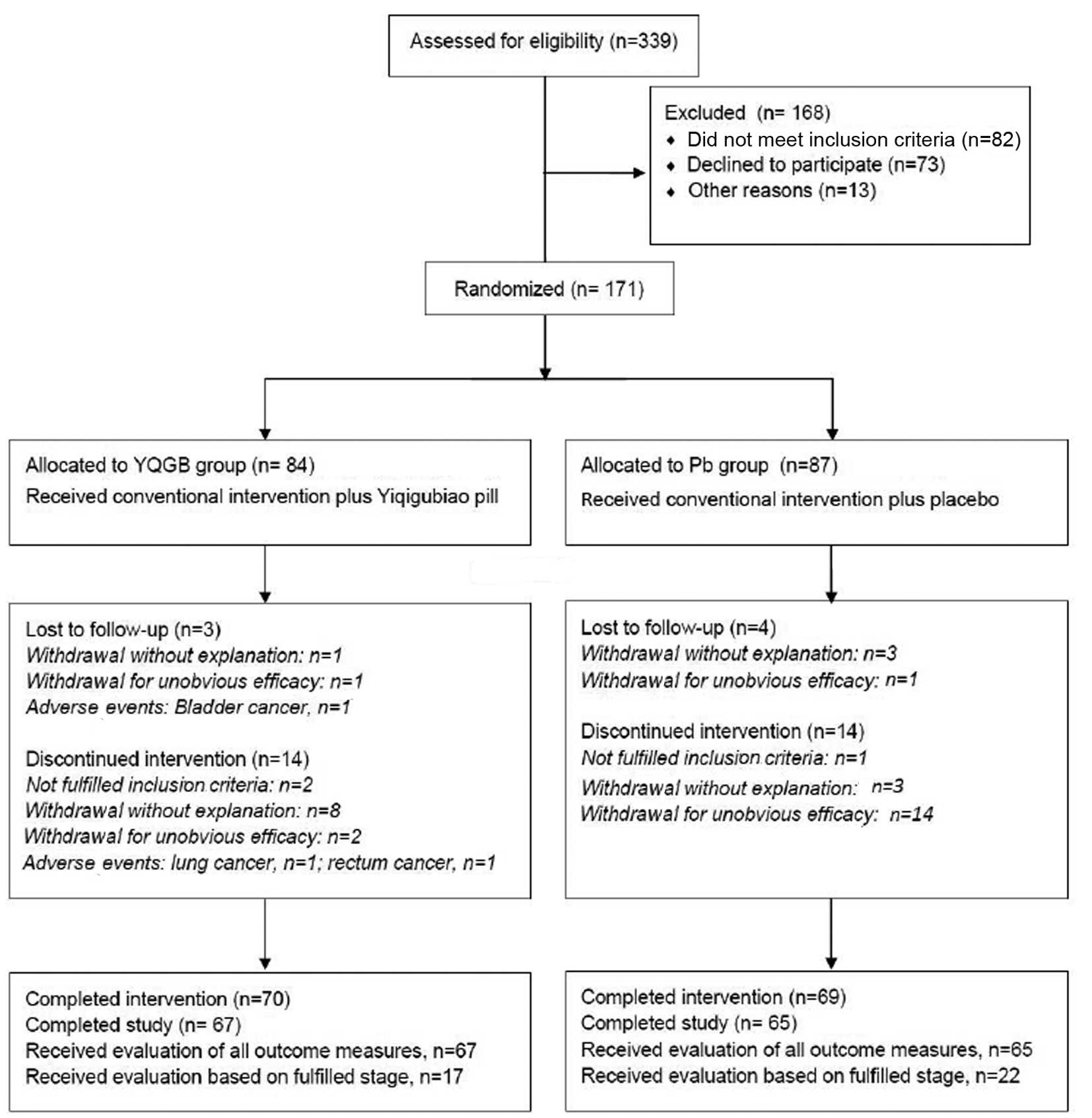
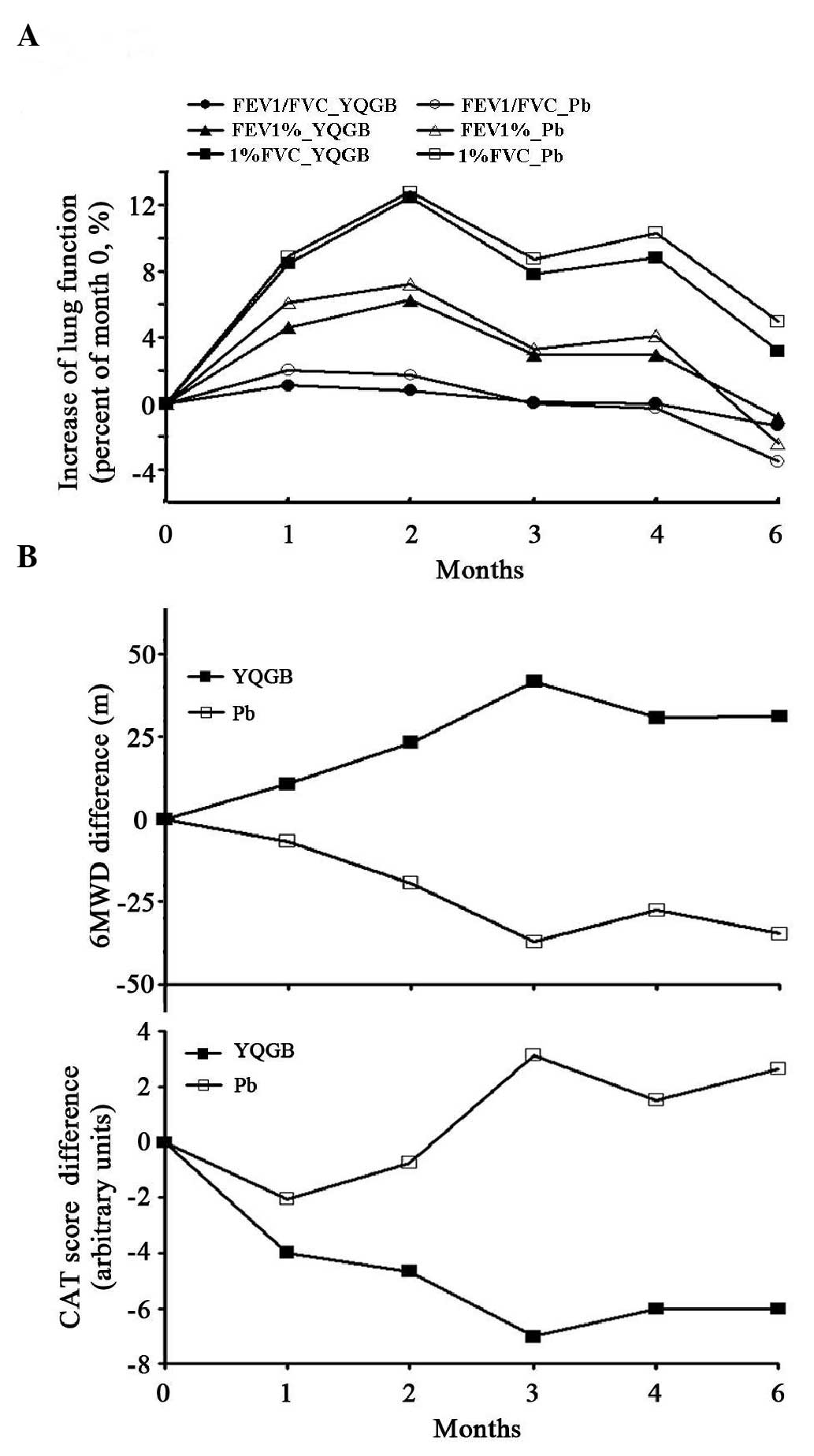
|
1
|
Lin HH, Murray M, Cohen T, Colijn C and
Ezzati M: Effects of smoking and solid-fuel use on COPD, lung
cancer, and tuberculosis in China: A time-based, multiple risk
factor, modelling study. Lancet. 372:1473–1483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chapman KR, Mannino DM, Soriano JB,
Vermeire PA, Buist AS, Thun MJ, Connell C, Jemal A, Lee TA,
Miravitlles M, et al: Epidemiology and costs of chronic obstructive
pulmonary disease. Eur Respir J. 27:188–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopez AD, Shibuya K, Rao C, Mathers CD,
Hansell AL, Held LS, Schmid V and Buist S: Chronic obstructive
pulmonary disease: Current burden and future projections. Eur
Respir J. 27:397–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Global Initiative for Chronic Obstructive
Lung Disease, . Global strategy for the diagnosis, management and
prevention of chronic obstructive pulmonary disease (Revised 2011).
http://www.goldcopd.com
|
|
5
|
Almansa R, Socias L, Andaluz-Ojeda D,
Martín-Loeches I, Bobillo F, Blanco J, Rico L, Berezo JÁ, Estella
Á, Sanchez-Garcia M, et al: Viral infection is associated with an
increased proinflammatory response in chronic obstructive pulmonary
disease. Viral Immunol. 25:249–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lugade AA, Bogner PN, Thatcher TH, Sime
PJ, Phipps RP and Thanavala Y: Cigarette smoke exposure exacerbates
lung inflammation and compromises immunity to bacterial infection.
