Introduction
Nasopharyngeal carcinoma (NPC) is a type of cancer
with a high prevalence rate (2.8/100,000 and 1.9/100,000
people/year in men and women, respectively, in 2008) in Southeast
China, particularly in the Guangxi, Guangdong, Hainan and the Hong
Kong Special Administrative Region (1). NPC is characterized by a high
metastatic potential, frequent initial dissemination to regional
lymph nodes and distant metastases, causing patients to succumb to
NPC (2). Early diagnosis of NPC and
chemoradiotherapy treatment enables the best outcome. The overall
five-year survival rate is associated with the NPC stage at
diagnosis, ranging from between 58% at stage IV and 90% in stage I.
However, in the advanced stages of NPC chemoradiotherapy is
impractical (3,4). Therefore, the induction of NPC cell
apoptosis is a strategy to control NPC and other malignancies in
clinical therapy (5).
B-cell lymphoma 2 (Bcl-2) is part of the Bcl-2
protein family, which regulates cell death by inducing or
inhibiting apoptosis. The Bcl-2 family is divided into
anti-apoptotic factors, including Bcl-2, Bcl-extra large (Bcl-xL)
and Bcl-2-like protein 2, and pro-apoptotic factors, such as
Bcl2-associated X protein (Bax), Bcl-2-associated death promoter,
Bcl-2-interacting mediator of cell death (Bim), Bcl-2 homologous
antagonist/killer and p53 upregulated modulator of apoptosis
(6). The extrinsic apoptosis
signaling pathway is mediated by receptor-ligand binding. In this
signaling pathway, the Fas receptor, Fas ligand (FasL),
Fas-associated death domain (FADD) and caspase-8 mediate apoptosis.
Alternatively, apoptotic stimuli can cause the depolarization of
the inner mitochondrial membrane, leading to the release of
cytochrome c (Cyt c) into the cytosol (7). Cyt c molecules induce the
activation of apoptotic protease activation factor-1 and
procaspase-9. Activated caspase-8 and −9 cleave and activate the
final executioner of apoptosis, caspase-3, resulting in chromatin
condensation and DNA fragmentation (8–10).
The seed of Nelumbo nucifera (Gaertn), also
known as the lotus, is traditionally used in Chinese folk medicine.
A number of previous studies have reported that the lotus seed
exhibits numerous health benefits and pharmacological effects, such
as anti-ischemic (11), antioxidant
(12–14), hepatoprotective (12), antiproliferative (15–19),
anti-inflammatory (20),
anti-infertility (21),
anti-arrhythmic (22–26), antifibrotic (27) and antiviral (28) activities. In the present study, the
anticancer activity of alkaloids extracted from the Ba lotus
seed (BLSA), a new variety of Nelumbo nucifera, which only
grows in Chongqing, a city located in the southwest of China, was
investigated in human NPC CNE-1 cells. In addition, the mechanism
underlying this activity was examined.
Materials and methods
Chemical reagents
TRIzol, OligodT18 primer, murine Maloney
leukemia virus (MMLV) reverse transcriptase, RNase inhibitor,
ethidium bromide (EtBr) and agarose were purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). All other
chemical reagents were of analytical grade and purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Preparation of alkaloids from
BLSA
Fresh BLSA was purchased from Chongqing Enterprise
Engineering Research Center of Ba-lotus Breeding and Deep
Processing (Chongqing, China), freeze-dried and ground into a fine
powder. Alkaloids were extracted from powdered Ba louts seed
(100 g) twice with 1,000 ml of ethanol (80% vol/vol) at 50°C for 1
h. Following filtering, the extraction solution was loaded into a
80 cm cation exchange resin 732 column at 50°C and the filtrate
collected 3 h later. Distilled water was used to wash away
water-soluble impurities and then an ethanol solution of BLSA
extract (80%, v/v) at 3 ml/min was used to elute the alkaloids. The
collected ethanol elucent was eluted by 80% ethanol solution
(containing 2% of ammonia water) and finely condensed using a
vacuum rotary evaporator at 37°C, then freeze-dried and stored at
−80°C until required.
Cell culture
Human NPC CNE-1 cells were obtained from the
Shanghai Institutes for Biological Sciences (Chinese Academy of
Sciences, Shanghai, China). The cells were routinely maintained in
Roswell Park Memorial Institute-1640 medium, supplemented with 10%
(v/v) fetal bovine serum and 1% penicillin-streptomycin, at 37°C in
a humidified 5% CO2 incubator at 95% relative humidity
(model 3154; Forma Scientific, Inc., Marietta, OH, USA).
Cell viability assay
Cell viability was measured using the MTT assay.
CNE-1 cells were seeded in 96-well plates (Nunc, Rochester, NY,
USA) at a density of 1×104 cells/well. Following
incubation for 24 h, cells were treated with a number of
concentrations (50, 100 and 200 µg/ml) of BLSA for a further 24 h.
Then, 0.5 mg/ml of MTT reagent (100 µl; Ekear, Shanghai, China;
cat. no. M0105) was added to each well and the cells incubated for
4 h at 37°C. The formazan crystals formed was dissolved in dimethyl
sulfoxide (100 µl/well). Then, the absorbance of the wells at 540
nm was measured using a micro plate reader (model 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Flow cytometry analysis
BLSA-treated CNE-1 cells were collected following
digestion with trypsin, washed twice with cold phosphate buffered
saline (PBS) and re-suspended in 2 ml PBS. Then, the DNA of
BLSA-treated cells was stained with propidium iodide using a
Cycletest Plus DNA Reagent Kit (BD Biosciences; Franklin Lakes, NJ,
USA; cat. no. 340242), according to the manufacturer's protocol.
Fluorescence intensity was determined using a FACSCalibur flow
cytometer and the data analyzed using Cell Quest Pro software
(version 5.2.1) (both BD Biosciences).
