Introduction
Iridium-192 (Ir192) is a radioactive isotope of
iridium with a half-life of 73.83 days, which emits gamma radiation
with a low level of linear energy transfer and a high level of
penetration in the human body (1).
Ir192 is frequently used as a gamma-ray source in industrial
radiography to detect flaws in metal components (2). It is also used as a radiation source in
radiotherapy; for example, it has been shown that Ir192 can reduce
recurrent coronary restenosis following the initial percutaneous
treatment of in-stent restenosis (3). However, exposure to high doses of Ir192
has a deleterious effect. It has been shown that the exposure of
fingertips to 20–30 Sv Ir192 can cause progressive tissue
deterioration and result in successive amputations of the fingers
being necessary (2). An
over-exposure to industrial radiography using Ir192 radionuclide
may result in skin erythema, skin necrosis, progressive tissue
deterioration and even malignant diseases such as myeloid leukemia,
lymphoma and multiple myeloma (4,5). It has
been shown that acute exposure to large- or medium-dose radiation
can seriously affect the blood and immune systems, leading to
hematopoietic failure or hematopoietic tumors (6). However, the effect of low-dose ionizing
radiation, particularly the effect of persistent low-dose
irradiation on the human body remains unclear.
More than 70 people were unintentionally exposed to
persistent low-dose Ir192 radiation in July 2002. The present study
was conducted as a 10-year follow-up of those people, and was
carried out to investigate the effect of persistent low-dose Ir192
exposure on immunological function, chromosome aberrations and the
telomerase activity of bone marrow mononuclear cells (BMNCs). The
effects of persistent low-dose Ir192 exposure on complement C3 and
C4 levels, CD3+, CD4+ and CD8+ T
cells, the lymphocyte transformation (LT) rate, and percentage of
natural killer (NK) cells were determined. The abnormality rates of
peripheral blood and bone marrow, the chromosome aberration rate,
and bone marrow mononuclear cell telomerase activity were also
analyzed.
Materials and methods
Patients
In total, 54 people exposed to persistent low-dose
Ir192 were included in this study. The inclusion criteria were as
follows: i) Worked within a radius of 10 meters from the radiation
source during accidental exposure (between May 9, 2002 and July 19,
2002); ii) diagnosed by the Chinese Institute of Radiation Medicine
(Beijing, China) as receiving an exposure dose of 0.05–0.65 Grey
(Gy). The exclusion criteria were as follows: i) Had been exposed
to radiation before: ii) had cancer, tuberculosis, hepatitis or
autoimmune disease; iii) had taken medicine in the previous 3
months. Among the 54 patients, there were 29 males and 25 females.
The median age of the patients was 31.5 years (range, 20–42 years).
The blood or bone marrow samples of the control group were obtained
from 20 healthy volunteer bone marrow donors. This study was
approved by the Institutional Review Boards (IRBs) of the General
Hospital of Guangzhou Military Command (Guangzhou, China). Written
informed consent was obtained from all patients in accordance with
the IRB regulations and the Declaration of Helsinki.
General information
Clinical symptoms, abnormality rates of peripheral
blood and bone marrow, immunoglobulin and complement (IgA, IgG,
IgM, C3 and C4) levels, T cell subsets (CD3+,
CD4+ and CD8+), the LT rate, percentage of NK
cells, chromosome aberration rate and BMNC telomerase activity were
recorded at different time points (1 month, and 1,3,5 and 10 years
after exposure).
Blood tests
An HMX LH500 blood cell analyzer (Beckman Coulter,
Inc., Brea, CA, USA) was used to analyze peripheral blood samples.
Bone marrow was taken by posterior superior iliac spine bone marrow
aspiration, smeared, Wright-Giemsa stained and observed under an
inverted microscope. Abnormal blood was defined by a white blood
cell (WBC) count of >10.0×109/l or
<4.0×109/l, hemoglobin (Hb) level of <120 g/l
(male) or <110 g/l (female), or platelet count of <100
109/l. Bone marrow suppression, active bone marrow
proliferation, and hyperactive bone marrow proliferation were
defined as myelodysplasia. Morphological abnormality was defined as
>2% of nucleated cells showing a morphological abnormality.
Tests of T cell subset
Cells were incubated with fluorescence-labeled
monoclonal antibodies and were observed under a fluorescence
microscope to calculate the percentage of positive cells. Briefly,
BD Pharm Lyse lysing buffer was applied to human whole blood red
blood cells (BD Biosciences, San Jose, CA, USA) with a monoclonal
antibody mixture and gently vortexed. Monoclonal antibodies include
anti-CD3 antibody (cat. no. ab16669; 1:1,000 dilution), anti-CD4
antibody (cat. no. ab133616; 1:100 dilution), anti-CD8 antibody
(cat. no. ab17147; 1:100 dilution), anti-CD57 antibody (cat. no.
ab25629; 1:100 dilution) (all purchased Abcam, Cambridge, MA, USA).
