Introduction
Inflammatory bowel disease (IBD) describes a group
of chronic diseases, including ulcerative colitis and Crohn's
disease, which have a significant effect on life quality (1). While the pathogeneses of these diseases
have not been fully identified, there is evidence to suggest that
the immune system may have a causative role (2). Tissue injury occurs not only as a
result of uncontrolled activation of the immune response, but also
due to an increase in oxygen and nitrogen metabolites (3). Within this mechanism, reactive oxygen
species (ROS) have been demonstrated to have a significant
propagation effect on the pathogenesis of IBD, and has a dominant
role in oxidative stress and apoptosis development (4). Oxidative stress, and the subsequent
increase in apoptosis, have constituted the basis of the treatment
strategy for the past 50 years; therefore, the majority of research
into therapeutic agents in this field has aimed to eliminate ROS
(5).
Acetic acid (AA)-induced colitis is one of several
models of experimental colitis used to investigate IBD, and is
propagated by intrarectal administration of AA to induce
inflammation and ulceration in the rectum and the colon in rats.
Oxidative destruction is thought to be the pathogenetic factor in
this scenario, and this damage is also observed in the gross
morphology of the colon (6). It is
assumed that the same result occurs in colitis in humans (7).
Dexpanthenol (Dxp) is a biologically active
alcohol-analog of pantothenic acid (PA) that is converted into PA
inside the cell (8). PA exerts its
anti-oxidative effects by increasing the synthesis of reduced
glutathione (GSH) and associated peroxidase enzymes, which serve as
the most important protective systems against oxidative stress and
lipid peroxidation (9).
Additionally, PA is incorporated into the structure of coenzyme A,
stimulates epithelization, and exerts anti-inflammatory effects
(10). The present study aimed to
investigate the effects of Dxp on tissue damage and oxidative
stress in a rat model of acetic acid-induced colitis, using
biochemical and histopathological analyses. This will elucidate
whether or not Dxp is a potential therapeutic agent for IBD.
Materials and methods
Animals
The present study was approved by the Institutional
Ethics Committee for Animal Experiments (2014/A-41). All
experiments were performed at the Inonu University Experimental
Animals Production Center (IUEAPC; Malatya, Turkey). The study
protocol was executed in accordance with the United States Animal
Welfare Act and the Guide for the Care and Use of Laboratory
Animals (National Institutes of Health, publication no. 5377-3,
1996), on the principles of animal research. A total of 32 Wistar
albino female rats (3–4 months old; weight, 200±20 g) were used.
Rats were purchased from the IUEAPC and randomly divided into
groups (n=8/group). Each rat was kept in a separate cage. All rats
were fed a standard rat pellet and tap water diet, with 12-h
fasting employed prior to the experiment. Rats were maintained in
an environment with a 12:12 light-dark cycle at 21±2°C room
temperature and 60±5% humidity.
Induction of colitis
AA was used to induce acute colitis in rats. Rats
were administered 4% AA at 24-h intervals for three days with a 6G
nelaton catheter, under mild anesthesia [ketamine hydrochloride (50
mg/kg) and xylazine (5 mg/kg) mixture]. A catheter was inserted 6
cm into the anus, and 1 ml AA was administered followed by 1 ml
air, after which rats were maintained in the Trendelenburg position
for 15 min. Dxp was administered as a single dose of 500 mg/kg for
three days, under mild anesthesia (as described above). An insulin
injector (Beybi Plastik Fabrikasi Sanayi A.Ş, Istanbul, Turkey) was
used to deliver Dxp into the peritoneum from the right lower
quadrant of the abdomen. Dosages of AA and Dxp were adjusted
according to previous dose-response studies (4,11).
Experimental protocol
A total of 32 rats were randomly divided into four
equal groups (n=8); i) group I, the control group, 1 ml saline
solution (0.9%) was administered intrarectally; ii) group II, rats
received 4% AA (1 ml/day into the colon intrarectally) as a single
dose for three consecutive days; iii) group III, rats received 4%
AA (1 ml/day into the colon intrarectally) as a single dose for
three consecutive days, and from day four, a single dose of Dxp
(500 mg/kg; Bepanthene ampule®; Bayer AG, Leverkusen,
Germany) was administered intraperitoneally (IP) for three days;
and iv) group IV, 500 mg/kg Dxp was administered IP as a single
dose for three consecutive days.
On day 7, laparotomy was performed under high-dose
anesthesia (70 mg/kg ketamine and 8 mg/kg xylazine; i.m.), and
tissue specimens were subsequently collected from the area 10-cm
distal from the colon. Oxidative stress markers, including
malondialdehyde (MDA), total oxidant status (TOS), oxidative stress
index (OSI), and anti-oxidant system markers, including superoxide
dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX),
glutathione (GSH) and total anti-oxidant capacity (TAC) levels,
were analyzed. Histopathological examination was performed under
light microscopy.