J Immunol. 192:5226–5235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calverley PM and Walker P: Chronic
obstructive pulmonary disease. Lancet. 362:1053–1061. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daga MK, Khan NA, Malhotra V, Kumar S,
Mawari G and Hira S: Study of body composition, lung function, and
quality of life following use of anabolic steroids in patients with
chronic obstructive pulmonary disease. Nutr Clin Pract. 29:238–245.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falk JA, Minai OA and Mosenifar Z: Inhaled
and systemic corticosteroids in chronic obstructive pulmonary
disease. Proc Am Thorac Soc. 5:506–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh D: New combination bronchodilators
for chronic obstructive pulmonary disease: Current evidence and
future perspectives. Br J Clin Pharmacol. 79:695–708. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greulich T, Koczulla AR, Nell C, Kehr K,
Vogelmeier CF, Stojanovic D, Wittmann M and Schultz K: Effect of a
three-week inpatient rehabilitation program on 544 consecutive
patients with very severe COPD: A retrospective analysis.
Respiration. 90:287–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta P and O'Mahony MS: Potential adverse
effects of bronchodilators in the treatment of airways obstruction
in older people: Recommendations for prescribing. Drugs Aging.
25:415–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh S, Loke YK and Furberg CD: Inhaled
anticholinergics and risk of major adverse cardiovascular events in
patients with chronic obstructive pulmonary disease: A systematic
review and meta-analysis. JAMA. 300:1439–1450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee TA, Pickard AS, Au DH, Bartle B and
Weiss KB: Risk for death associated with medications for recently
diagnosed chronic obstructive pulmonary disease. Ann Intern Med.
149:380–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jian XY, Huang ZQ, Lai X, Chen WY and
Jiang RB: A clinical study on the effects of Pingchuan capsule on
BODE index in COPD patients at stable stage. Journal of Beijing
University of Traditional Chinese Medicine. 19:32–34. 2012.(In
Chinese).
|
|
16
|
Jiang FC, Yan Y, Yang L, Song Q and Li YL:
Impact of chinese herb on quality of life of stable chronic
obstructive pulmonary disease: A randomized controlled study.
Zhongguo Zhong Yao Za Zhi. 36:3203–3206. 2011.(In Chinese).
PubMed/NCBI
|
|
17
|
Yu XQ, Li JS and Li L: A study on
distributional regularity of TCM syndromes in chronic obstructive
pulmonary disease patients. China Journal of Chinese Medicine.
4:44–46. 2003.(In Chinese).
|
|
18
|
Yu QH and Qiu ZN: Analysis of diagnosis
and treatment of chronic obstructive pulmonary disease. Chinese
Archives of Traditional Chinese Medicine. 21:11902003.(In
Chinese).
|
|
19
|
Li HW and Li HJ: A report on 150 cases of
recurrent airway inflammation treated with Yiqigubiao formula in
teenagers. Lishizhen Medicine and Materia Medica Research.
17:626–627. 2006.(In Chinese).
|
|
20
|
Li M, Qu CY and Zhui AQ: A clinical study
on 80 cases of allergic rhintis treated with Yiqigubiao therapy.
China Modern Doctor. 46:104–111. 2008.(In Chinese).
|
|
21
|
Yang Q, Xu Y, Feng G, Hu C, Zhang Y, Cheng
S, Wang Y and Gong X: p38 MAPK signal pathway involved in
anti-inflammatory effect of Chaihu-Shugan-San and
Shen-ling-bai-zhu-San on hepatocyte in non-alcoholic
steatohepatitis rats. Afr J Tradit Complement Altern Med.
11:213–221. 2014.PubMed/NCBI
|
|
22
|
Zhu Y and Liu X: Treatment of chronic
bronchitis with modified ma xing shi gan tang and er chen tang. J
Tradit Chin Med. 24:12–13. 2004.PubMed/NCBI
|
|
23
|
Zheng YS, Wu ZS, Ni HB, Ke L, Tong ZH, Li
WQ, Li N and Li JS: Codonopsis pilosula polysaccharide attenuates
cecal ligation and puncture sepsis via circuiting regulatory T
cells in mice. Shock. 41:250–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu L and Yu M: New progression on study of
Poria cocos. Xinjiang Journal of Traditional Chinese Medicine.
23:79–82. 2005.(In Chinese).
|
|
25
|
Hong MH, Kim JH, Bae H, Lee NY, Shin YC,
Kim SH and Ko SG: Atractylodes japonica Koidzumi inhibits the
production of proinflammatory cytokines through inhibition of the
NF-kappaB/IkappaB signal pathway in HMC-1 human mast cells. Arch
Pharm Res. 33:843–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Celli BR and MacNee W: ATS/ERS Task Force:
Standards for the diagnosis and treatment of patients with COPD: A
summary of the ATS/ERS position paper. Eur Respir J. 23:932–946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morales-Blanhir JE, Vidal CD Palafox, Mde
J Rosas Romero, Castro MM Garcia, Villegas A Londoño and Zamboni M:
Six-minute walk test: A valuable tool for assessing pulmonary
impairment. J Bras Pneumol. 37:110–117. 2011.(In Portuguese).
