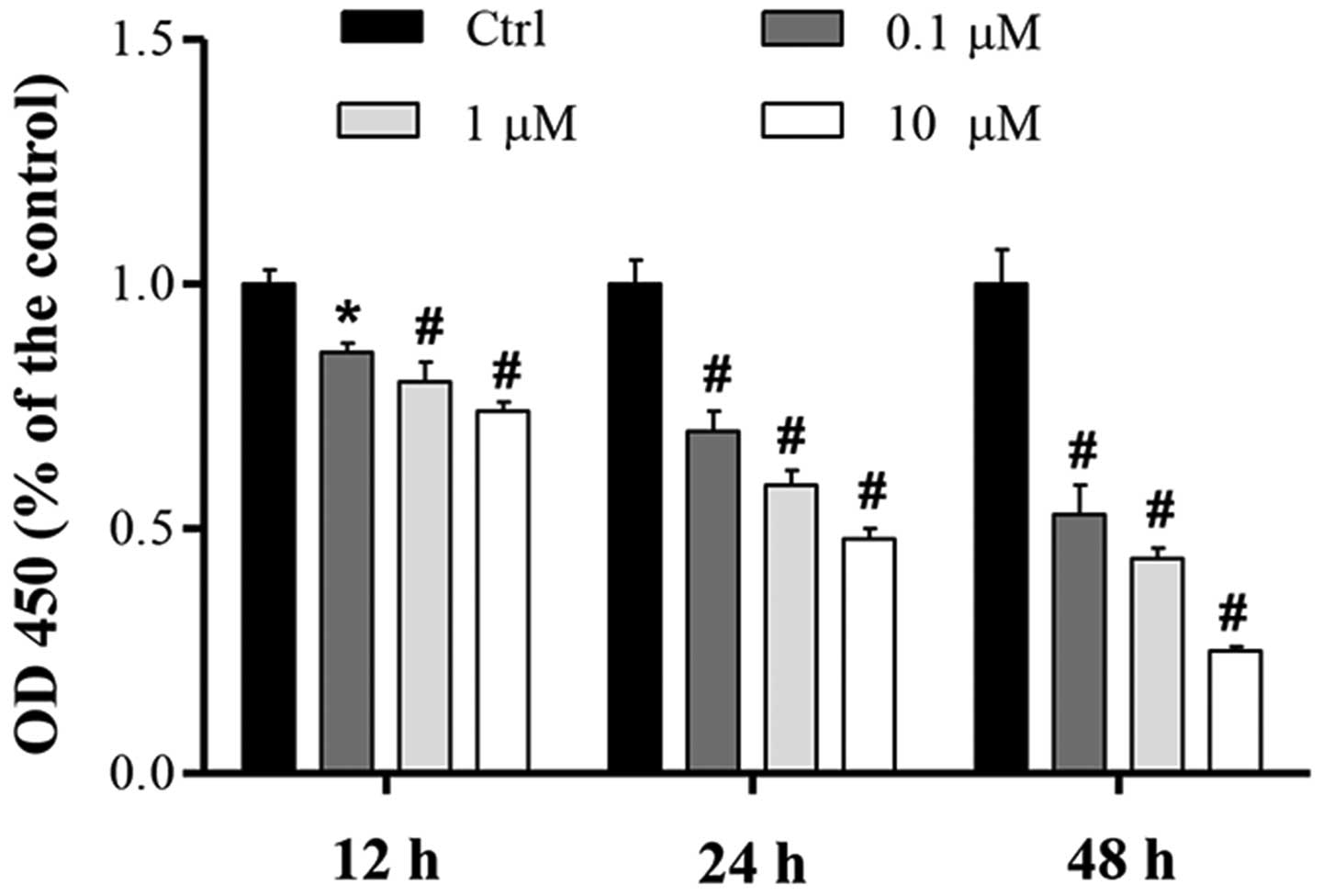
To determine the effect of cucurbitacin B on MKN-45
cell proliferation, the effect of various doses of cucurbitacin B
(0.1–10 µM) over 12, 24 and 48 h was investigated using a CCK-8
assay. Compared with the control, the proliferation of MKN-45 cells
was significantly inhibited in a concentration-dependent manner,
and the greatest level of proliferation suppression was induced by
cucurbitacin B at a concentration of 10 µM (Fig. 1). Results also showed that
cucurbitacin B demonstrated a time-dependent inhibition of cell
proliferation (Fig. 1).