Reverse transcription polymerase chain
reaction (RT-PCR)
RT-PCR was performed for the following genes:
Caspase-3, −8 and −9, Bax, Bcl-2, Bcl-xL, Fas, FasL, NF-κB, IkB-α
and GADPH. Total RNA was isolated from BLSA-treated CNE-1 cells
using TRIzol reagent, according to the manufacturer's
recommendations, and centrifuged at 12,000 × g for 15 min at
25°C following the addition of chloroform. Isopropanol was added to
the supernatant in a 1:1 ratio and the RNA pelleted by
centrifugation (12,000 × g for 15 min at 4°C). The RNA was
washed with ethanol, solubilized in diethyl pyrocarbonate-treated
RNase-free water and quantified by measuring the absorbance at 260
nm using a UV-1750 spectrophotometer (Shimadzu Corporation, Kyoto,
Japan). RNA (1 µg) was reverse transcribed using a PCR master mix
[1X reverse transcriptase buffer, dNTPs (1 mM),
oligo(dT)18 primers (500 ng), MMLV reverse transcriptase
(140 units) and RNase inhibitor (40 units)] for 45 min at 42°C.
Then, cDNA (2 µl) was mixed with 1 µl of each primer (10 µM) and 16
µl of DNase-free water in a PCR premix tube (AccuPower PCR PreMix;
Bioneer Corporation, Daejeon, Korea) and PCR was performed in an
automatic thermocycler (Bioneer Corporation, Daejeon, South Korea)
for 40 cycles of 94°C for 5 min, 58°C for 30 sec, and 72°C for 90
sec, followed by a 10 min cycle at 95°C. Sequences of the primers
used in PCR are presented at Table
I. The PCR products were separated on 2% agarose gels and
visualized by EtBr staining. GAPDH was used for normalization of
the results. Gene expression was quantified using ImageJ software
(version 1.44; National Institutes of Health, Bethesda, MD, USA)
and results presented as fold change compared to the control
group.
 | Table I.RT-PCR primer sequences. |
Table I.
RT-PCR primer sequences.
| Gene name | Primer
sequences |
|---|
| Caspase-3 | Forward:
5′-CAAACTTTTTCAGAGGGGATCG-3′ |
|
| Reverse:
5′-GCATACTGTTTCAGCATGGCA-3′ |
| Caspase-8 | Forward:
5′-CCCCACCCTCACTTTGCT-3′ |
|
| Reverse:
5′-GGAGGACCAGGCTCACTTA-3′ |
| Caspase-9 | Forward:
5′-GGCCCTTCCTCGCTTCATCTC-3′ |
|
| Reverse:
5′-GGTCCTTGGGCCTTCCTGGTAT-3′ |
| Bax | Forward:
5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ |
|
| Reverse:
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-2 | Forward:
5′-CTCGTCGCTACCGTCGTGACTTGG-3′ |
|
| Reverse:
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-xL | Forward:
5′-CCCAGAAAGGATACAGCTGG-3′ |
|
| Reverse:
5′-GCGATCCGACTCACCAATAC-3′ |
| Fas | Forward:
5′-GAAATGAAATCCAAAGCT-3′ |
|
| Reverse:
5′-TAATTTAGAGGCAAAGTGGC-3′ |
| FasL | Forward:
5′-GGATTGGGCCTGGGGATGTTTCA-3′ |
|
| Reverse:
5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′ |
| NF-κB | Forward:
5′-CACTTATGGACAACTATGAGGTCTCTGG-3′ |
|
| Reverse:
5′-CTGTCTTGTGGACAACGCAGTGGAATTTTAGG-3′ |
| IκB-α | Forward:
5′-GCTGAAGAAGGAGCGGCTACT-3′ |
|
| Reverse:
5′-TCGTACTCCTCGTCTTTCATGGA-3′ |
| GAPDH | Forward:
5′-CGGAGTCAACGGATTTGGTC-3′ |
|
| Reverse:
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Protein extraction and western blot
analysis
For protein extraction, BLSA-treated CNE-1 cells
were washed with ice-cold PBS, homogenized with ice-cold
radioimmunoprecipitation assay (RIPA) buffer and centrifuged at
13,000 × g for 30 min at 4°C. Protein concentrations were
determined using the Bradford Protein Assay kit (Bio-Rad
Laboratories, Inc.; cat. no. 5000001). For Western blot analysis,
30 µg of protein extract was separated by SDS-PAGE (10% gel) and
then electrotransferred onto a nitrocellulose membrane (Schleicher
& Schuell Bioscience, Inc., Keene, NH, USA). Blocking and
antibody treatment were conducted in 10% skimmed milk for 2 h at
4°C. The blots were incubated for 4 h at 4°C with primary
antibodies against caspase-3 (rabbit monoclonal; 1:1,000; cat. no.
14220S), caspase-8 (rabbit monoclonal; 1:1,000; cat. no. 9478S) and
caspase-9 (rabbit monoclonal; 1:1,000; cat. no. 9508S), and Bax
(rabbit monoclonal; 1:1,000; cat. no. 14796S), Bcl-2 (rabbit
monoclonal; 1:1,000; cat. no. 4223S), Bcl-xL (rabbit monoclonal;
1:1,000; cat. no. 2764S), Fas (mouse monoclonal; 1:1,000; cat. no.
8023S), FasL (rabbit polyclonal; 1:1,000; cat. no. 4273S), NF-κB
(mouse monoclonal; 1:1,000; cat. no. 13681S), IκB-α (rabbit
monoclonal; 1:1,000; cat. no. 4812S) and β-actin (mouse monoclonal;
1:1,000; cat. no. 12262S) (all Cell Signaling Technology, Inc.,
Danvers, MA, USA). Following washing with PBS containing 0.05%
Tween 20 (PBS-T), the blots were incubated with horseradish
peroxidase-conjugated goat anti-rabbit (cat. no. 7074S) or horse
anti-mouse antibodies (cat. no. 7076S) at a dilution of 1:5,000
(both Cell Signaling Technology, Inc.) for 1 h at room temperature.
Then, blots were washed three times with PBS-T and antibody binding
visualized by enhanced chemiluminescence (ECL Western Blotting
Detection kit; GE Healthcare Life Sciences, Little Chalfont, UK;
cat. no. RPN2108). Protein expression was quantified using ImageJ
software (version 1.44; National Institutes of Health).
Statistical analysis
Results are presented as the mean ± standard
deviation. Differences between the mean values of groups were
assessed by one-way analysis of the variance, followed by a
post-hoc Duncan's new multiple range test. P<0.05 was considered
to indicate a statistically significant difference. SAS software
(version 9.1; SAS Institute, Inc., Cary, NC, USA) was used for
statistical analysis.