Histological analyses
For light microscopic analysis, samples harvested
from the distal colon were fixed in 10% formalin for 48 h,
dehydrated in an ascending alcohol series, and subsequently
embedded in paraffin. Using a microtome, paraffin blocks were
prepared for sectioning at 5-µm thickness. Sections were stained
with hematoxylin and eosin to assess the general morphology and
Periodic acid-Schiff was employed for goblet cell secretion
assessment.
Assessment of colonic injury was performed using the
following criteria: Mucosal epithelium (ulceration); lamina propria
(polymorphonuclear cell infiltrate); crypts loss, submucosa
(hemorrhage, edema, infiltration of inflammatory cells); and
caspase-3 immuno-reactivity (according to the extent of cell
staining in all layers of the colon). Colonic mucosal damage was
evaluated on a 0–4 scale by two histologists, who were blinded to
the experimental group. Grading criteria were as follows: Grade 0,
normal appearance; grade 1, slight injury (0–25% involvement);
grade 2, moderate injury (25–50% involvement); grade 3, severe
injury (50–75% involvement); and grade 4, extensive full thickness
injury (>75%) (12). The
microscopic grade of each tissue was calculated as the sum of the
scores given to each criteria; therefore, the maximum score that
could be obtained was 28. Surface goblet cells and mitosis figures
in crypts were counted using a Leica Q Win image analysis system
(Leica Microsystems Ltd., Milton Keynes, UK) in 20 areas under ×40
objective.
For immunohistochemical analysis, sections of 4 µm
thickness were placed on polylysine-coated slides. Following
rehydration, samples were immersed in a citrate buffer (pH 7.6),
heated in a microwave oven for 20 min, cooled for 20 min at room
temperature, and subsequently washed with phosphate-buffered saline
(PBS; three 2 min washes). Washed sections were immersed in 0.3%
H2O2 for 7 min prior to washing again with
PBS. Sections were incubated with a primary caspase-3 (CPP32) Ab-4
rabbit polyclonal antibody (cat no. RB-1197-R7; ready-to-use;
Thermo Fisher Scientific, Inc.) for 30 min at room temperature,
rinsed in PBS and subsequently incubated with biotinylated goat
anti-polyvalent (cat. no. TP-125-BN; ready-to-use) antibody and
streptavidin peroxidase (cat no. TS-125-HR) (both Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 10 min at room temperature.
Staining was completed with chromogen substrate for 15 min and
slides were counter-stained with Mayer's hematoxylin for 1 min,
rinsed in tap water and dehydrated. Sections were examined by a
histopathologist unaware of the experimental protocol using a Leica
DFC 280 light microscope (Leica Microsystems, Ltd.).
Caspase-3-positive cells in the cytoplasm were stained brown.
Biochemical analysis
A total of 200 mg frozen colonic tissue was cut into
sections using liquid nitrogen and homogenized in 10 volumes of
ice-cold Tris-HCl buffer with respect to tissue weight (50 mmol/l;
pH 7.4) using a homogenizer (Ultra Turrax IKAT18 basic
homogenization; Werke, Staufen, Germany) for 3 min at 7,500 ×
g and 4°C. The supernatant solution was extracted with an
equal volume of ethanol/chloroform mixture (3/5; v/v). Following
centrifugation at 3,000 × g for 30 min at 4°C, the upper
layer was used to analyze total tissue protein levels.
Determination of MDA
MDA content in the homogenates was determined
spectrophotometrically by measuring the presence of thiobarbituric
acid reactive substances (TBARS) (13). A total of 3 ml 1% phosphoric acid and
1 ml 0.6% thiobarbituric acid solution were added to 0.5 ml
homogenate and pipetted into a tube. The mixture was heated in
boiling water for 45 min and, following cooling, the colored part
was supplemented with 4 ml n–butanol. Absorbance was
measured using a spectrophotometer (UV-1601; Shimadzu Corporation,
Kyoto, Japan) at 532 and 520 nm. Amount of lipid peroxide was
calculated as TBARS of lipid peroxidation. Results were expressed
in nmol/g tissue according to a prepared standard graph, prepared
using the measurements of the standard solutions
(1,1,3,3-tetramethoxypropane).
Determination of protein content
Protein content of the samples was determined by the
method outlined by Lowry et al (14), using bovine serum albumin as a
standard.