Cucurbitacin B inhibits cell cycle
progression from G0/G1 to S phase
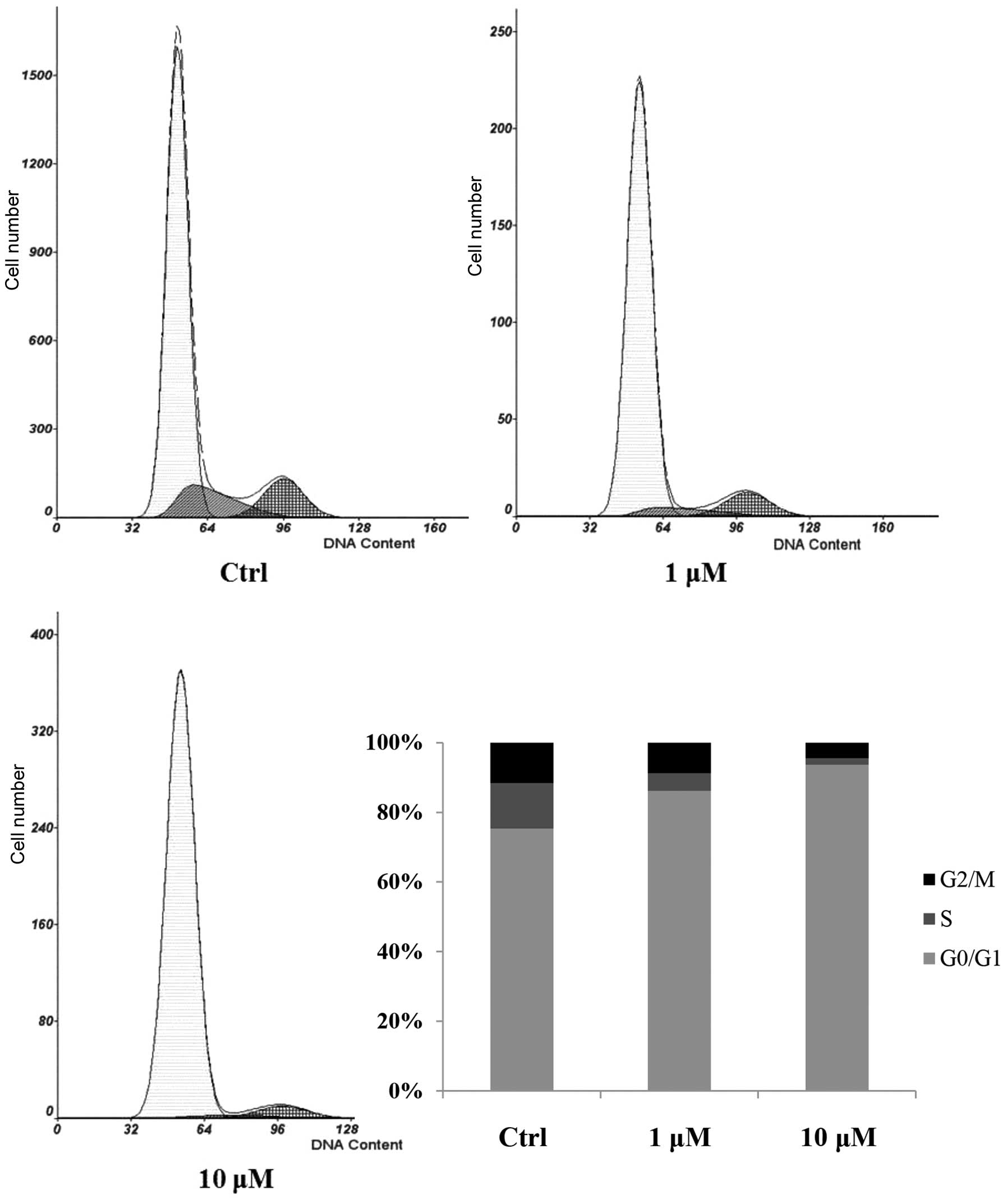
The effect of cucurbitacin B on cell cycle
progression was analyzed using flow cytometric analysis. The
control group showed an increased percentage of cells in S phase
and decreased G0/G1 populations, while the cucurbitacin B-treated
cells showed a suppression of cell cycle progression. Cucurbitacin
B at a dose of 10 µM reduced the percentage of cells in S phase and
increased the G0/G1 populations compared with the control group
(Fig. 2), suggesting that
cucurbitacin B affected the G0/G1 to S phase transition rather than
being involved in the S or G2/M phases.