Results
BLSA decreases CNE-1 cell
proliferation
BLSA was found to significantly reduce CNE-1 cell
proliferation in vitro, in a dose-dependent manner, at all
concentrations tested compared with the control group (P<0.05;
Table II). The highest dose of BLSA
(200 µg/ml) showed the greatest inhibitory activity (81.3±0.2%;
Table II).
 | Table II.Growth inhibition of human NPC CNE-1
cells by alkaloids of BLSA evaluated by the MTT assay. |
Table II.
Growth inhibition of human NPC CNE-1
cells by alkaloids of BLSA evaluated by the MTT assay.
| Treatment
(µg/ml) |
OD540 | Inhibitory rate
(%) |
|---|
|
0 |
0.471±0.005a | – |
| 50 |
0.376±0.010a |
20.2±0.2a |
| 100 |
0.249±0.014b |
47.1±0.3b |
| 200 |
0.088±0.012b |
81.3±0.2b |
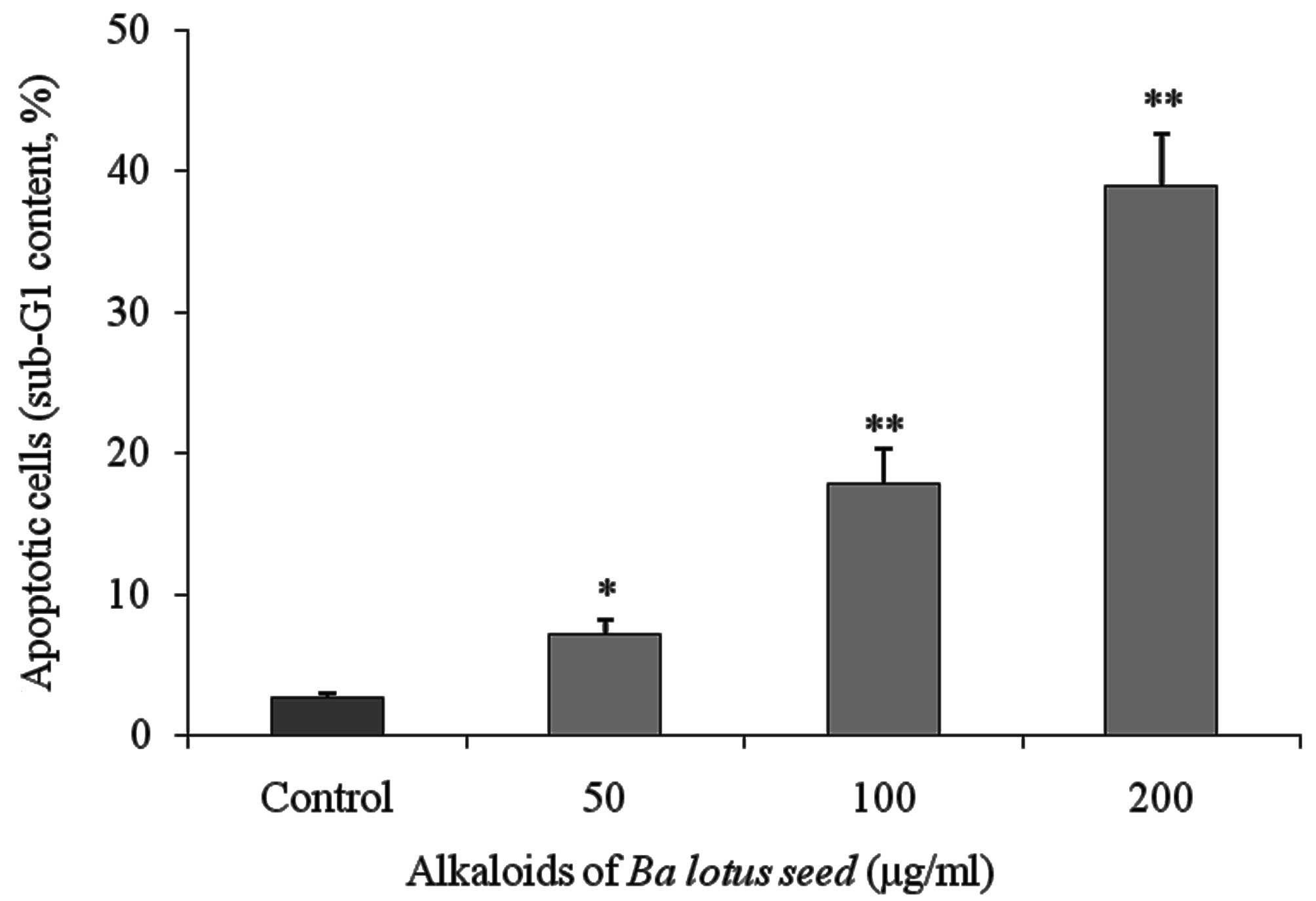
BLSA induces apoptosis in CNE-1
cells
Flow cytometry analysis identified that BLSA
increased apoptosis in CNE-1 cells in a dose-dependent manner. BLSA
treatment (50, 100 and 200 µg/ml) significantly increased the
sub-G1 DNA content of CNE-1 cells to 7.1 (P<0.05), 17.8
(P<0.01) and 38.9% (P<0.01), respectively, compared with 2.6%
in the untreated control group (Fig.
1).