Determination of SOD activity
Total SOD activity was determined according to the
method used by Sun et al (15), which is based upon the principle of
the inhibition of nitroblue-tetrazolium (NBT) reduction by the
xanthine-xanthine oxidase system as a superoxide generator. One
unit of SOD was defined as the enzyme amount required to induce a
50% reduction in the NBT reduction rate. SOD activity was
calculated as U/mg protein.
Determination of CAT activity
CAT activity was determined according to the method
outlined by Aebi and Suter (16).
The principle of the assay is based on the determination of the
rate constant (k, s−1) or H2O2
decomposition rate at 240 nm. Results are provided as k/g
protein.
Determination of GPX activity
GPX activity was measured according to the method
used by Paglia and Valentine (17).
Briefly, in a tube containing NADPH, GSH, sodiumazide and
glutathione reductase, an enzymatic reaction was initiated by the
addition of H2O2, and the change in
absorbance at 340 nm was recorded using a spectrophotometer.
Activity was expressed in U/g protein.
Determination of GSH content
Concentration of GSH in the homogenate was measured
spectrophotometrically according to Ellman's method (17). Briefly, aliquots of tissue homogenate
were mixed with distilled water and 50% trichloroacetic acid in
glass tubes and centrifuged at 2,000 × g for 15 min at 4°C.
Supernatants were mixed with 0.4 mol Tris buffer (pH 8.9) and 0.01
mol 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) was added.
Following agitation of the reaction mixture, absorbance was
measured at 412 nm within 5 min of the addition of DTNB against a
blank sample that contained no homogenate. Absorbance values were
extrapolated from a glutathione standard curve and provided as GSH
(µM/g tissue).
Determination of TAC
TAC levels were determined using a novel automated
colorimetric measurement method developed by Erel (18). In this method, a hydroxyl radical is
produced by the Fenton reaction, which reacts with the colorless
substrate, O–dianisidine, to produce a dianisyl radical that
is bright yellow-brown in color. Following the addition of the
sample, the oxidative reactions initiated by the hydroxyl radicals
present in the reaction mix are suppressed by the antioxidant
components of the sample. In turn, this prevents the color change
and thereby provides an effective measurement of the total
antioxidant capacity of the sample. This assay has been
demonstrated to have excellent precision values (<3%). Results
were presented as mmol Trolox equivalent/l.
Determination of TOS
TOS was determined using a novel automated
measurement method, developed by Erel (18). Oxidants present in the sample oxidize
the ferrous ion-O-dianisidine complex to a ferric ion. The
oxidation reaction is enhanced by glycerol molecules, which are
abundantly present in the reaction medium. The ferric ion forms a
colored complex with xylenol orange in an acidic medium. Color
intensity, which can be measured spectrophotometrically, is
correlated to the total amount of oxidant molecules present in the
sample. The assay was calibrated with H2O2
and the results were expressed in terms of µmol
H2O2 equivalent/l.
Measurement of OSI
The TOS to TAC ratio was accepted as the OSI, which
is an indicator of the degree of the oxidative stress (20). The OSI value was calculated using the
following formula: OSI (arbitrary unit)=TOS/TAC. The OSI value of
the distal colon samples was also calculated as OSI (arbitrary
unit).
Statistical analysis
Data were expressed as median (min-max) values or
mean ± standard deviation depending upon the overall variable
distribution. Normality was assessed using the Shapiro-Wilk test.
Normally distributed data were analyzed by one-way analysis of
variance, followed by Tukey's post-hoc tests. Non-normally
distributed data were compared by Kruskal Wallis H test among the
groups. When significant differences were determined, multiple
comparisons were carried out using the Mann Whitney U test with
Bonferroni correction. Statistical analyses were performed using
SPSS version 22.0 for Windows (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Body weight
No animal mortality occurred during the experimental
period. A decrease in the body weights was observed among the
groups prior to and following the experiments (data not shown),
however, this difference was not significant.
Biochemical assessment
As demonstrated in Table
I, a significant difference was detected in the MDA levels
between group AA and the control group (P<0.05), and between the
MDA levels in Group AA and Group AA + Dxp (P<0.05). A
significant decrease was detected in SOD, CAT and GPX levels in
Group AA, compared with the control group (P<0.05). When Group
AA + Dxp and Group AA were compared, significant improvements were
observed in SOD, CAT, GPX, and GSH parameters in Group AA + Dxp
(P<0.05). Significant differences in TOS and OSI levels were
also detected between Group AA and the control group (P<0.05).
Furthermore, there were marked differences in TOS, TAC, and OSI
levels, and a significant difference in TOS levels between Group AA
and Group AA + Dxp (P<0.05).
 | Table I.Comparison of tissue biochemical
parameters among the study groups (n=8). |
Table I.