Cucurbitacin B upregulates p27 and
downregulates CDK4, CDK2, cyclin D1 and cyclin E mRNA
expression
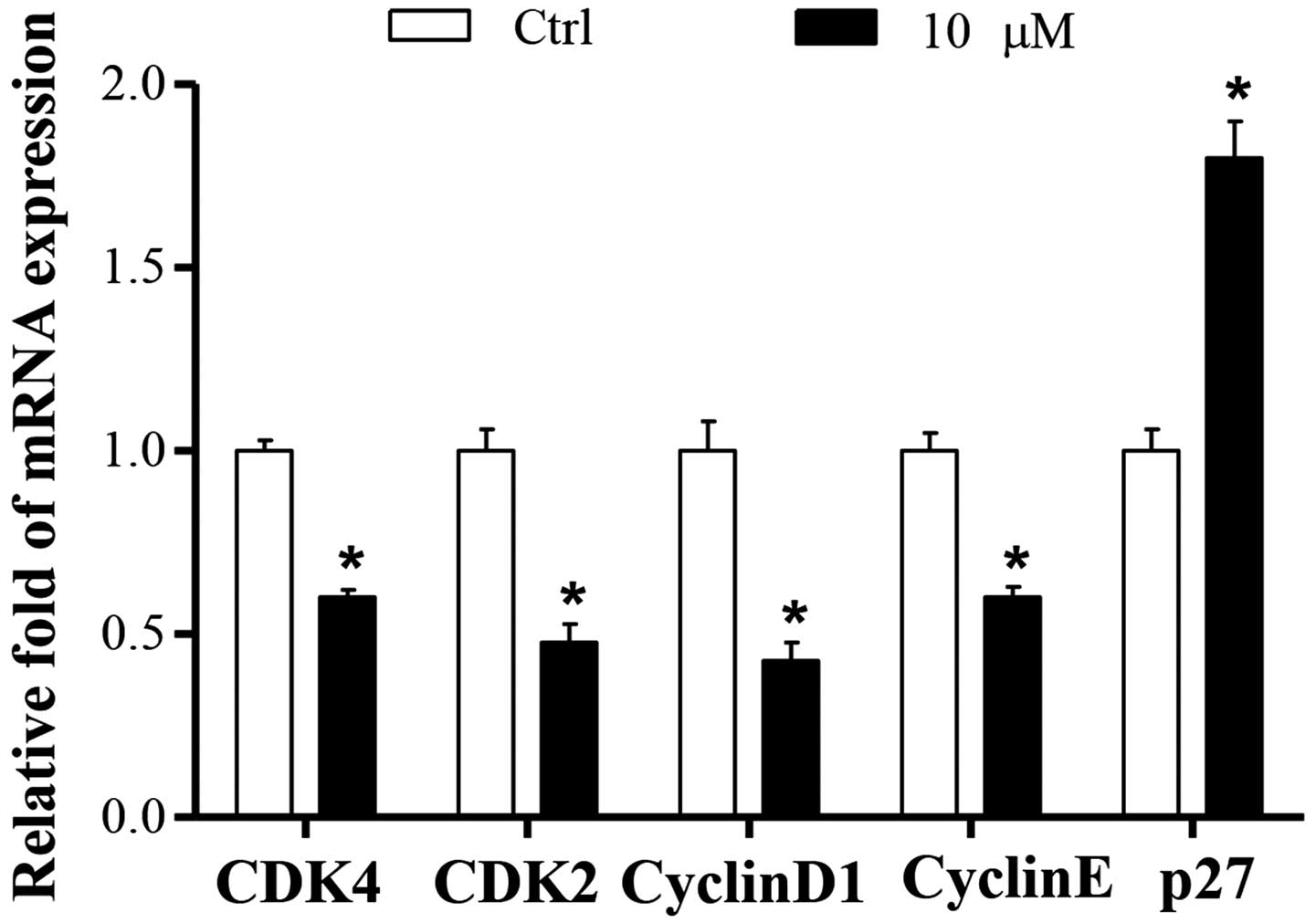
To further characterize the potential mechanism
underlying cucurbitacin B-induced cell cycle arrest, the effects of
cucurbitacin B on cell cycle-associated genes, including cyclins,
CDKs and cell cycle inhibitory expression, were analyzed using
RT-qPCR. The results demonstrated that the mRNA expression levels
of CDK4, CDK2, cyclin D1 and Cyclin E were significantly decreased
in the 10 µM cucurbitacin B group compared with the control group
(P<0.01; Fig. 3).
CDK activity is additionally regulated by small
proteins such as p27, which inactivates the cyclin-CDK complexes in
the G1 phase, leading to cell cycle arrest. The present results
demonstrated that the mRNA expression level of p27 in control group
was low, whereas it was significantly increased by co-treatment