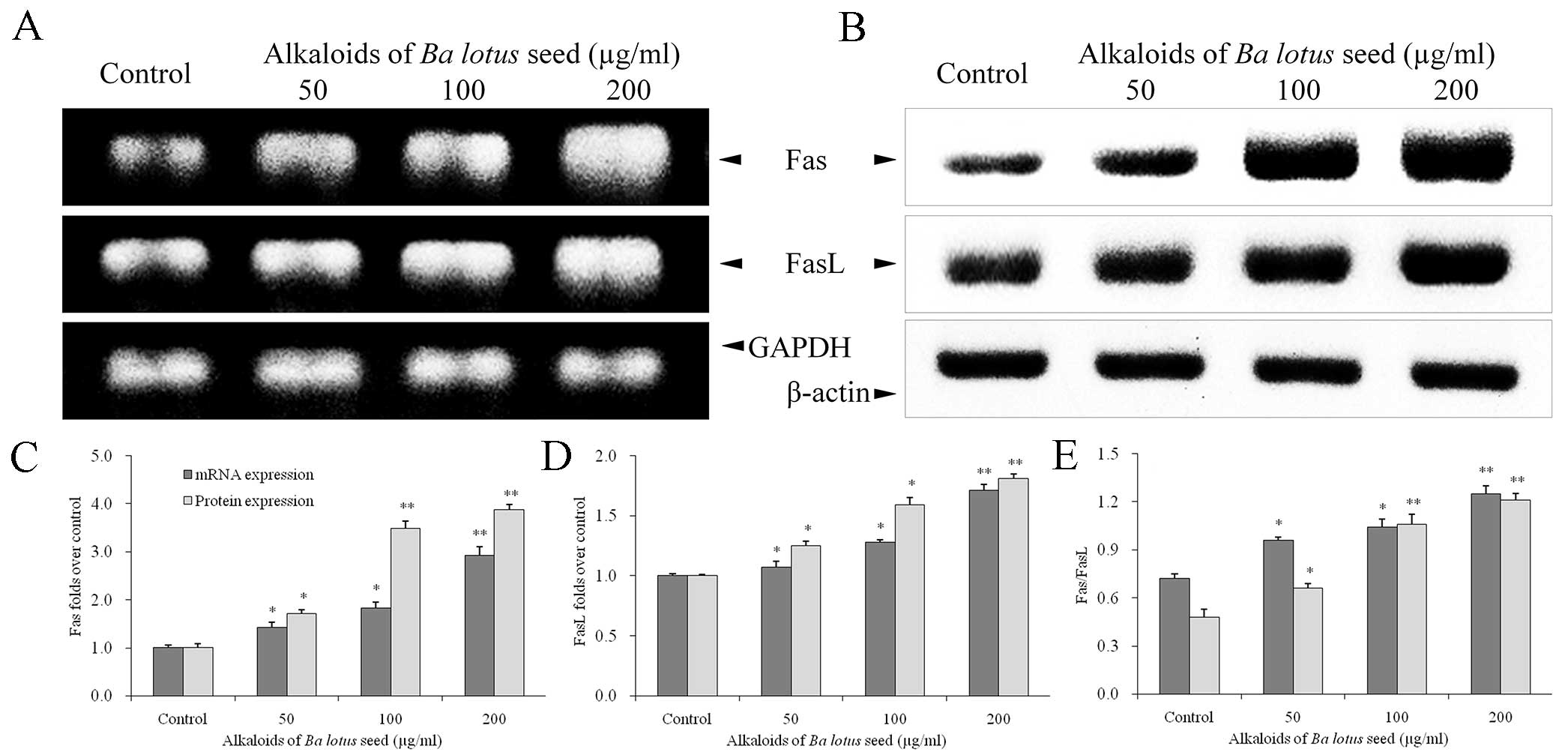
BLSA increases Fas and FasL protein
expression in CNE-1 cells
The effect of BLSA on mRNA and protein levels of
specific genes was determined by RT-PCR and western blot analysis,
respectively. BLSA treatment was identified to significantly
increase mRNA and protein levels of Fas and FasL in CNE-1 cells, in
a dose-dependent manner, at all concentrations tested compared with
the control (P<0.05; Fig. 2). The
highest dose of BLSA (200 µg/ml) significantly up-regulated Fas
(2.9 fold) and FasL (1.7 fold) mRNA levels, and Fas (1.5 fold) and
FasL (3.3 fold) protein levels compared with the control group (all
P<0.01; Fig. 2C and D).
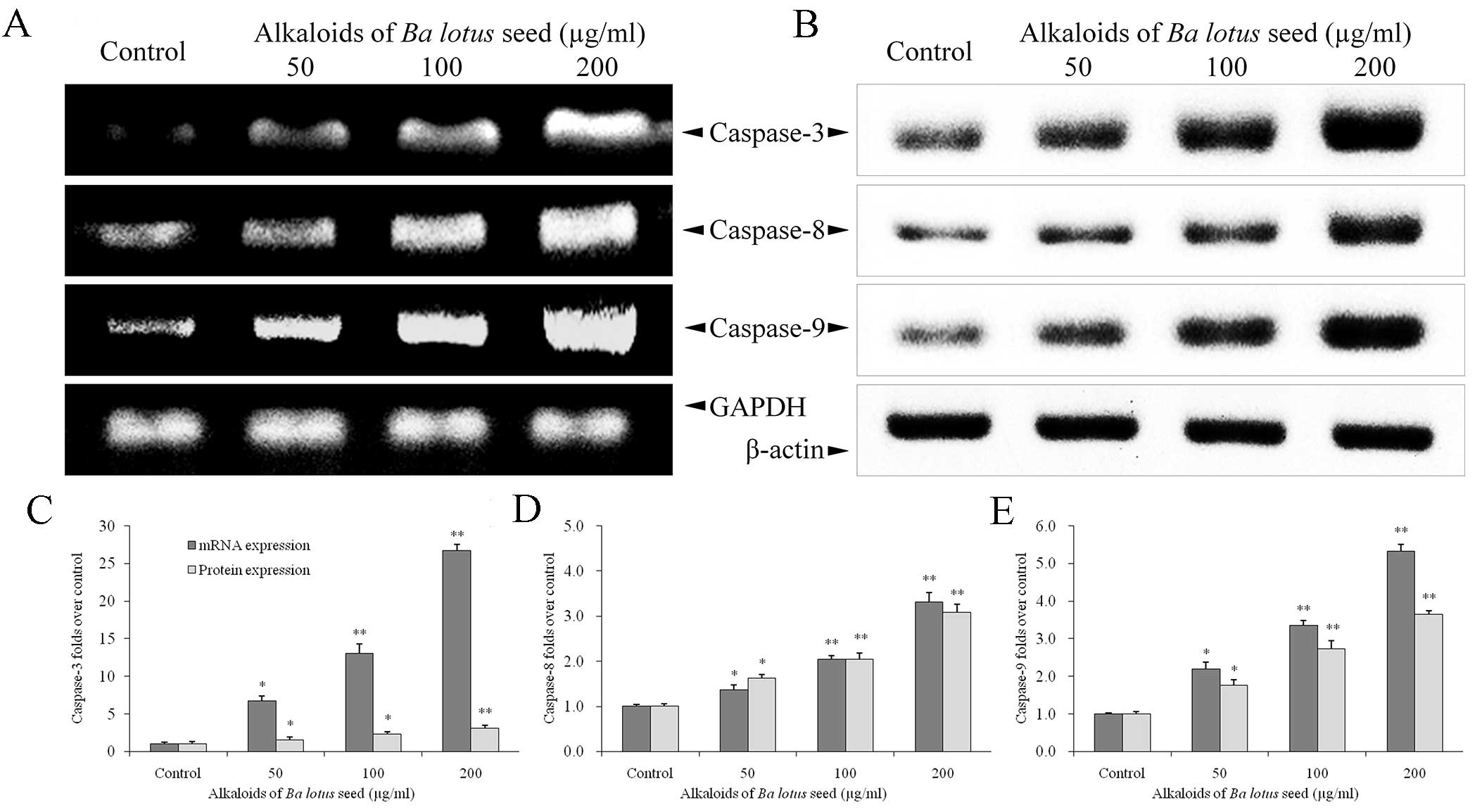
BLSA increases caspase-3, −8 and −9
expression in CNE-1 cells
BLSA treatment significantly increased mRNA and
protein expression levels of caspase-3, −8 and −9 in CNE-1 cells,
at all concentrations tested compared with the control (P<0.05;
Fig. 3). The highest dose of BLSA