Comparison of tissue biochemical
parameters among the study groups (n=8).
| Group | MDA (nmol/g) | SOD (U/mg
protein) | CAT (k/g
protein) | GPX (U/g
protein) | GSH
(micromol/g) | TOS
(micromol/g) | TAC (mmol
troloxEq/l) | OSI (arbitrary
unit) |
|---|
| Control | 3.18
(2.52–5.20) | 0.68
(0.61–0.97) | 1.36
(0.86–1.95) | 285.43
(198.53–395.44) | 2.69
(2.13–3.20) | 1.78
(1.38–2.15) | 0.95
(0.81–1.12) | 1.86
(1.25–2.42) |
| AA | 5.89
(4.97–7.52)a | 0.41
(0.24–0.59)a | 0.49
(0.25–0.84)a | 143.51
(92.54–150.7)a | 1.91
(1.15–2.81) | 2.56
(1.58–4.14)a | 0.87
(0.63–1.05) | 2.76
(2.09–6.47)a |
| AA+Dxp | 4.04
(3.42–6.06)b | 0.69
(0.58–0.97)b | 1.12
(1.05–1.92)b | 290.91
(204.62–293.59)b | 2.81
(2.47–3.08)b | 1.80
(1.34–2.41)b | 0.98
(0.81–1.07) | 1.96
(1.40–2.42) |
| Dxp | 4.16
(3.13–6.06) | 0.68
(0.51–0.93)b | 1.15
(0.77–1.92)b | 250.30
(201.00–294.57)b | 2.86
(2.00–3.27)b | 2.09
(1.38–2.26) | 0.91
(0.73–0.98) | 2.03
(1.40–2.69) |
| P-value | <0.05 | < 0.01 | <0.01 | <0.001 | <0.01 | <0.05 | >0.05 | <0.05 |
Histological results
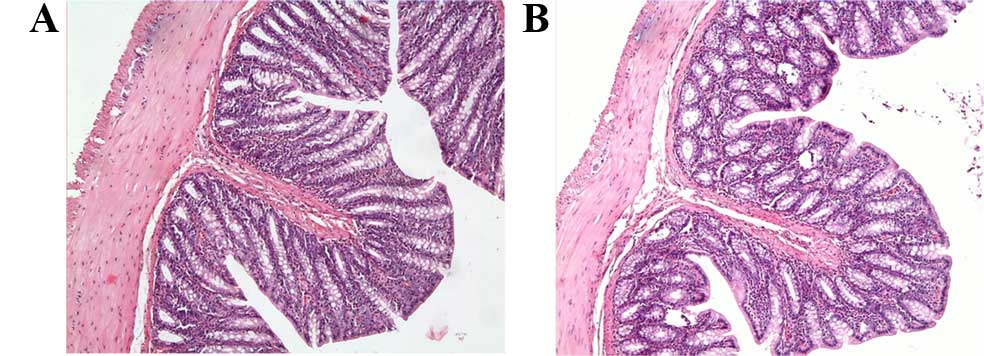
Tissue sections of rats in the control and Dxp
groups exhibited normal large bowel structures, and the colonic
mucosa was observed as intact (Fig. 1A
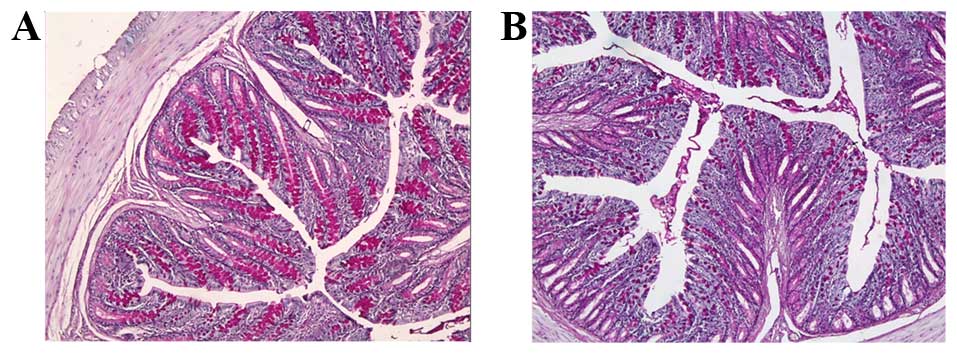
and B). Numerous goblet cells were identified on the surface
epithelium and crypt in the control and Dxp groups (Fig. 2A and B). Notable histological damage
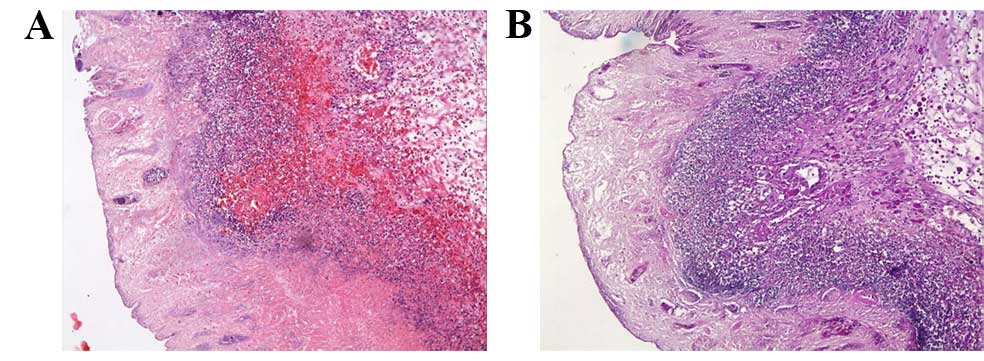
occurred in the AA group (score, 12.5±2.3). The lamina
epithelialis, lamina propria, muscularis mucosae, and the submucosa
of the colon layers could not be distinguished from each other due
to extensive inflammatory cell infiltration and ulceration
(Fig. 3A). Diffuse inflammatory
cells in the mucosa were predominantly composed of neutrophils. In
addition to severe goblet cell depletion, an absence of crypts was
observed (Fig. 3B).