Following incubation for 15 min at room temperature, the mixture
was centrifuged at 200 × g for 5 min and the supernatant was
aspirated. It was added to phosphate buffered saline-fetal bovine
serum (PBS-FBS; containing 1% heat-inactivated fetal bovine serum;
Gibco; Thermo Fisher Scientific, Waltham, MA, USA; and 0.1% sodium
azide), the pellet was re-suspended with PBS-FBS after
centrifugation at 200 × g for flow cytometric analysis. The
absolute cell count and the marker percentage were calculated using
FACSCalibur (BD Biosciences).
Chromosome aberration assay
Peripheral blood (3 ml) was collected from each
patient and incubated at 37°C for 2 h to allow it naturally
stratify. Cells from the interfacial layer (0.1 ml) were cultured
in RPMI-1640 (Thermo Fisher Scientific, Inc.) containing 15% fetal
bovine serum (Sigma-Aldrich, St. Louis, MO, USA) in a 37°C
incubator. Colchicine was added to the cells at a concentration of
0.05 µg/ml 24 h after incubation. Cells were collected 48 h after
incubation, stained with Giemsa, and observed under an inverted
microscope. A total of 200 metaphase cells were counted and
chromosome aberrations were analyzed.
Automatic karyotype analysis
(G-binding)
The bone marrow culture was applied for the
preparation of conventional chromosomes. Based on G-banding
technology, chromosomal karyotypes analysis was developed and 20
mitoses were counted. Abnormal karyotypes were determined according
to the International System for Human Cytogenetic Nomenclature 1995
(ISCN 1995). An AMMS-1 karyotype analyzer (Q500; Leica Microsystems
GbmH, Wetzlar, Germany) was used in this experiment.
Measurement of BMNC telomerase
activity
A telomeric repeat amplification protocol-enzyme
linked immunosorbent assay (TRAP-ELISA; cat. no. CB42542175; EMD
Millipore, Billerica, MA, USA) was used in this experiment.
Briefly, BMNCs were isolated by Ficoll density gradient
centrifugation (room temperature, 400 × g, 20 min). TRAP-ELISA was
performed according to the manufacturer's protocol.
Statistical analysis
SPSS 11.0 software (SPSS Inc., Chicago, IL, USA) was
used to perform the statistical analysis. All data are expressed as
the mean ± standard deviation. The results were analyzed using
Student's t-test or one-way analysis of variance followed by
Student-Newman-Keuls post-hoc test. In all tests, P<0.05 was
considered to indicate a statistically significant difference.
Results
Follow-up of clinical symptoms
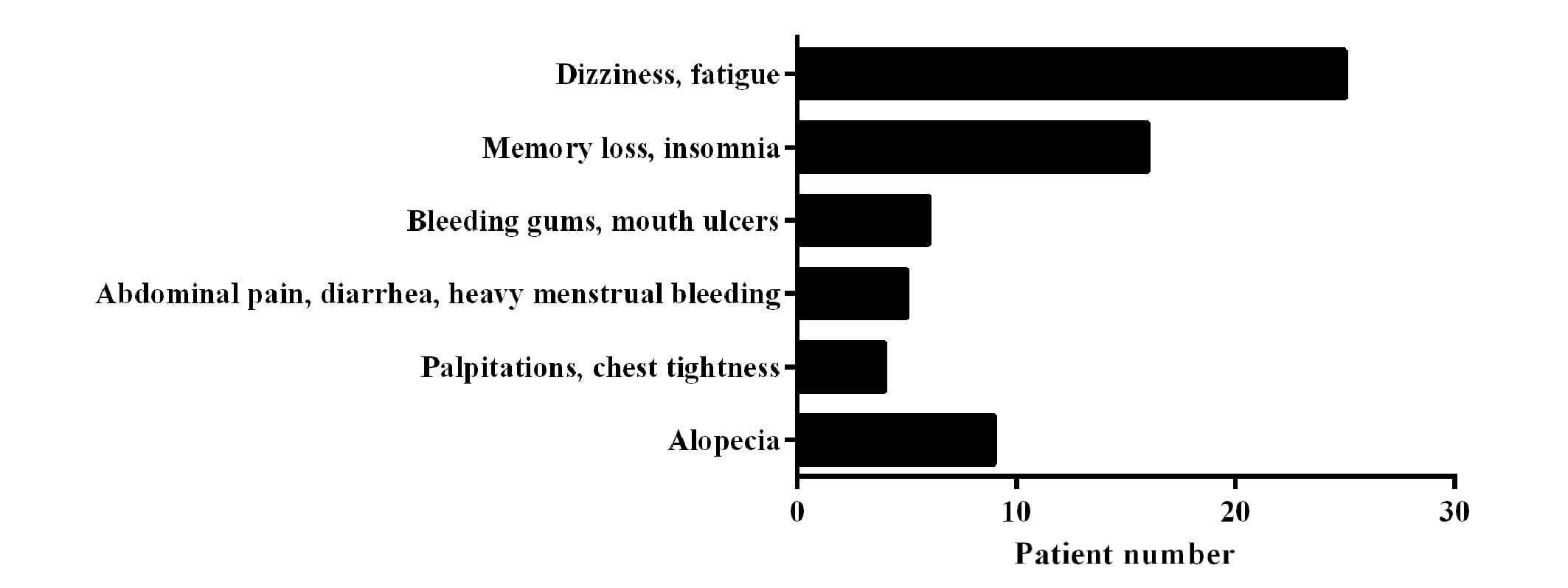
The results shown in Fig.