(200 µg/ml) significantly increased mRNA and protein levels of
caspase-3 (26.7 and 1.4 fold, respectively; Fig. 3C), −8 (3.3 and 1.5 fold,
respectively; Fig. 3D) and −9 (5.3
and 1.6 fold, respectively; Fig. 3E)
compared with the untreated control group (all P<0.01).
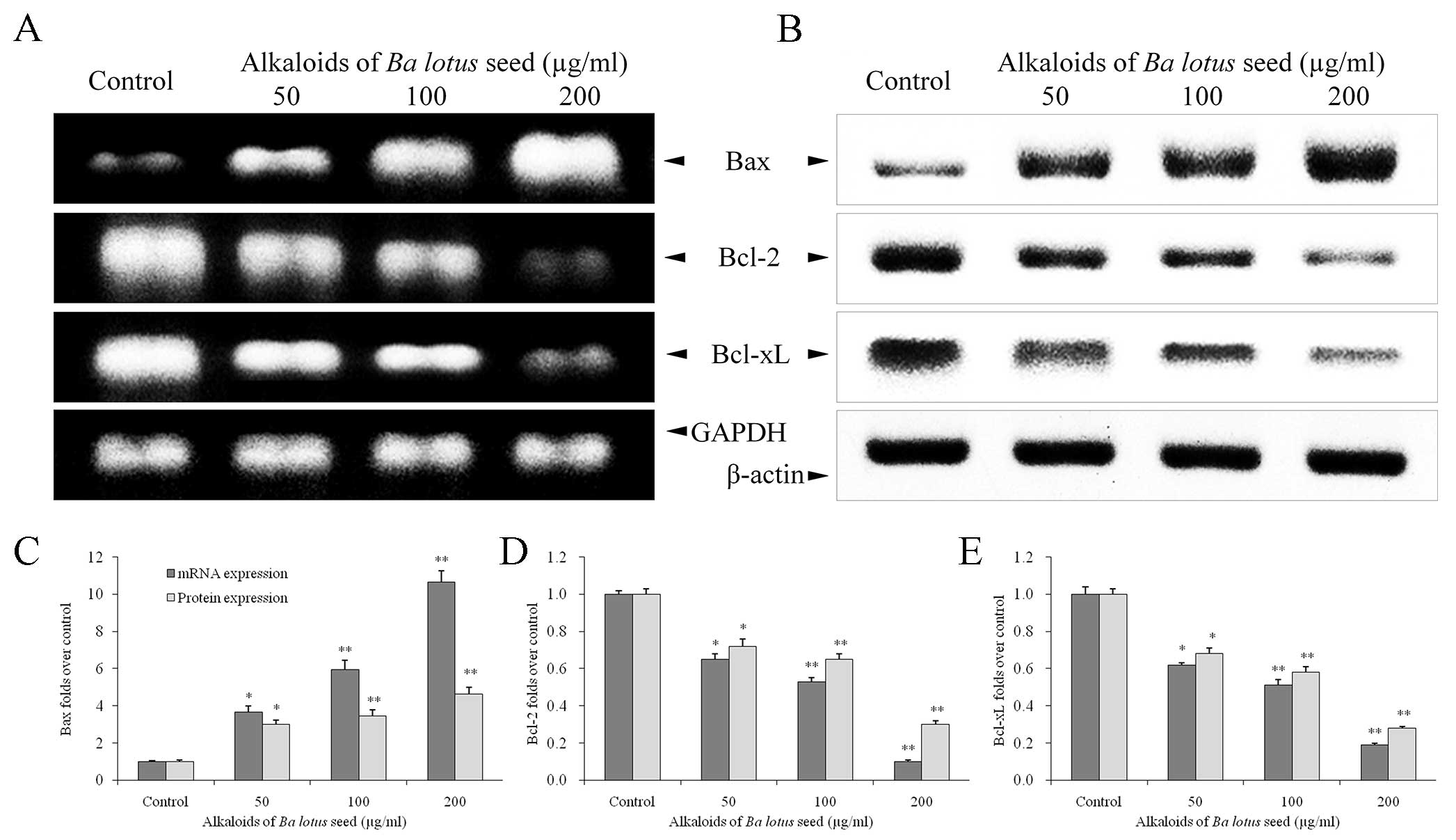
BLSA modulates Bcl-2, Bcl-xL and Bax
expression in CNE-1 cells
Compared with the control group, BLSA treatment
significantly decreased expression of Bcl-2 and Bcl-xL mRNA and
protein, and increased expression of Bax mRNA and protein, in a
dose dependent manner, in CNE-1 cells, at all concentrations tested
(P<0.05; Fig. 4). At the highest
dose (200 µg/ml), BLSA significantly reduced mRNA and protein
levels of Bcl-2 (90 and 94%, respectively) and Bcl-xL (81 and 75%,
respectively) compared with the control group (P<0.01; Fig. 4D and E). In contrast, 200 µg/ml BLSA
enhanced mRNA (10.6 fold; P<0.01) and protein (1.6 fold;
P<0.05) levels of Bax in CNE-1 cells (Fig. 4C).