with cucurbitacin B (P<0.01; Fig.
3).
Cucurbitacin B induces apoptosis of
MKN-45 cells
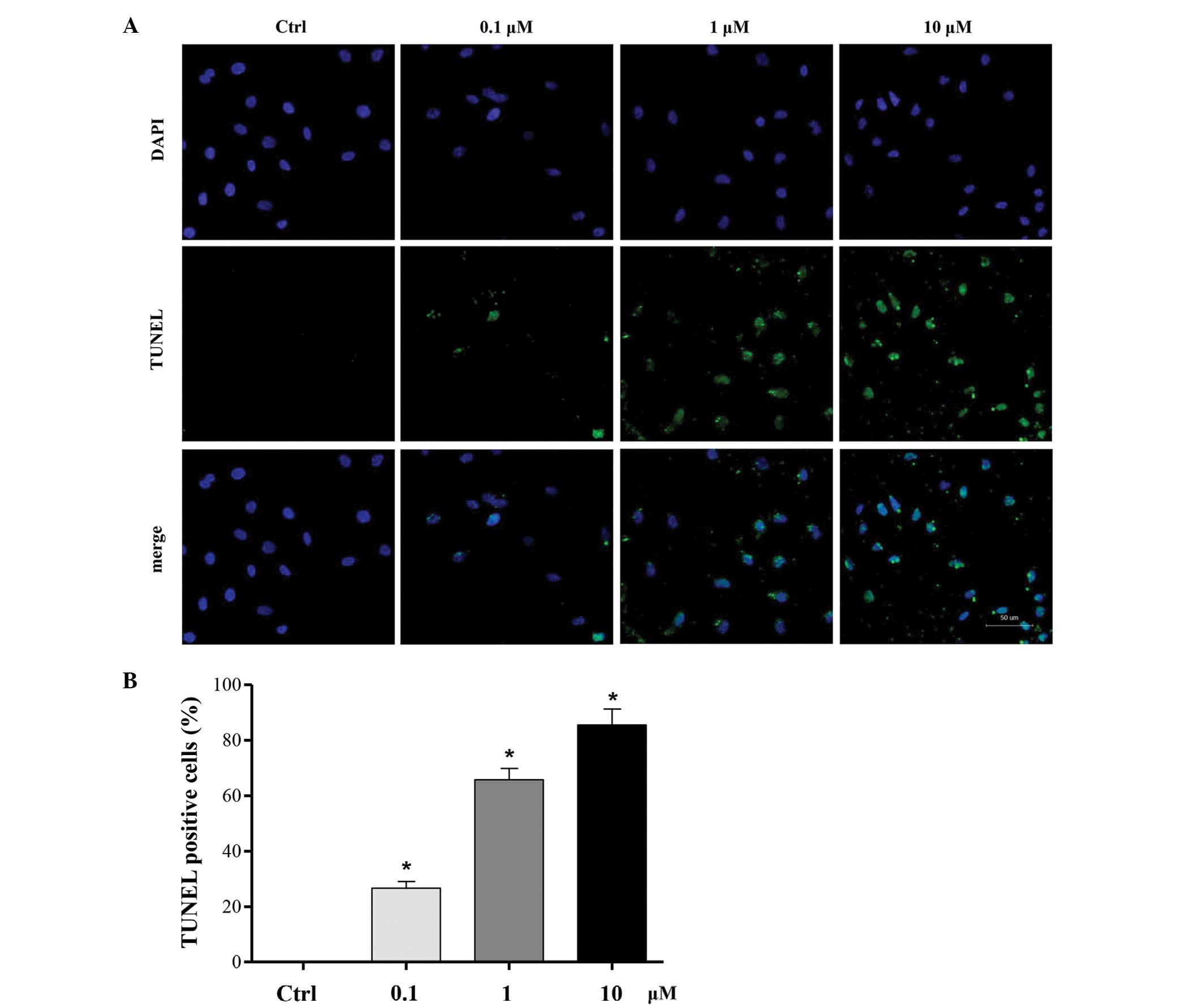
To investigate the effect of cucurbitacin B on
MKN-45 cells apoptosis, a TUNEL assay was used to stain the
apoptotic nuclei. Compared with the control group, a noticeable
increase of cell apoptosis was observed in MKN-45 cells, which was
significantly promoted by co-treatment with 0.1, 1 and 10 µM
cucurbitacin B for 24 h in a concentration-dependent manner. The
highest level of apoptosis promotion was induced by cucurbitacin B
at a concentration of 10 µM (P<0.01; Fig. 4).
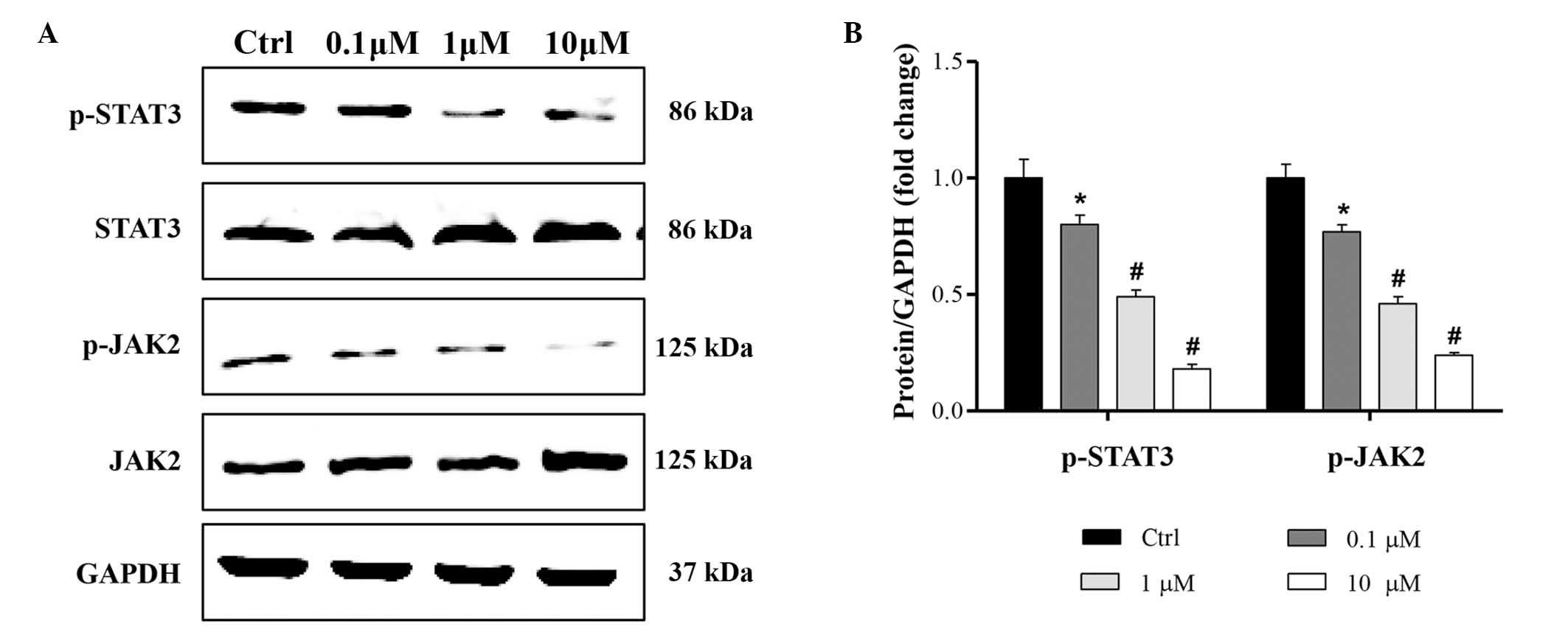
Cucurbitacin B regulates JAK2/STAT3
signaling pathway
To investigate the molecular mechanisms underlying
the anti-proliferation and proapoptotic effects of cucurbitacin B,
JAK2/STAT3 signaling pathway expression was evaluated. The
activation of JAK2/STAT3 was assessed using western blot analysis.