1 show that 1 month after exposure, 90.7% patients exhibited
clinical symptoms of various degrees, including dizziness, fatigue,
memory loss or insomnia, bleeding gums, mouth ulcers, abdominal
pain, diarrhea, heavy menstrual bleeding, palpitations or chest
tightness, and alopecia. These symptoms were only observed in 7/54
patients (13.0%), 3/42 patients (7.1%) and 3/31 patients (9.7%) in
the follow-up checks at years 1, 5 and 10 after exposure. Among the
3 patients who exhibited prolonged symptoms, 1 patient was exposed
to a large dose (>1.5 Gy). No aplastic anemia, bone marrow
failure diseases or leukemia were found in this 10-year follow-up
study.
Parameters in blood and bone
marrow
The proportion of patients with an abnormal WBC
count was 44.4% (24/54) 1 month after exposure, and this decreased
to 24.1% (13/54) 1 year post exposure, 18.0% (9/50) 3 years post
exposure, 16.7% (7/42) 5 years post exposure and 9.7% (3/31) 10
years post exposure. Morphological abnormalities were observed in
bone marrow granulocytes, erythrocytes and megakaryocytes. The
morphological abnormality rate was 90.7% at 1 month after exposure,
and this decreased to 18.5% 1 year post exposure, 12.0% 3 years
post exposure, 9.5% 5 years post exposure and 12.9% 10 years post
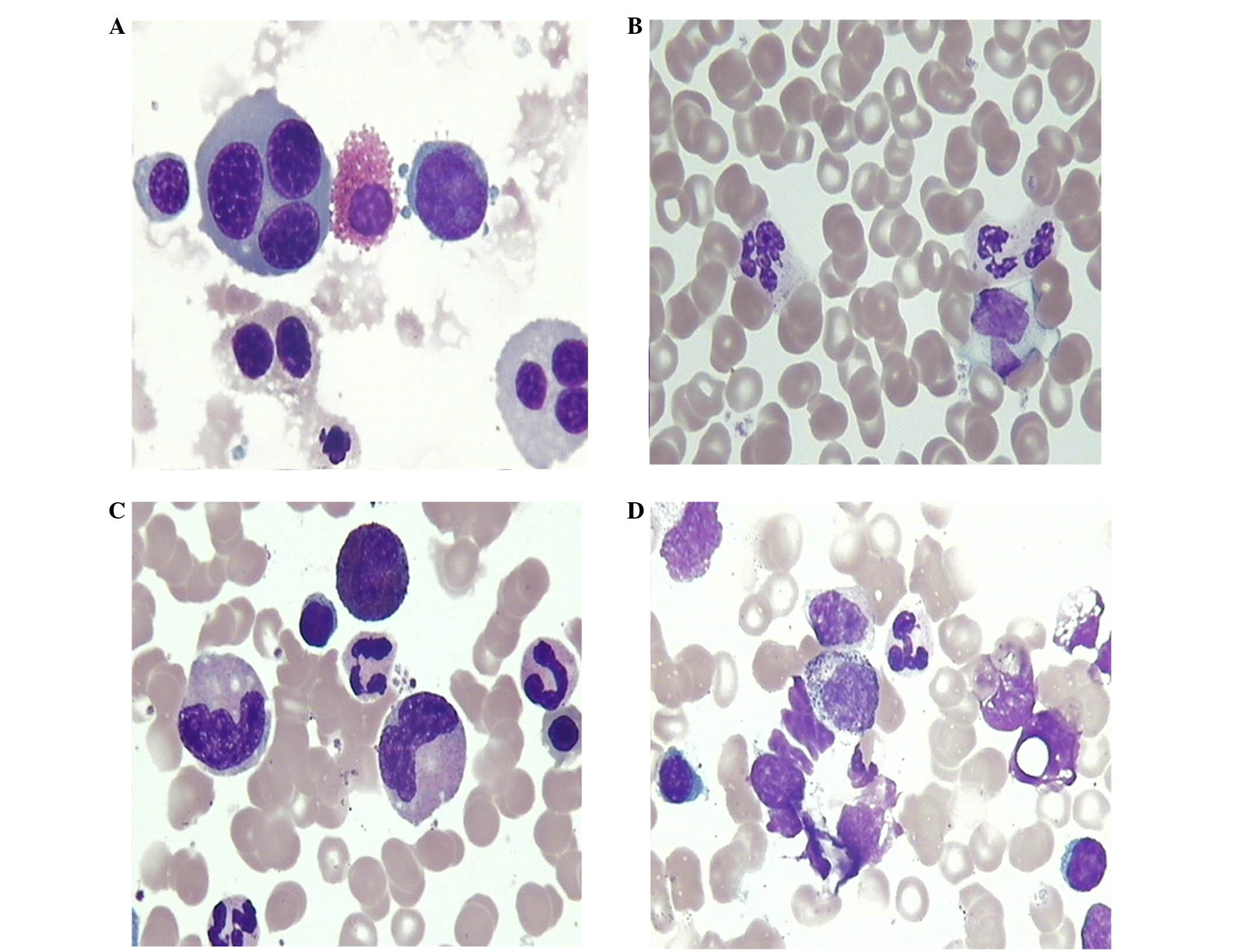
exposure (Table I). Erythrocyte
morphological abnormalities included small red blood cells, giant
red blood cells and immature red blood cells (Fig. 2A). Megakaryocyte morphological
abnormalities were mainly exhibited as increased number of small
megakaryocytes and original megakaryocytes. Granulocyte
morphological abnormalities that were observed were mainly cell
swelling, reduced number of cytoplasmic granules, karyopyknosis,
spinous changes (Fig. 2B), vacuolar
degeneration (Fig. 2C), toxic
granulation and increasing number of myeloblasts and promyelocytes
(Fig. 2D).