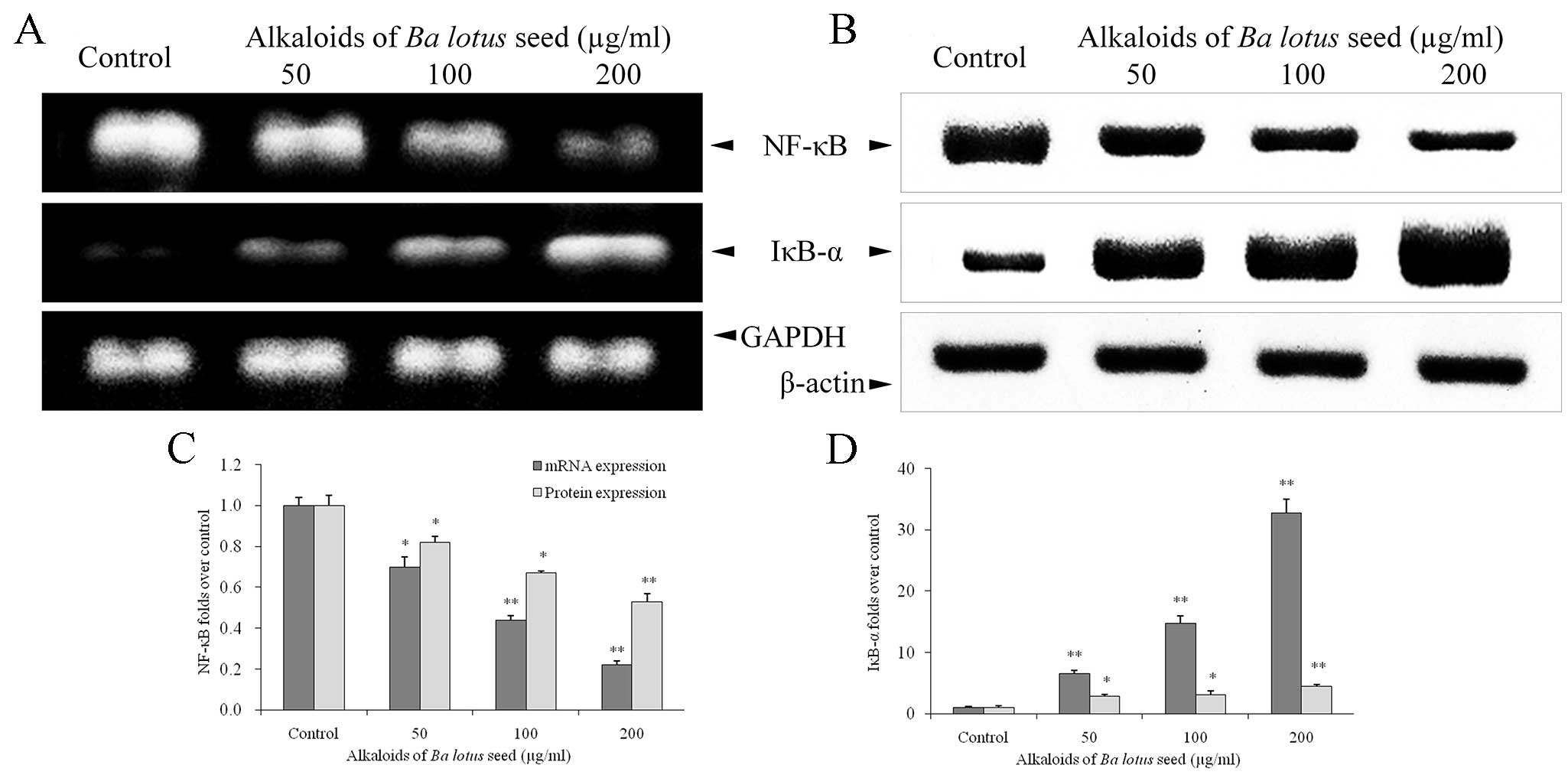
BLSA modulates NF-κB and IκB-α
expression in CNE-1 cells
BLSA treatment significantly decreased NF-κB mRNA
and protein expression, and increased IκB-α mRNA and protein
expression, in a dose dependent manner in CNE-1 cells, at all
concentrations tested (P<0.05 vs. the control group; Fig. 5). Following treatment with 200 µg/ml
BLSA, NF-κB mRNA and protein levels were significantly decreased by
78% and 35%, respectively, compared with the control group
(P<0.01; Fig. 5C). In addition,
BLSA increased mRNA and protein levels of IκB-α by 32.7 and
2.3-fold, respectively, compared with the control group (P<0.01;
Fig. 5D).
Discussion
Alkaloids, isolated from herbs, may possess
anti-cancer activities, including induction of cell cycle arrest,
apoptosis, autophagy, and inhibition of angiogenesis and metastasis
(29). A recent study reported that
a number of alkaloids isolated from N. nucifera Gaertn. cv.
Rosa-plena exhibited antioxidant and anticancer activity
in vitro (30). In the
present study, BLSA exhibited anti-cancer effects, associated with
the induction of apoptosis, in CNE-1 cells. BLSA significantly
reduced CNE-1 cell proliferation and promoted transition into the
sub-G1 phase. These results indicate that the anti-CNE-1 effects of
BLSA are associated with apoptosis.
In the current study, mRNA and proteins expression
levels of a number of apoptosis-associated genes in BLSA-treated
CNE-1 cells were investigated using RT-PCR and western blotting,
respectively. Following treatment for 24 h with BLSA, mRNA and
protein levels of Fas and FasL were significantly increased
compared with untreated cells. Fas and FasL are inducers of
apoptosis that serve a primary role in death receptor-mediated
apoptosis (31). Activation of
Fas/FasL recruits FADD and the death domain, which subsequently
induce the activation of caspase-8, −9 and −10, key regulators that
promote cellar apoptosis (32).