The results indicate that the phosphorylation of JAK2/STAT3 was
significantly reduced by 0.1, 1 and 10 µM cucurbitacin B, in a
concentration-dependent manner (Fig.
5).
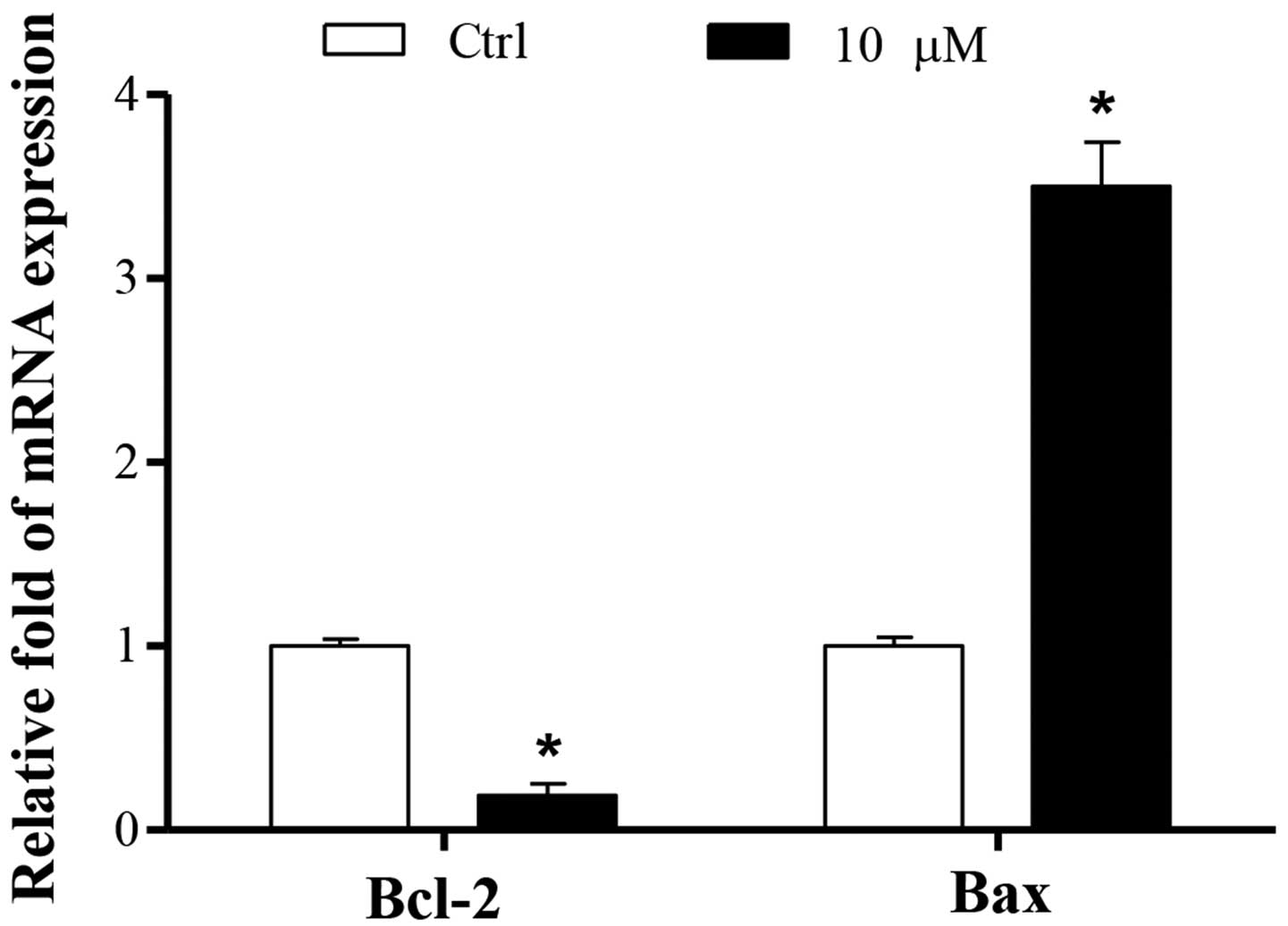
Cucurbitacin B promotes the mRNA
expression levels of Bax and reduced those of Bcl-2
To investigate the molecular mechanisms underlying
the proapoptotic effects of cucurbitacin B, the apoptotic pathway
was examined. The mRNA expression of the antiapoptotic protein
Bcl-2 was decreased, while the expression of the proapoptotic
protein Bax was increased (P<0.01). These results were
consistent with the finding that cucurbitacin B induced MKN-45 cell
apoptosis (Fig. 6).