 | Table I.Parameters of the blood and bone
marrow of patients following Ir192 exposure. |
Table I.
Parameters of the blood and bone
marrow of patients following Ir192 exposure.
|
|
| Abnormal blood
results, n (%) | Abnormal bone marrow,
n (%) |
|---|
|
|
|
|
|
|---|
| Time point | No. of patients | WBC | Hb | PLT | Morphology | Hyperplasia |
|---|
| 1 month | 54 | 24 (44.4) | 7 (13.0) | 7 (13.0) | 49 (90.7) | 3 (5.6) |
| 1 year | 54 | 13 (24.1) | 5 (9.2) | 3 (5.6) | 10 (18.5) | 3 (5.6) |
| 3 years | 50 | 9 (18.0) | 4 (8.0) | 2 (4.0) | 6 (12.0) | 3 (6.0) |
| 5 years | 42 | 7 (16.7) | 2 (4.8) | 2 (4.8) | 4 (9.5) | 3 (7.1) |
| 10 years | 31 | 3 (9.6) | 2 (6.5) | 0 (0) | 4 (12.9) | 3 (9.7) |
Immune function parameters
Complement C3 and C4 levels were significantly
decreased 1 month after exposure when compared with those of normal
controls (P<0.01), and recovered to a normal level 1 year after
exposure. No significant changes were found in IgA, IgG or IgM
levels (Table II). At 1 month after
exposure, the CD3+, CD4+, CD8+ and
CD4+/CD8+ T cell levels, LT rate and
percentage of NK cells were significantly lower than those of the
controls (P<0.01), and these values had recovered to normal
levels by 3 years after exposure (Table III).
 | Table II.Immunoglobulin and complement changes
of patients following Ir192 exposure (g/l). |
Table II.
Immunoglobulin and complement changes
of patients following Ir192 exposure (g/l).
| Group | No. of patients | IgA | IgM | C3 | C4 |
|---|
| Control | 20 | 1.88±0.35 | 10.37±2.33 | 0.95±0.18 | 0.18±0.08 |
| Experimental
groups |
|
|
|
|
|
| 1
month | 54 | 1.96±0.34 | 9.59±1.43 |
0.62±0.14a |
0.05±0.03a |
| 1
year | 54 | 2.14±0.32 | 10.39±2.04 | 0.94±0.33 | 0.16±0.01 |
| 3
years | 50 | 1.94±0.42 | 10.20±1.62 | 0.87±0.14 | 0.18±0.03 |
| 5
years | 42 | 1.93±0.35 | 9.92±1.33 | 1.04±0.47 | 0.21±0.03 |
| 10
years | 31 | 1.91±0.29 | 10.20±2.07 | 1.01±0.29 | 0.24±0.04 |
 | Table III.Changes in the T cell subsets, LT
rate and percentage of NK cells of patients following Ir192
exposure. |
Table III.
Changes in the T cell subsets, LT
rate and percentage of NK cells of patients following Ir192
exposure.
| Groups | No. of
patients | CD3+
(%) | CD4+
(%) | CD8+
(%) |
CD4+/CD8+ | LT rate (%) | NK cells (%) |
|---|
| Control | 20 | 74.3±7.6 | 49.9±4.2 | 29.5±2.6 | 1.69±0.3 | 84.5±5.1 | 13.7±0.1 |
| Experimental
groups |
|
|
1 month | 54 |
49.1±5.1a |
42.0±4.7a |
20.8±2.7a |
1.97±0.1a |
48.6±2.5a |
8.43±0.2a |
|
1 year | 54 |
52.3±4.8a |
43.1±4.1a |
22.1±3.8a |
2.01±0.2a |
60.5±4.1a |
8.85±1.6a |
|
3 years | 50 | 70.3±4.2 | 48.5±2.9 | 29.4±3.5 | 1.77±0.2 | 80.5±3.1 | 12.7±0.3 |
|
5 years | 42 | 71.1±4.0 | 49.4±2.3 | 28.3±2.5 | 1.80±0.3 | 86.9±3.8 | 12.1±0.2 |
| 10
years | 31 | 70.9±3.0 | 50.0±3.4 | 30.1±2.6 | 1.73±0.4 | 88.3±5.7 | 13.1±0.4 |
Chromosome aberrations
The chromosome aberration rates of the patients were
determined at 1 month, and at 1, 3, 5 and 10 years after exposure.