The results of the present study determined that
mRNA and protein levels of caspase-3, −8 and −9 were significantly
increased in BLSA-treated CNE-1 cells compared with the control
group. The caspase signaling cascade is a key event in extrinsic
and intrinsic apoptosis, which is characterized by the activation
of caspase-8 and −9, respectively (9). Caspase-8, the initiator caspase in Fas
signaling, is recruited to the activated Fas receptor and
facilitates death receptor-mediated apoptosis (33). Caspase-9, an apoptotic effector
molecule in intrinsic apoptosis, initiates programmed cell death
following activation (10).
Activated caspase-8 and −9 activate caspase-3, an executioner
caspase that subsequently induces apoptosis (8). These results indicate that BLSA induces
CNE-1 cell apoptosis through activating extrinsic (Fas/FasL) and
intrinsic apoptotic signaling pathways.
The Bcl-2 family, a well-known family of apoptosis
regulators, serves a primary role in intrinsic apoptosis (34). Bcl-2 and Bcl-xL are typically
anti-apoptotic factors that block the release of Cyt c from
mitochondria and thus promote cell survival. Bcl-2 can reduce the
release of Cyt c from the mitochondria, thus inhibiting
apoptosis (6). In contrast, Bax is a
pro-apoptotic factor that promotes apoptosis (6,7). The
balance between anti- and pro-apoptotic factors influences the
occurrence of apoptosis, and is associated with the success rate of
chemotherapy in cancer patients (35). In the present study, BLSA treatment
significantly increased mRNA and protein levels of pro-apoptotic
Bax, and reduced mRNA and protein levels of anti-apoptotic Bcl-2
and Bcl-xL in CNE-1 cells. Activated Bax is directly engaged by Bim
to promote apoptosis (36). In
addition, caspases-8 may activate Bax and induce the release of Cyt
c from the mitochondria, causing the cleavage of caspase-9
and contributing to the activation of caspase-3 (6,37). The
results of the current study suggest that BLSA modulates the ratio
of anti-apoptotic to pro-apoptotic factors, in particular by
enhancing the expression Bax to promote the apoptosis of CNE-1
cells.
NF-κB reduces tumor necrosis factor (TNF)-α-induced
cell apoptosis (38) and is an
important negative regulator of apoptosis in cancer cells (39). Deregulation of NF-κB expression has
been found in a number of human cancers (40,41).
Overexpression of NF-κB promotes cell proliferation and reduces
cell death (42). In addition, NF-κB
can directly activate Bcl-xL (43)
and suppress a number of anti-apoptotic factors, such as inhibitor
of apoptosis, caspase-8-like FADD-like interleukin-1β-converting
enzyme inhibitory protein, TNF receptor associated factor 1 (TRAF1)
and TRAF2, to regulate apoptosis (44). Following treatment with BLSA, mRNA
and protein levels of NF-κB were significantly reduced in CNE-1
cells. In addition, BLSA treatment significantly increased mRNA and
protein levels of IκB-α. Increasing IκB-α levels is a therapeutic
strategy to reduce cancer cell growth in clinical chemotherapy
(45–47).
In conclusion, the results of the present study
indicate that BLSA suppresses the proliferation of human CNE-1 NPC
cells in vitro. In addition, the results indicate that BLSA
induces apoptosis, through reducing the ratio of anti-apoptotic
(Bcl-2 and Bcl-xL) to pro-apoptotic (Bax) factors, increasing mRNA
and protein expression levels of Fas/FasL and promoting cleavage of
caspase-3, −8 and −9 in CNE-1 cells. BLSA, as an inducer of
apoptosis, may have future applications as an adjuvant in clinical
therapy for NPC patients.
Acknowledegments
The present study was supported by Chongqing
Engineering Research Center for Functional Food (grant no.
cstc2015yfpt_gcjsyjzx0027) and the Program for Innovation and Team
Building at the Chongqing Institute of Higher Education (grant no.
CXTDX201601040).
References
|
1
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su J, Xu XH, Huang Q, Lu MQ, Li DJ, Xue F,
Yi F, Ren JH and Wu YP: Identification of cancer stem-like
CD44+ cells in human nasopharyngeal carcinoma cell line.
Arch Med Res. 42:15–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peter ME and Krammer PH: The CD95
(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cullen SP and Martin SJ: Caspase
activation pathways: Some recent progress. Cell Death Differ.
16:935–938. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Lei M, Wang Z, Qiao G, Yang T and
Zhang J: TCR-induced, PKC-θ-mediated NF-κB activation is regulated
by a caspase-8-caspase-9-caspase-3 cascade. Biochem Biophys Res
Commun. 450:526–531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Kang M, Cho C, Chung HS, Kang CW,
Parvez S and Bae H: Effects of Nelumbinis semen on contractile
dysfunction in ischemic and reperfused rat heart. Arch Pharm Res.
29:777–785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sohn DH, Kim YC, Oh SH, Park EJ, Li X and
Lee BH: Hepato-protective and free radical scavenging effects of
Nelumbo nucifera. Phytomedicine. 10:165–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rai S, Wahile A, Mukherjee K, Saha BP and
Mukherjee PK: Antioxidant activity of Nelumbo nucifera (sacred
lotus) seeds. J Ethnopharmacol. 104:322–327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling ZQ, Xie BJ and Yang EL: Isolation,
characterization, and determination of anti-oxidative activity of
oligomericprocyanidins from the seedpod of Nelumbo nucifera Gaertn.
J Agric Food Chem. 53:2441–2445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu CP, Tsai WJ, Lin YL, Liao JF, Chen CF
and Kuo YC: The extracts from Nelumbo nucifera suppress cell cycle
progression, cytokine genes expression, and cell proliferation in
human peripheral blood mononuclear cells. Life Sci. 75:699–716.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu CP, Tsai WJ, Shen CC, Lin YL, Liao JF,
Chen CF and Kuo YC: Inhibition of (S)-armepavine from Nelumbo
nucifera on autoimmune disease of MRL/MpJ-lpr/lpr mice. Eur J
Pharmacol. 531:270–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu CP, Kuo YC, Shen CC, Wu MH, Liao JF,
Lin YL, Chen CF and Tsai WJ: (S)-armepavine inhibits human
peripheral blood mononuclear cell activation by regulating Itk and
PLCgamma activation in a PI-3K-dependent manner. J Leukoc Biol.