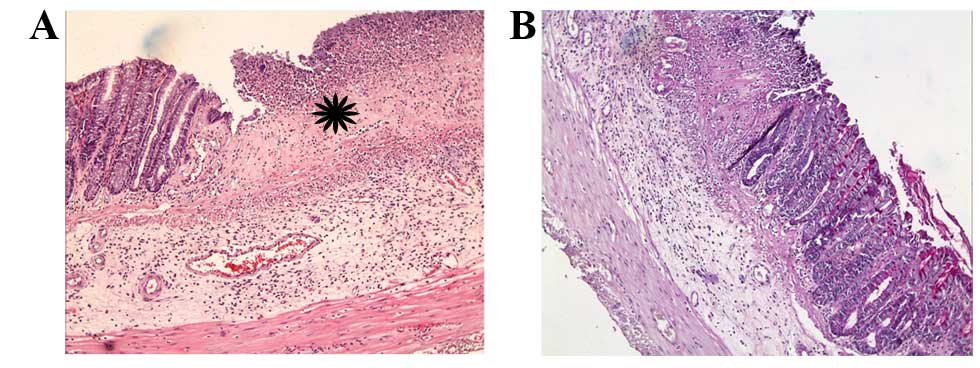
The microscopic score of the AA + Dxp group was
significantly reduced compared to the AA group (P<0.01);
however, Dxp treatment did not completely alleviate lesions, it
merely restricted them. Ulceration, inflammatory cell infiltration
and loss of crypt were still present in the affected areas
(Fig. 4A). In some areas, the mucosa
remained intact in this group. In intact fields, goblet cells were
detected on the surface and crypt epithelium (Fig. 4B). Additionally, the number of
mitotic figures was significantly increased when compared with the
AA group (P<0.0001).
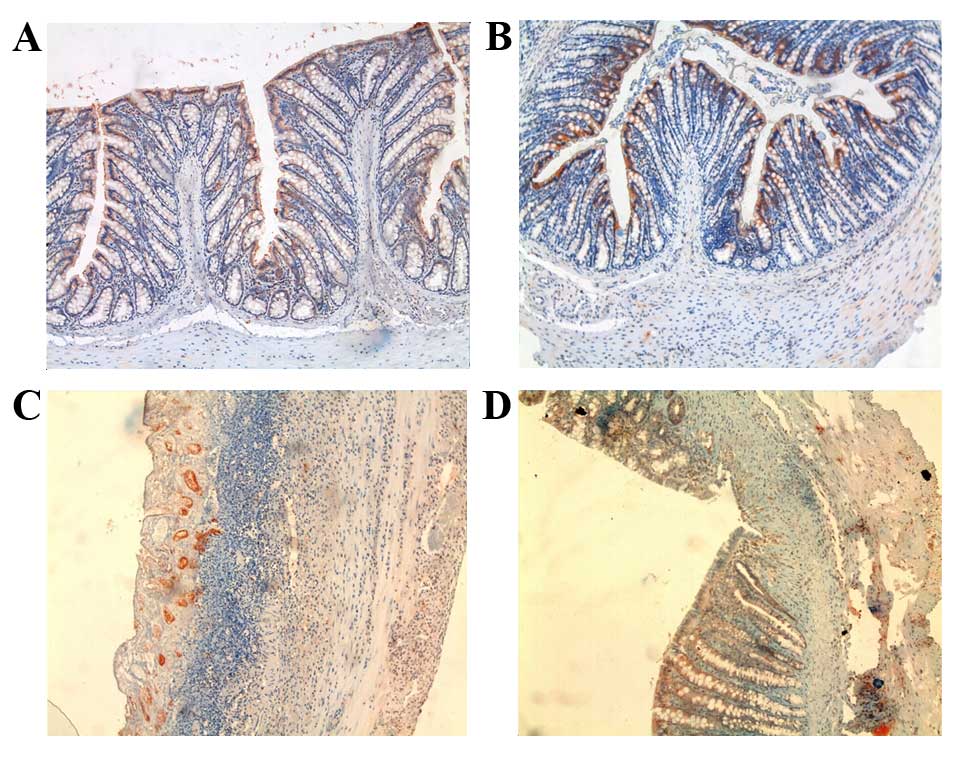
Caspase-3 immunostaining was only detected in the
epithelial cells on the luminal surface in the control and Dxp
groups (Fig. 5A and B). Conversely,
caspase-3-positive cells were observed only in epithelial crypt
cells due to superficial epithelial ulceration in the AA group
(Fig. 5C). The appearance of cells
stained with caspase-3 in the AA + Dxp group was consistent with
the control group (Fig. 5D). Results
of histological grading and the number of goblet cells and mitotic
figures in all groups are shown in Tables II and III.
 | Table II.Colonic injury grades among the study
groups. |
Table II.
Colonic injury grades among the study
groups.
| Group | Control | AA | AA + Dxp | Dxp |
|---|
| Microscopic
grade | 1.1±0.1 |
12.5±2.3a |
6.8±2.3b | 1.4±0.2 |
 | Table III.Number of goblet cells and mitotic
figures. |
Table III.
Number of goblet cells and mitotic
figures.
| Group | Goblet cell | Mitotic figure |
|---|
| Control | 62.0±17.3 | 1.3±1.1 |
| AA |
15.1±19.6a |
0.7±1.0a |
| AA + Dxp |
37.7±25.5b |
1.2±1.1b |
| Dxp | 61.8±17.2 | 1.2±1.2 |
Discussion
IBD describes a group of chronic diseases that have
marked effects on the quality of life of those affected. In recent
years, the incidence of IBD has gradually increased (19). Despite the potential role of genetic,
immunologic, and environmental factors, the etiology of IBD remains
unclear; however, free oxygen radicals are considered to be a
causal factor for IBD (20).
Therefore, several studies have aimed to identify potential IBD
therapies. A number of previous studies have focused on oxidative
stress, which is important in mucosal injury pathogenesis (21) and is an initiator of apoptosis