The results demonstrated that at 1 month after exposure, the rate
of dicentric and centric-ring aberrations was 4.76±0.37%, and the
total rate of chromosome aberrations was 9.62±0.52%. The rate of
dicentric and centric-ring aberrations was decreased to 0.96±0.15%
at 1 year after exposure (Table
IV). The karyotypes of 4 patients at 1 month after exposure are
shown in Fig. 3. The chromosome
karyotype of one of these patients exhibited three anomalies. These
anomalies were Y chromosome deletions (Fig. 3B), Another patient exhibited X
chromosome, and chromosome 4, 5, 7, 8, 10, 12, 13 deletions, and
chromosomes 11 and 14 had a derivative chromosome (Fig. 3D). The remaining two individuals
appeared to have normal karyotypes (Fig.
3A and C).
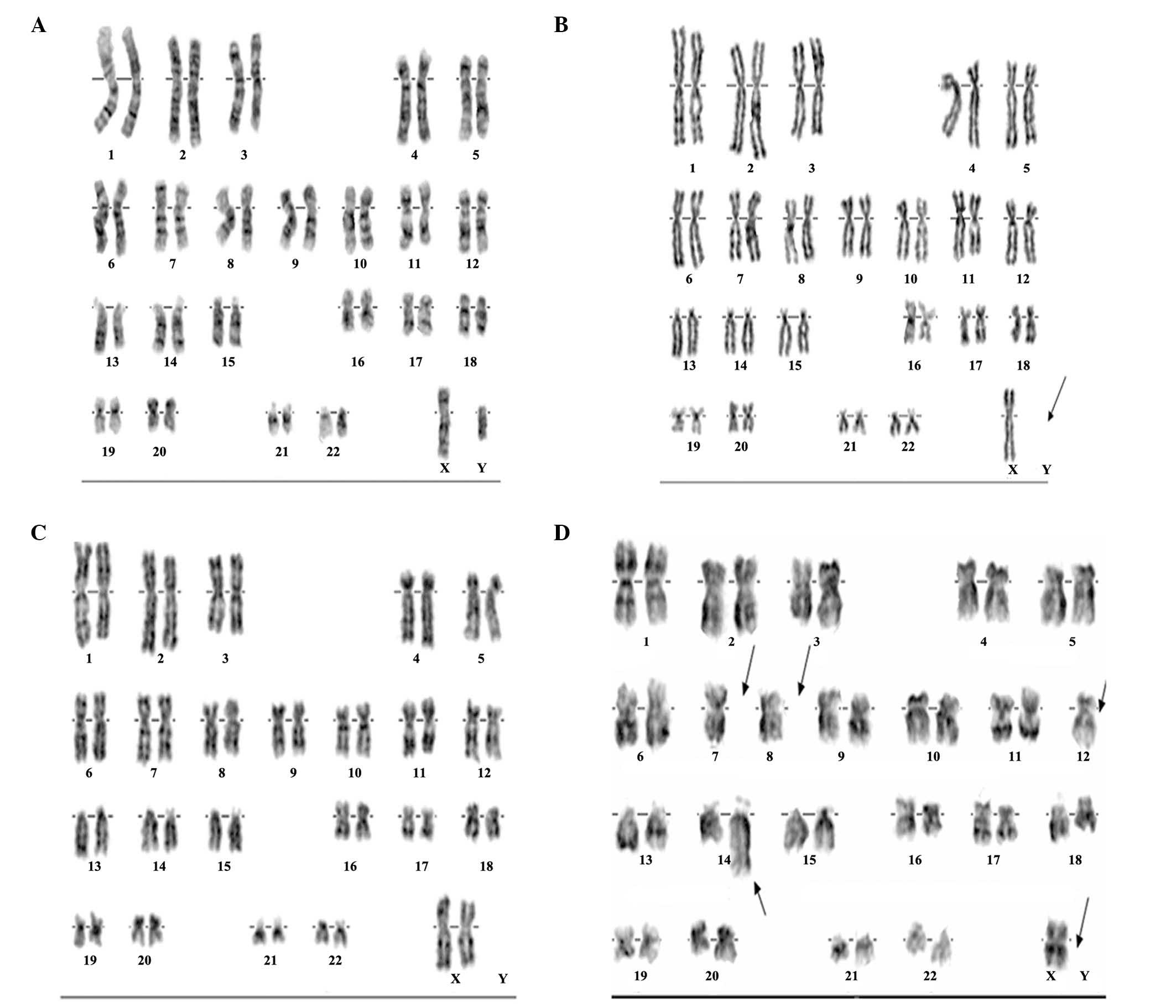 | Figure 3.Automatic karyotype analysis of
chromosome aberrations. Four representative images of chromosome
aberrations from 4 patients at 1 month after iridium-192 exposure.