Discussions
The results of the present study demonstrated that
cucurbitacin B decreased MKN-45 cell proliferation in a
concentration-dependent manner. It was also found that cucurbitacin
B suppressed the MKN-45 cell cycle at the G0/G1 to S phase by
inhibiting the mRNA expression of CDK4, CDK2, cyclin D1 and cyclin
E, and increasing that of p27. Furthermore, the apoptosis rate of
the MKN-45 cells was increased by cucurbitacin B in a
concentration-dependent manner, which was verified by TUNEL
staining. This result was further suggested by the inhibition of
Bcl-2 and increase of Bax mRNA expression levels. These beneficial
effects of cucurbitacin B on the MKN-45 cells were associated with
the inhibition of the JAK2/STAT3 signaling pathway. Thus, the
present study revealed the anticancer effects of cucurbitacin B on
gastric cancer cells, which may lead to a novel strategy for
gastric carcinoma treatment.
Cell proliferation is an important process for cell
survival, which is also an important biological characteristic of
tumor formation (13). Therefore,
the inhibition of tumor proliferation is a crucial aim of tumor
treatment. The present study found that cucurbitacin B was able to
reduce viable cells in vitro in a dose-dependent manner, and
that the underlying mechanism involved cell cycle arrest. Following
cucurbitacin B treatment, the percentage of cells in the G0/G1
phase was markedly increased.
Cell cycle checkpoints are control mechanisms which
ensure proper DNA replication and chromosome division (24). Specifically, they are negative
feedback mechanisms, which are activated in case of abnormal cell
cycle events, such as DNA damage or obstructed DNA replication, to
induce cell cycle arrest (25). The
molecules driving the cell cycle are CDK/cyclin complexes, which
contribute to the binding of cyclins to CDKs and show the activity
of protein kinase (24,25). In these complexes, cyclins function
as the regulatory subunit, and CDKs as the catalytic subunit. The
activity of CDKs may be inhibited by CDK inhibitors (CKIs)
(24,25). CDK4 and CDK2 are known to form
complexes with cyclin D1 and cyclin E, which are essential for the
regulation of cell cycle progression from the G0/G1 to S phase
(26,27). Another regulator controlling cell
cycle progression is the CKI of p27, which forms heterotrimeric
complexes with CDKs/cyclins to inhibit their activity, such as
cyclin D1-CDK4 and cyclin E-CDK2 (28). In the present study, the expression
of cell cycle regulatory genes in response to cucurbitacin B in
MKN-45 cells was investigated. Cucurbitacin B reduced the mRNA
expression levels of cyclin D1, cyclin E, CDK4 and CDK2. Consistent
with these changes, the mRNA expression of p27 was increased. These
observations suggest that the antiproliferative activity of
cucurbitacin B has a multifaceted effect on numerous target
molecules critically involved in growth inhibition.
Tumor cells possess unlimited replication potential;
and the occurrence of tumors is associated with the abnormal
proliferation and differentiation of cells in addition to abnormal
apoptosis (8). Loss of tumor cells
via apoptosis is considered to be a contributing factor in tumor
therapy and cancer molecular biology (8). There are two major apoptotic pathways,
initiated by caspase-9 and caspase-8 respectively, which can
activate caspase cascades (29).
Apoptosis triggered by activation of the mitochondria-dependent
caspase pathway represents the primary programmed cell death
mechanism (29). This is activated
by various intracellular stresses that induce permeabilization of
the mitochondrial membrane (30).
The Bcl-2 family consists of apoptosis regulators which serve a
crucial function in the mitochondrial pathway of apoptosis. Various
precipitating factors alter the intracellular expression of Bcl-2
family or protein structure (30).
Through regulating permeability of the mitochondrial membrane,
particularly the outer mitochondrial membrane, members of the Bcl-2
family induce the release of mitochondrial intermembrane space
proteins (31). In the present
study, cucurbitacin B downregulated the mRNA expression levels of
Bcl-2 and upregulated the expression of Bax in the MKN-45 cells,
which may regulate the proapoptotic effect of cucurbitacin B,
suggesting that cucurbitacin B induced the apoptosis via the
endogenous apoptosis pathway. These findings indicated that through
its proapoptotic property, cucurbitacin B may be beneficial in
gastric cancer treatment.
JAK/STAT pathway plays a crucial role in numerous
signal transductions in vivo. The JAKs activation has an
important role in cell differentiation, proliferation, apoptosis
and migration (18). Structure
activation of JAKs contributes to phosphorylation of the STAT
family. STAT3 is a member of STAT family, which has been
intensively studied in recent years (13–17). As
confirmed by cell culture and animal models, the STAT3 signaling
pathway provides an effective molecular target in the treatment of
various human cancers (13–17). STAT3 signal is present in the
epithelial cells of normal gastric mucosa and human gastric cancer
cells (17). STAT3 activation
mediates normal physiological processes of gastric mucosa (32). Overactivation of STAT3 is found in
various types of gastric cancer cells (33). Pathological detection of gastric
cancer indicated that STAT3 protein expression was obviously higher
than that in normal gastric mucosa, the former being 2.14 times of
that of the latter on average (34,35).