(22,23). Apoptosis is important in tissue
homeostasis (24); insufficient or
excessive apoptosis disrupts equilibrium and causes various
diseases (25). Apoptotic cell death
also alters epithelial barrier function and allows infiltration by
pathogenic microorganisms. While the details remain unclear,
several studies (26–28) have suggested that apoptosis has a
role in IBD pathogenesis, together with oxidative stress (29). Therefore, the majority of studies
have focused on substances with anti-oxidant and anti-inflammatory
properties.
In the present study, the oxidative stress,
apoptosis and anti-oxidant properties of Dxp were investigated in a
rat model of AA-induced colitis. The AA-induced colitis model used,
is beneficial for the investigation of IBD pathogenesis and novel
treatment options for ulcerative colitis, since it induces
inflammation and ulceration (7).
Colon tissue injury was detected in the colitis
model with a significant increase in oxidative stress markers and
histopathological changes. Oxidative stress is considered to be a
causative factor of AA-associated alterations in the colon tissue.
Experimental studies have indicated that oxidative stress results
from the shift of equilibrium between the pro-oxidant and
anti-oxidant systems in favor of the pro-oxidant system as a result
of excessive production of free oxygen radicals (30).
In the present study, distal colitis was induced via
AA treatment, which revealed that AA treatment caused prominent
tissue injury. When the results of the colonic tissue in the
control group and Group AA were compared, AA treatment was
demonstrated to induce an increase in MDA levels in the tissue.
Therefore, it was determined that AA may have caused oxidative
stress by leading to a marked increase in MDA levels in the colon
tissue. MDA is the toxic end-product of lipid peroxidation, and
reflects the level of lipid peroxidation in the tissue; hence its
common usage as a marker of lipid peroxidation (31). MDA is secreted due to the toxic
effects of active free oxygen radicals. While ROS affect all
biomolecules in the organic environment, their major targets are
membrane lipids, other lipids, proteins and DNA (32). ROS initiate lipid peroxidation by
removing a hydrogen atom (33).