(A) Male patient, 32 years old, 46, XY. (B) Male patient, 23 years
old, 45, X, -Y. (C) Female patient, 42 years old, 46, XX. (D)
Female patient, 25 years old, 45, X, -X. |
 | Table IV.Chromosomal aberrations of patients
following Ir192 exposure. |
Table IV.
Chromosomal aberrations of patients
following Ir192 exposure.
| Time point | Number | C+D (%) | AA (%) | SA (%) | Chromosome
aberration (%) |
|---|
| 1 month |
9,400 | 4.82±0.65 | 3.06±0.23 | 1.05±0.05 | 9.62±0.52 |
| 1 year | 10,400 | 0.96±0.15 | 0.81±0.13 | 0.19±0.01 | 1.83±0.39 |
| 3 years |
9,200 | 0.42±0.05 | 0.58±0.15 | 0.13±0.02 | 1.36±0.15 |
| 5 years |
8,400 | 0.20±0.05 | 0.47±0.32 | 0.09±0.01 | 0.82±0.24 |
| 10 years |
6,200 | 0.05±0.01 | 0.40±0.08 | 0.02±0.00 | 0.47±0.15 |
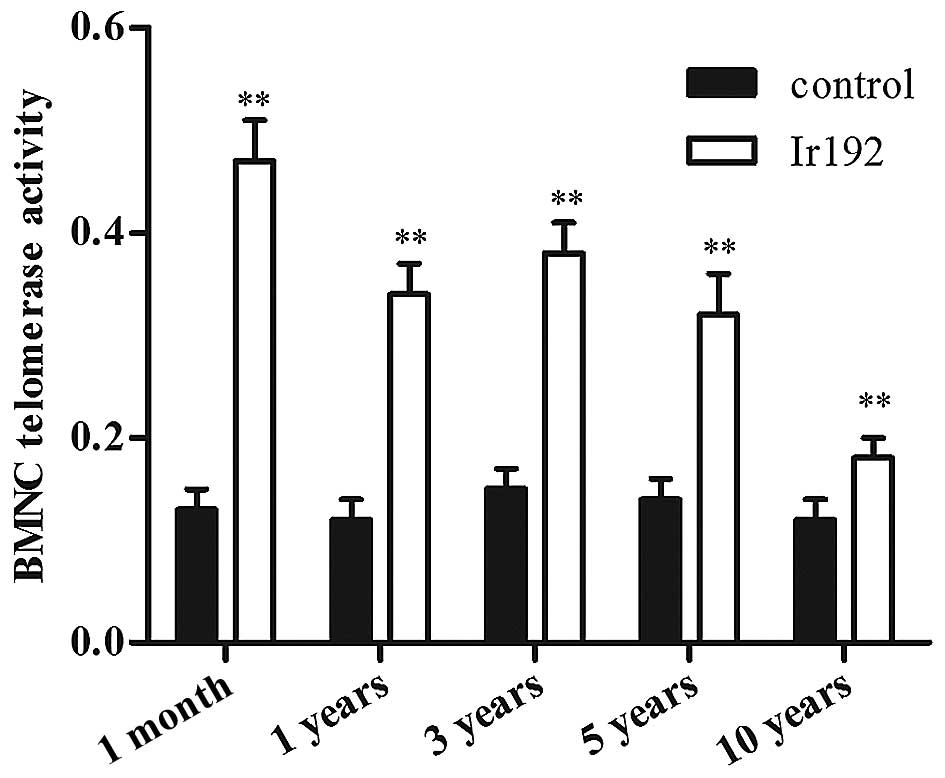
Changes in the telomerase activity of
BMNCs
The telomerase activity of BMNCs in the experimental
group (n=54) was 0.47±0.04 at 1 month after exposure, which was
significantly higher than that of the controls. At 5 and 10 years
after exposure, the telomerase activity of BMNCs in the
experimental group was 0.32±0.03 and 0.18±0.02, respectively, and
remained significantly higher than that of the controls (Fig. 4).
Discussion
Although the clinical symptoms of persistent
low-dose radiation exposure vary due to differences in doses and
the duration of exposure, the biological effects are mainly
excitatory effects and adaptation effects. The irradiation accident
in this study lasted 70 days; however, it was not possible to
accurately define the exposure time and dose. According to the
analysis of lymphocyte chromosome aberrations, the Chinese
Institute of Radiation Medicine diagnosed that the exposure dose
was 0.05–0.65 Gy. The majority of the patients' clinical symptoms
had disappeared by 1 year after exposure. The reason why there were
3 patients who continued to exhibit clinical symptoms might be that
these 3 patients were exposed to a large dose (>1.5 Gy). The
hematopoietic system is highly sensitive to ionizing radiation.