81:1276–1286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiao JH, Zhang YL, Feng XL, Wang JL and
Qian JQ: Effects of isoliensinine on angiotensin II-induced
proliferation of porcine coronary arterial smooth muscle cells. J
Asian Nat Pro Res. 8:209–216. 2006. View Article : Google Scholar
|
|
19
|
Yu J and Hu WS: Effects of neferine on
platelet aggregation in rabbits. Acta Pharm Sin. 32:1–4. 1997.(In
Chinese).
|
|
20
|
Lin JY, Wu AR, Liu CJ and Ying S:
Suppressive effects of lotus plumule (Nelumbo nucifera Geartn.)
supplementation on LPS-induced systemic inflammation in a BALB/c
mouse model. J Food Drug Anal. 14:273–278. 2006.
|
|
21
|
Mazumder UK, Gupta M, Pramanik G,
Mukhopadhyay RK and Sarkar S: Antifertility activity of seed of
Nelumbo nucifera in mice. Ind J Exp Biol. 30:533–534. 1992.
|
|
22
|
Li G, Li X and Lü F: Effects of neferine
on transmembrane potentials of guinea pig myocardium. Zhongguo Yao
Li Xue Bao. 10:406–410. 1989.(In Chinese). PubMed/NCBI
|
|
23
|
Li GR, Li XG and Lu FH: Effects of
neferine on transmembrane potential in rabbit sinoatrial nodes and
clusters of cultured myocardial cells from neonatal rats. Zhongguo
Yao Li Xue Bao. 10:328–331. 1989.(In Chinese). PubMed/NCBI
|
|
24
|
Li GR, Qian JQ and Lü FH: Effects of
neferine on heart electromechanical activity in anaesthetized cats.
Zhongguo Yao Li Xue Bao. 11:158–161. 1990.(In Chinese). PubMed/NCBI
|
|
25
|
Wang JL, Nong Y and Jiang MX: Effects of
liensinine on haemodynamics in rats and the physiologic properties
of isolated rabbit atria. Yao Xue Xue Bao. 27:881–885. 1992.(In
Chinese). PubMed/NCBI
|
|
26
|
Wang JL, Nong Y, Xia GJ, Yao WX and Jiang
MX: Effects of liensinine on slow action potentials in myocardium
and slow inward current in canine cardiac Purkinje fibers. Yao Xue
Xue Bao. 28:812–816. 1993.(In Chinese). PubMed/NCBI
|
|
27
|
Xiao JH, Zhang JH, Chen HL, Feng XL and
Wang JL: Inhibitory effect of isoliensinine on bleomycin-induced
pulmonary fibrosis in mice. Planta Med. 71:225–230. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuo YC, Lin YL, Liu CP and Tsai WJ: Herpes
simplex virus type 1 propagation in HeLa cells interrupted by
Nelumbo nucifera. J Biomed Sci. 12:1021–1034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu JJ, Bao JL, Chen XP, Huang M and Wang
YT: Alkaloids isolated from natural herbs as the anticancer agents.
Evid Based Complement Alternat Med. 2012:4850422012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu CM, Kao CL, Wu HM, Li WJ, Huang CT, Li
HT and Chen CY: Antioxidant and anticancer aporphine alkaloids from
the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules.
19:17829–17838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O' Reilly LA, Tai L, Lee L, Kruse EA,
Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT,
et al: Membrane-bound Fas ligand is essential for Fas-induced
apoptosis. Nature. 461:659–663. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Waring P and Müllbacher A: Cell death
induced by the Fas/Fas ligand pathway and its role in pathology.
Immunol Cell Biol. 77:312–317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scaffidi C, Medema JP, Krammer PH and
Peter ME: FLICE is predominantly expressed as two functionally
active isoforms, caspase-8/a and caspase-8/b. J Biol Chem.
272:26953–26958. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Volkmann N, Marassi FM, Newmeyer DD and
Hanein D: The rheostat in the membrane: BCL-2 family proteins and
apoptosis. Cell Death Differ. 21:206–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Letai A, Bassik MC, Walensky LD,
Sorcinelli MD, Weiler S and Korsmeyer SJ: Distinct BH3 domains
either sensitize or activate mitochondrial apoptosis, serving as
prototype cancer therapeutics. Cancer Cell. 2:183–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Finucane DM, Bossy-Wetzel E, Waterhouse
NJ, Cotter TG and Green DR: Bax-induced caspase activation and
apoptosis via cytochrome c released from mitochondria is inhibited
by Bcl-xL. J Biol Chem. 274:2225–2233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Antwerp DJ, Martin SJ, Verma IM and
Green DR: Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell
Biol. 8:107–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ohshima K, Sugihara M, Haraoka S, Suzumiya
J, Kanda M, Kawasaki C, Shimazaki K and Kikuchi M: Possible
immortalization of Hodgkin and Reed-Sternberg cells: Telomerase
expression, lengthening of telomere and inhibition of apoptosis by
NF-kappaB expression. Leuk Lymphoma. 41:367–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rayet B and Gélinas C: Aberrant rel/nfkb
genes and activity in human cancer. Oncogene. 18:6938–6947. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-kB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109:(Suppl). S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen C, Edelstein LC and Gélinas C: The
Rel/NF-kappaB family directly activates expression of the apoptosis
inhibitor Bcl-x(L). Mol Cell Biol. 20:2687–2695. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin A and Karin M: NF-κB in cancer: A
marked target. Semin Cancer Biol. 13:107–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tergaonkar V, Bottero V, Ikawa M, Li Q and
Verma IM: IkappaB kinase-independent IkappaBalpha degradation
pathway: Functional NF-kappaB activity and implications for cancer
therapy. Mol Cell Biol. 23:8070–8083. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee CH, Jeon YT, Kim SH and Song YS:
NF-kappaB as a potential molecular target for cancer therapy.
Biofactors. 29:19–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gilmore TD and Garbati MR: Inhibition of
NF-κB signaling as a strategy in disease therapy. Curr Top
Microbiol Immunol. 349:245–263. 2011.PubMed/NCBI
|