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mahler DA and Wells CK: Evaluation of
clinical methods for rating dyspnea. Chest. 93:580–586. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jones PW, Harding G, Berry P, Wiklund I,
Chen WH and Leidy N Kline: Development and first validation of the
COPD Assessment Test. Eur Respir J. 34:648–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Celli BR, Cote CG, Marin JM, Casanova C,
de Oca M Montes, Mendez RA, Plata V Pinto and Cabral HJ: The
body-mass index, airflow obstruction, dyspnea and exercise capacity
index in chronic obstructive pulmonary disease. N Engl J Med.
350:1005–1012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Richardson K..Weinberg A: Dynamics of
regulatory T-cells during pregnancy: Effect of HIV infection and
correlations with other immune parameters. PLoS One. 6:e281722011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li JS and Wang MH: Clinical significance
of acute exacerbation in chronic obstructive pulmonary disease.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 19:572–573. 2007.(In
Chinese). PubMed/NCBI
|
|
33
|
The Lung Health Study Research Group, .
Effect of inhaled triamcinolone on the decline in pulmonary
function in chronic obstructive pulmonary disease. N Engl J Med.
343:1902–1909. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tashkin DP, Celli B, Senn S, Burkhart D,
Kesten S, Menjoge S and Decramer M: UPLIFT Study Investigators: A
4-year trial of tiotropium in chronic obstructive pulmonary
disease. N Engl J Med. 359:1543–1554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang JJ, Rochtchina E, Tan AG, Cumming RG,
Leeder SR and Mitchell P: Use of inhaled and oral corticosteroids
and the long-term risk of cataract. Ophthalmology. 116:652–657.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hougardy DM, Peterson GM, Bleasel MD and
Randall CT: Is enough attention being given to the adverse effects
of corticosteroid therapy? J Clin Pharm Ther. 25:227–234. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Zhang F and Li J: The
immunoregulatory effects of traditional Chinese medicine on
treatment of asthma or asthmatic Inflammation. Am J Chin Med.
43:1059–1081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang HF, Zhang HL, Li JS, Yu YQ, Lu SY, Li
B, Xie Y and Bai YP: Effectiveness and safety of traditional
Chinese medicine on stable chronic obstructive pulmonary disease: A
systematic review and meta-analysis. Complement Ther Med.
23:603–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
The Committee of Pulmonary Disease, .
China Association of Traditional Chinese Medicine: The diagnostic
standard on symptoms of COPD in Chinese medicine (version 2011).
Journal of Traditional Chinese Medicine. 2:177–178. 2012.(In
Chinese).
|
|
40
|
Hu J and Meek P: Health-related quality of
life in individuals with chronic obstructive pulmonary disease.
Heart Lung. 34:415–422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
O'Donnell DE, Lam M and Webb KA:
Measurement of symptoms, lung hyperinflation, and endurance during
exercise in chronic obstructive pulmonary disease. Am J Respir Crit
Care Med. 158:1557–1565. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hogg JC, Chu F, Utokaparch S, Woods R,
Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson
HO and Par PD: The nature of small-airway obstruction in chronic
obstructive pulmonary disease. N Engl J Med. 350:2645–2653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Babusyte A, Jeroch J, Stakauskas R,
Stravinskaite K, Malakauskas K and Sakalauskas R: The effect of
induced sputum and bronchoalveolar lavage fluid from patients with
chronic obstructive pulmonary disease on neutrophil migration in
vitro. Medicina (Kaunas). 46:315–322. 2010.PubMed/NCBI
|
|
44
|
Lin WS: Clinical study of allergic
rhinitis treated by ‘bu qi gu biao’. Zhong Xi Yi Jie He Za Zhi.
9:263–265. 1989.(In Chinese). PubMed/NCBI
|
|
45
|
Rios JL: Chemical constituents and
pharmacological properties of Poria cocos. Planta Med. 77:681–691.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li CQ, He LC, Dong HY and Jin JQ:
Screening for the anti-inflammatory activity of fractions and
compounds from Atractylodes macrocephala koidz. J Ethnopharmacol.
114:212–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hyam SR, Jang SE, Jeong JJ, Joh EH, Han MJ
and Kim DH: Echinocystic acid, a metabolite of lancemaside A,
inhibits TNBS-induced colitis in mice. Int Immunopharmacol.
15:433–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dimopoulos G, Tsiodras S, Lerikou M,
Chranioti A, Perros E, Anagnostopoulou U, Karakitsos P and
Armaganidis A: Viral profile of COPD exacerbations according to
patients. Open Respir Med J. 9:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|