STAT3 protein expression in mildly differentiated gastric cancer
was generally higher than that in highly differentiated gastric
cancer (34,35).
It has been shown that cucurbitacin B has potent
antitumor activity against human pancreatic cancer cells by
inhibiting JAK/STAT signaling pathway (36), and may also inhibit growth and induce
apoptosis through JAK2/STAT3 signaling in SH-SY5Y human
neuroblastoma cells (37).
Therefore, we further aimed to determine whether the JAK2/STAT3
signaling pathway could be affected by cucurbitacin B. It was found
that cucurbitacin B directly inhibited the phosphorylation of JAK2
and the downstream STAT3.
In conclusion, the present study demonstrated for
the first time that cucurbitacin B inhibits the proliferation and
promotes apoptosis of MKN-45 cells in vitro. These
beneficial effects of cucurbitacin B on the MKN-45 cells were
associated with the inhibition of the JAK2/STAT3 signaling pathway.
The results of this study suggest that the use of cucurbitacin B to
treat gastric carcinoma diseases warrants further
investigation.
References
|
1
|
Allum WH, Blazeby JM, Griffin SM,
Cunningham D, Jankowski JA and Wong R: Association of Upper
Gastrointestinal Surgeons of Great Britain and Ireland, the British
Society of Gastroenterology and the British Association of Surgical
Oncology: Guidelines for the management of oesophageal and gastric
cancer. Gut. 60:1449–1472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernini M and Bencini L: Multimodal
treatment of gastric cancer: Surgery, chemotherapy, radiotherapy
and timing. Int J Surg Oncol. 2012:2462902012.PubMed/NCBI
|
|
3
|
Yu C, Yu R, Zhu W, Song Y and Li T:
Intensity-modulated radiotherapy combined with chemotherapy for the
treatment of gastric cancer patients after standard D1/D2 surgery.
J Cancer Res Clin Oncol. 138:255–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roukos DH: Current advances and changes in
treatment strategy may improve survival and quality of life in
patients with potentially curable gastric cancer. Ann Surg Oncol.
6:46–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu CD: Clinical study of nimotuzumab
combined with chemotherapy in the treatment of late stage gastric
cancer. Asian Pac J Cancer Prev. 15:10273–10276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao SR, Li LM, Xia HP, Wang GM, Xu HY and
Wang AR: Clinical observation on recombinant human endostatin
combined with chemotherapy for advanced gastrointestinal cancer.
Asian Pac J Cancer Prev. 16:4037–4040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Zhong L, Liu BZ, Gao YJ, Gao YM
and Hu XX: Effect of GINS2 on proliferation and apoptosis in
leukemic cell line. Int J Med Sci. 10:1795–1804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu YJ, Li YM, Hou XD, Guo C, Cao N and
Jiao ZY: Effect of tissue factor on invasion inhibition and
apoptosis inducing effect of oxaliplatin in human gastric cancer
cell. Asian Pac J Cancer Prev. 13:1845–1849. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan B, Liu L, Zhao Y, Xiu LJ, Sun DZ, Liu
X, Lu Y, Shi J, Zhang YC, Li YJ, et al: Xiaotan Sanjie decoction
attenuates tumor angiogenesis by manipulating Notch-1-regulated
proliferation of gastric cancer stem-like cells. World J
Gastroenterol. 20:13105–13118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Research on the efficacy of Celastrus
orbiculatus in suppressing TGF-β1-induced epithelial-mesenchymal
transition by inhibiting HSP27 and TNF-α-induced NF-κ B/ Snail
signaling pathway in human gastric adenocarcinoma. BMC Complement
Altern Med. 14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hung MH, Tai WT, Shiau CW and Chen KF:
Downregulation of signal transducer and activator of transcription
3 by sorafenib: A novel mechanism for hepatocellular carcinoma
therapy. World J Gastroenterol. 20:15269–15274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Ren G, Wang T, Chen Y, Gong C, Bai
Y, Wang B, Qi H, Shen J, Zhu L, et al: Aberrantly expressed Fra-1
by IL-6/STAT3 transactivation promotes colorectal cancer
aggressiveness through epithelial-mesenchymal transition.
Carcinogenesis. 36:459–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao X, Liu H, Zhang X, Zhang L, Li X, Wang
C and Sun S: Cell surface GRP78 accelerated breast cancer cell
proliferation and migration by activating STAT3. PLoS One.
10:e01256342015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peyser ND, Freilino M, Wang L, Zeng Y, Li
H, Johnson DE and Grandis JR: Frequent promoter hypermethylation of
PTPRT increases STAT3 activation and sensitivity to STAT3
inhibition in head and neck cancer. Oncogene. 2015.PubMed/NCBI
|
|
15
|
Wen W, Wu J, Liu L, Tian Y, Buettner R,
Hsieh MY, Horne D, Dellinger TH, Han ES, Jove R and Yim JH:
Synergistic anti-tumor effect of combined inhibition of EGFR and
JAK/STAT3 pathways in human ovarian cancer. Mol Cancer. 14:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hossain DM, Pal SK, Moreira D, Duttagupta
P, Zhang Q, Won H, Jones J, D'Apuzzo M, Forman S and Kortylewski M:
TLR9-targeted STAT3 silencing abrogates immunosuppressive activity
of myeloid-derived suppressor cells from prostate cancer patients.