Previous studies have shown that MDA levels are elevated in IBD
(34–36). Furthermore increased lipid
peroxidation has been reported in AA-associated tissue injuries,
which is consistent with the findings of the present study. The
present study demonstrated that Dxp treatment caused a significant
decrease in AA-associated oxidative stress.
Another important finding of the present study was
that AA treatment leads to a decrease in SOD, CAT and GPX levels in
the tissue. It is well-known that enzymatic and non-enzymatic
anti-oxidant systems exist to protect tissues from pro-oxidants
and, as there is a balance between these systems, the imbalance has
a role in the development of various diseases (37). SOD, CAT and GPX are endogenous
enzymatic anti-oxidants, whereas GSH is a non-enzymatic
anti-oxidant (38). These molecules
protect cells and organisms from cytotoxic free oxygen radicals.
SOD is among the most important enzymatic anti-oxidants. Oxygen
radicals are converted into H2O2 by SOD.
H2O2 is subsequently detoxified by CAT and
GPX, and is converted into H2O and O2
molecules. The release of ROS in IBD disrupts the anti-oxidant
system during mucosal inflammation and leads to oxidative damage.
Decreased anti-oxidant levels have been reported in patients with
ulcerative colitis (39,40). Kruidenier et al (41) demonstrated that Cu/Zn SOD and MnSOD
levels were elevated in patients with IBD, compared with the
control group. Kuralay et al (35) established an experimental model of
colitis and indicated that SOD levels increased as a response to
oxidative stress, and this increase was successfully reduced after
treatment with anti-oxidant agents. The findings of the present
study are consistent with the existing hypothesis of the mechanism
of colitis tissue injury, which occurs as a result of ROS that
weaken the anti-oxidant system. Furthermore, the results of the
present study are consistent with previous studies, which have
demonstrated increased lipid peroxidation and decreased
anti-oxidant systems in AA-induced colitis (42).
The GSH cycle is the other important intracellular
anti-oxidant defense system. It is used as a substrate for the
activity of several anti-oxidant enzymes. In particular, GPX is a
glutathione-dependent enzyme. In the presence of GSH, GPX
detoxifies H2O2 into H2O and
O2 molecules. GSH subsequently loses a hydrogen atom and
oxidized glutathione (GSSG) is formed (43). Glutathione reductase forms GSH from
GSSG. A decrease in GSH activity increases oxidative stress and
leads to the accumulation of toxic products (44). In the present study, AA led to a
decrease in GSH levels.
In the present study, increased levels of TOS and
OSI, and decreased TAC levels were detected in Group AA. TOS and
OSI levels in Group AA + Dxp were reduced compared with Group AA;
however, no significant increase in TAC levels was detected. These
findings are consistent with the results of a previous study
(45), suggesting that AA increases
oxidative stress, whereas Dxp decreases it.
Macroscopic and histopathological examinations are
the gold standards for evaluating inflammatory injury in the colon.
The present study demonstrated that caspase-3 activity in the colon
was considerably increased in Group AA compared with the control
group. When Group AA + Dxp and Group AA were compared, Dxp
treatment significantly decreased caspase-3 activity. This effect
can be attributed to the anti-inflammatory and anti-apoptotic
effects of Dxp. Altintas et al (39) have previously demonstrated that Dxp
treatment significantly decreases tissue injury and apoptosis. The
findings of the present study are consistent with this study.
Despite these effects of Dxp, it is still unclear how Dxp exerts
these effects at the molecular level.
The present study indicated that when Dxp was IP
administered to rats with AA-induced colitis can reduce the extent
of colonic mucosal damage, abate the increase in MDA, TOS and OSI
levels, and restore diminished antioxidant enzymes and substances
such as SOD, CAT, GPX, GSH and TAC. These effects can be attributed
to the anti-oxidant, anti-inflammatory and anti-apoptotic effects
of Dxp. These data also suggest that Dxp is more effective when
administered after the induction of colitis.
In conclusion, the present study employed
biochemical and histopathological analysis to demonstrate that
oxidative stress and apoptosis are elevated in IBD. This, in turn,
showed that oxidative stress and apoptosis may have a major role in
IBD pathogenesis. These findings indicate that studies on IBD
treatment will continue to aim to reduce the effects of the ROS.
The beneficial effects of Dxp on tissue lipid peroxidation, protein
oxidation, and anti-oxidant systems suggested that it may represent
a treatment option to alleviate the spread of IBD.
References
|
1
|
Khor B, Gardet A and Xavier RJ: Genetics
and pathogenesis of inflammatory bowel disease. Nature.