Large or medium-dose acute exposure can seriously damage the body's
hematopoietic function, leading to hematopoietic failure or
hematopoietic tumors (7). However,
the impact of low-dose ionizing radiation on the hematopoietic
system has not been fully evaluated. It has been observed that WBC
counts exhibit a significant reduction following gamma-radiation
(8,9). Researchers showed that in the Chernobyl
accident, changes occurred in red blood cells, Hb levels, platelet
quality and quantity in people exposed to <1 Gy radiation. They
also found that the incidence of thyroid cancer and acute leukemia
was significantly increased accompanied by an increase in P53 gene
mutation among those who were irradiated during their childhood
(10–12), indicating that age, dose, frequency,
time and different stages after exposure could significantly affect
the quality and quantity of blood cells and the incidence of
tumors. The results of the present study indicated that persistent
0.05–0.65 Gy Ir192 did not cause irreversible inhibition or damage
of the hematopoietic system in the affected population, and it may
be speculated that the human hematopoietic system is effectively
able to compensate for the damage caused by persistent 0.05–0.65 Gy
Ir192 exposure.
The body's immune system is also highly sensitive to
ionizing radiation. Low-dose long-term radiation has been shown to
enhance immune function (13),
whereas high-dose exposure may inhibit immune function (13,14). The
results of the present study indicated that humoral immunity can be
restored in a short time period, and lymphocytes are extremely
sensitive to radiation and their self-healing rate is very slow. It
has been reported that immune disorders may be sustained for more
than 30 years; the IgG, CD4+, CD8+ and
CD16+ levels of individuals involved in the clean-up of
the Chernobyl nuclear leak were significantly lowered and thyroid
disease was identified in 59 cases in 385 participants (10). However, immune stimulatory and
excitatory effects of low-dose irradiation were not observed in the
present study, which may be associated with the special nature of
the observed population.
The chromosomes of peripheral blood lymphocytes may
be damaged by small doses of radiation leading to chromosomal
aberrations. Thus, the estimation of a radiation dose by analyzing
chromosome aberrations in peripheral blood lymphocytes has been
widely recognized as a diagnostic method (15–17). In
the present study, the rate of dicentric and centric-ring
aberrations was 4.82±0.65% at 1 month after exposure, and was
decreased by 80% at 1 year after exposure. No aberrations were
found 10 years after exposure.
It has previously been shown that ionizing radiation
can cause DNA damage, and ultimately lead to nucleus swelling,
shrinkage, dissolution and then apoptosis (18,19).
However, DNA damage can activate telomerase activity, that is small
doses of radiation are able to induce the expression of telomerase
(20,21). Highly expressed telomerase may be
able to lengthen telomeres to repair radiation-damaged cellular
DNA, and has the potential to induce malignant hematopoietic cell
clones (22–24). The telomerase activity of BMNCs in
the experimental group was significantly higher than that of
controls even at 5 or 10 years after exposure, suggesting that
after receiving the same dose of radiation, the recovery of BMNC
telomerase activity takes more time than the recovery of
hematopoietic and immune systems. Although no new aberrations or
leukemia were found in the patients in the present study at 10
years after exposure, a longer follow-up time is required to
conclude whether sustained high expression of telomerase is a risk
factor of cancers or not.
In the present study, doctor billing records were
used to determine when follow-ups occurred, but no information
about the intents for these visits was available. Therefore, some
visits may have been routine appointments, and some patients may
have scheduled an appointment but not have agreed to follow-up.
The results of the present study indicate that
exposure to persistent low-dose Ir192 radiation resulted in
different degrees of immune dysfunction, abnormalities of blood
cells, abnormalities of bone marrow, chromosome aberrations, and
changes in the telomerase activity of BMNCs. Immune dysfunction,
abnormalities of blood cells and abnormalities of bone marrow were
recovered within 1–3 years in the majority of cases. Chromosome
aberrations took 5–10 years for recovery. However, it appears to
take >10 years for the telomerase activity of BMNCs to recover.
A longer follow-up time is required in order to monitor the
development of clonal proliferative diseases such as leukemia.
Acknowledgements
This study was supported by funds from the PLA
Medical Science and Technology Research 12th Five-Year Plan key
project funded projects (BWS11J071), the Natural Science Foundation
of Guangdong Province (05000138), and the Guangzhou Health
Collaborative Innovation Major Projects (201400000003-1 and
201400000003-4).
References
|
1
|
Milacic S and Simic J: Case report:
Iridium 192 - health effects during 20 years after irradiation.
Kobe J Med Sci. 54:E108–E113. 2008.PubMed/NCBI
|
|
2
|
Jalil A and Molla MA: Accidental
overexposure to 192Ir source in industrial radiography: A follow-up
study. Health Phys. 62:74–76. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moses JW, Moussa I, Leon MB, Teirstein PS,
Fish RD, Ellis SG, Nawas D, Kluck B, Giorgianni JA, Donohoe D and
Kuntz RE: Effect of catheter-based iridium-192 gamma brachytherapy
on the added risk of restenosis from diabetes mellitus after
intervention for in-stent restenosis (subanalysis of the GAMMA I
Randomized Trial). Am J Cardiol. 90:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inskip PD, Kleinerman RA, Stovall M,
Cookfair DL, Hadjimichael O, Moloney WC, Monson RR, Thompson WD,
Wactawski-Wende J and Wagoner JK: Leukemia, lymphoma and multiple
myeloma after pelvic radiotherapy for benign disease. Radiat Res.