Clin Cancer Res. 21:3771–3782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon J, Ko YS, Cho SJ, Park J, Choi YS,
Choi Y, Pyo JS, Ye SK, Youn HD, Lee JS, et al: Signal transducers
and activators of transcription 3-induced metastatic potential in
gastric cancer cells is enhanced by glycogen synthase kinase-3β.
APMIS. 123:373–382. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YX, Cai H, Jiang G, Zhou TB and Wu H:
Silibinin inhibits proliferation, induces apoptosis and causes cell
cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway
inhibition. Asian Pac J Cancer Prev. 15:6791–6798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silva IT, Carvalho A, Lang KL, Dudek SE,
Masemann D, Durán FJ, Caro MS, Rapp UR, Wixler V, Schenkel EP, et
al: In vitro and in vivo antitumor activity of a novel
semisynthetic derivative of cucurbitacin B. PLoS One.
10:e01177942015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li ZJ, Shin JM, Choi DK, Lim SK, Yoon TJ,
Lee YH, Sohn KC, Im M, Lee Y, Seo YJ, et al: Inhibitory effect of
cucurbitacin B on imiquimod-induced skin inflammation. Biochem
Biophys Res Commun. 459:673–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang M, Bian ZG, Zhang Y, Wang JH, Kan L,
Wang X, Niu HY and He P: Cucurbitacin B inhibits proliferation and
induces apoptosis via STAT3 pathway inhibition in A549 lung cancer
cells. Mol Med Rep. 10:2905–2911. 2014.PubMed/NCBI
|
|
22
|
Iwanski GB, Lee DH, En-Gal S, Doan NB,
Castor B, Vogt M, Toh M, Bokemeyer C, Said JW, Thoennissen NH and
Koeffler HP: Cucurbitacin B, a novel in vivo potentiator of
gemcitabine with low toxicity in the treatment of pancreatic
cancer. Br J Pharmacol. 160:998–1007. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu W and McArthur G: Cell cycle regulation
and melanoma. Curr Oncol Rep. 18:342016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan YB: Creatine kinase in cell cycle
regulation and cancer. Amino acids. 48:1775–1784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jirawatnotai S, Aziyu A, Osmundson EC,
Moons DS, Zou X, Kineman RD and Kiyokawa H: Cdk4 is indispensable
for postnatal proliferation of the anterior pituitary. J Biol Chem.
279:51100–51106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martín A, Odajima J, Hunt SL, Dubus P,
Ortega S, Malumbres M and Barbacid M: Cdk2 is dispensable for cell
cycle inhibition and tumor suppression mediated by p27 (Kip1) and
p21 (Cip1). Cancer Cell. 7:591–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abukhdeir AM and Park BH: P21 and p27:
Roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiraz Y, Adan A, Yandim M Kartal and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Monian P and Jiang X: Clearing the final
hurdles to mitochondrial apoptosis: Regulation post cytochrome C
release. Exp Oncol. 34:185–191. 2012.PubMed/NCBI
|
|
31
|
Huber HJ, Duessmann H, Wenus J, Kilbride
SM and Prehn JH: Mathematical modelling of the mitochondrial
apoptosis pathway. Biochim Biophys Acta. 1813:608–615. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanai M, Konda Y, Nakajima T, Izumi Y,
Kanda N, Nanakin A, Kubohara Y and Chiba T:
Differentiation-inducing factor-1 (DIF-1) inhibits STAT3 activity
involved in gastric cancer cell proliferation via MEK-ERK-dependent
pathway. Oncogene. 22:548–554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu LF, Cheng Y, Qiao MM, Zhang YP and Wu
YL: Activation of STAT3 signaling in human stomach adenocarcinoma
drug-resistant cell line and its relationship with expression of
vascular endothelial growth factor. World J Gastroenterol.
11:875–879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schauer M, Janssen KP, Rimkus C, Raggi M,
Feith M, Friess H and Theisen J: Microarray-based response
prediction in esophageal adenocarcinoma. Clin Cancer Res.
16:330–337. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu LF, Zhu YB, Qiao MM, Zhong J, Tu SP and
Wu YL: Constitutive activation and clinical significance of Stat3
in human gastric cancer tissues and cell lines. Zhonghua Yi Xue Za
Zhi. 84:2064–2069. 2004.(In Chinese). PubMed/NCBI
|
|
36
|
Thoennissen NH, Iwanski GB, Doan NB,
Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, et al:
Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT
pathway and potentiates antiproliferative effects of gemcitabine on
pancreatic cancer cells. Cancer Res. 69:5876–5884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng Q, Liu Y, Liu W, Ma F, Zhou Y, Chen
M, Chang J, Wang Y, Yang G and He G: Cucurbitacin B inhibits growth
and induces apoptosis through the JAK2/STAT3 and MAPK pathways in
SH-SY5Y human neuroblastoma cells. Mol Med Rep. 10:89–94.
2014.PubMed/NCBI
|