135:108–124. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Butenko ZA, Smirnova IA, Zak KP,
Kishinskaja EG and Janok EA: Leukemia-associated gene
rearrangements in blood mononuclears of subjects in long terms
after radiation exposure. J Exp Clin Cancer Res. 19:57–59.
2000.PubMed/NCBI
|
|
6
|
Samarth RM: Protection against radiation
induced hematopoietic damage in bone marrow of Swiss albino mice by
Mentha piperita (Linn). J Radiat Res. 48:523–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Sun H, Xiao F, Wang X, Yang Y, Liu
Y, Zhang Q, Wu C, Wang H and Wang LS: Protection against
radiation-induced hematopoietic damage in bone marrow by hepatocyte
growth factor gene transfer. Int J Radiat Biol. 90:36–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maks CJ, Wan XS, Ware JH, Romero-Weaver
AL, Sanzari JK, Wilson JM, Rightnar S, Wroe AJ, Koss P, Gridley DS,
et al: Analysis of white blood cell counts in mice after gamma-or
proton-radiation exposure. Radiat Res. 176:170–176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanzari JK, Wan XS, Krigsfeld GS, Wroe AJ,
Gridley DS and Kennedy AR: The effects of gamma and proton
radiation exposure on hematopoietic cell counts in the ferret
model. Gravit Space Res. 1:79–94. 2013.PubMed/NCBI
|
|
10
|
Kurjane N, Bruvere R, Shitova O, Romanova
T, Jaunalksne I, Kirschfink M and Sochnevs A: Analysis of the
immune status in Latvian Chernobyl clean-up workers with
nononcological thyroid diseases. Scand J Immunol. 54:528–533. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ivanov VK, Tsyb AF, Gorsky AI, Maksyutov
MA, Rastopchin EM, Konogorov AP, Korelo AM, Biryukov AP and Matyash
VA: Leukaemia and thyroid cancer in emergency workers of the
Chernobyl accident: Estimation of radiation risks (1986-1995).
Radiat Environ Biophys. 36:9–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikiforov YE, Nikiforova MN, Gnepp DR and
Fagin JA: Prevalence of mutations of ras and p53 in benign and
malignant thyroid tumors from children exposed to radiation after
the Chernobyl nuclear accident. Oncogene. 13:687–693.
1996.PubMed/NCBI
|
|
13
|
Bogdándi EN, Balogh A, Felgyinszki N,
Szatmári T, Persa E, Hildebrandt G, Sáfrány G and Lumniczky K:
Effects of low-dose radiation on the immune system of mice after
total-body irradiation. Radiat Res. 174:480–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cui YF, Ding YQ, Xu H, Liu XL, Jin W, Mao
JP and Mao BZ: The effects of acute large dose of gamma-irradiation
on immune function of mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
20:675–677. 2004.(In Chinese). PubMed/NCBI
|
|
15
|
Agrawala PK, Adhikari JS and Chaudhury NK:
Lymphocyte chromosomal aberration assay in radiation biodosimetry.
J Pharm Bioallied Sci. 2:197–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Q, Cao J, Wang ZQ, Bai YS, Lü YM,
Huang QL, Zhao WZ, Li J, Jiang LP, Tang WS, et al: Dose estimation
by chromosome aberration analysis and micronucleus assays in
victims accidentally exposed to (60)Co radiation. Br J Radiol.
82:1027–1032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rozgaj R, Kasuba V and Simić D: The
frequency of dicentrics and acentrics and the incidence of rogue
cells in radiation workers. Mutagenesis. 17:135–139. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watters D: Molecular mechanisms of
ionizing radiation-induced apoptosis. Immunol Cell Biol.
77:263–271. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Kim SY, Kil IS and Park JW:
Regulation of ionizing radiation-induced apoptosis by mitochondrial
NADP+-dependent isocitrate dehydrogenase. J Biol Chem.
282:13385–13394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neuhof D, Ruess A, Wenz F and Weber KJ:
Induction of telomerase activity by irradiation in human
lymphoblasts. Radiat Res. 155:693–697. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Liu Y, Chow LS, Wong SC, Tsao GS,
Kwong DL, Sham JS and Nicholls JM: Regulation of telomerase
activity by gamma-radiation in nasopharyngeal carcinoma cells.
Anticancer Res. 20:433–437. 2000.PubMed/NCBI
|
|
22
|
Akiyama M, Ozaki K, Kawano T, Yamada O,
Kawauchi K, Ida H and Yamada H: Telomerase activation as a repair
response to radiation-induced DNA damage in Y79 retinoblastoma
cells. Cancer Lett. 340:82–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun J, Huang H and Zhu YY: Study on the
expression of tankyrase in malignant hematopoietic cells and its
relation with telomerase activity. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 12:11–15. 2004.(In Chinese). PubMed/NCBI
|
|
24
|
Wang L, Xiao H, Zhang X, Wang C and Huang
H: The role of telomeres and telomerase in hematologic malignancies
and hematopoietic stem cell transplantation. J Hematol Oncol.
7:612014. View Article : Google Scholar : PubMed/NCBI
|