474:307–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karlinger K, Györke T, Makö E, Mester A
and Tarján Z: The epidemiology and the pathogenesis of inflammatory
bowel disease. Eur J Radiol. 35:154–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pavlick KP, Laroux FS, Fuseler J, Wolf RE,
Gray L, Hoffman J and Grisham MB: Role of reactive metabolites of
oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol
Med. 33:311–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harputluoglu M, Demirel U, Yücel N,
Karadağ N, Temel I, Firat S, Ara C, Aladağ M, Karincaoğlu M and
Hilmioğlu F: The effects of Gingko biloba extract on acetic
acid-induced colitis in rats. Turk J Gastroenterol. 17:177–182.
2006.PubMed/NCBI
|
|
5
|
Zhu H and Li YR: Oxidative stress and
redox signaling mechanisms of inflammatory bowel disease: Updated
experimental and clinical evidence. Exp Biol Med (Maywood).
237:474–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pereira C, Grácio D, Teixeira JP and Magro
F: Oxidative stress and DNA damage: Implications in inflammatory
bowel disease. Inflamm Bowel Dis. 21:2403–2417. 2015.PubMed/NCBI
|
|
7
|
Vishwakarma N, Ganeshpurkar A, Pandey V,
Dubey N and Bansal D: Mesalazine-probiotics beads for acetic acid
experimental colitis: Formulation and characterization of a
promising new therapeutic strategy for ulcerative colitis. Drug
Deliv. 22:94–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slyshenkov VS, Rakowska M, Moiseenok AG
and Wojtczak L: Pantothenic acid and its derivatives protect
Ehrlich ascites tumor cells against lipid peroxidation. Free Radic
Biol Med. 19:767–772. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wojtczak L and Slyshenkov VS: Protection
by pantothenic acid against apoptosis and cell damage by oxygen
free radicals-the role of glutathione. Biofactors. 17:61–73. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jessop CE and Bulleid NJ: Glutathione
directly reduces an oxidoreductase in the endoplasmic reticulum of
mammalian cells. J Biol Chem. 279:55341–55347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ceylan H, Yapici S, Tutar E, Ceylan NO,
Tarakçıoğlu M and Demiryurek AT: Protective effects of dexpanthenol
and y-27632 on stricture formation in a rat model of caustic
esophageal injury. J Surg Res. 171:517–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arribas B, Suárez-Pereira E, Mellet C
Ortiz, García Fernández JM, Buttersack C, Rodríguez-Cabezas ME,
Garrido-Mesa N, Bailon E, Guerra-Hernández E, Zarzuelo A and Gálvez
J: Di-D-fructose dianhydride-enriched caramels: Effect on colon
microbiota, inflammation, and tissue damage in
trinitrobenzenesulfonic acid-induced colitic rats. J Agric Food
Chem. 58:6476–6484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mihara M and Uchiyama M: Determination of
malonaldehyde precursor in tissues by thiobarbituric acid test.
Anal Biochem. 86:271–278. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
15
|
Sun Y, Oberley LW and Li Y: A simple
method for clinical assay of superoxide dismutase. Clin Chem.
34:497–500. 1988.PubMed/NCBI
|
|
16
|
Aebi H and Suter H: CatalaseMethods of
Enzymatic Analysis. Bergmeyer HU: Academic Press; New York, NY: pp.
3251969
|
|
17
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
18
|
Erel O: A novel automated method to
measure total antioxidant response against potent free radical
reactions. Clin Biochem. 37:112–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burisch J, Jess T, Martinato M and Lakatos
PL: ECCO-EpiCom: The burden of inflammatory bowel disease in
Europe. J Crohns Colitis. 7:322–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martinez CA, Ribeiro ML, Gambero A,
Miranda DD, Pereira JA and Nadal SR: The importance of oxygen free
radicals in the etiopathogenesis of diversion colitis in rats. Acta
Cir Bras. 25:387–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YJ, Kim EH and Hahm KB: Oxidative
stress in inflammation-based gastrointestinal tract diseases:
Challenges and opportunities. J Gastroenterol Hepatol.
27:1004–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franco R, Sánchez-Olea R, Reyes-Reyes EM
and Panayiotidis MI: Environmental toxicity, oxidative stress and
apoptosis: Ménage à trois. Mutat Res. 674:3–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Souza HS, Tortori CJ, Castelo-Branco MT,
Carvalho AT, Margallo VS, Delgado CF, Dines I and Elia CC:
Apoptosis in the intestinal mucosa of patients with inflammatory
bowel disease: Evidence of altered expression of FasL and perforin
cytotoxic pathways. Int J Colorectal Dis. 20:277–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Myers BS, Martin JS, Dempsey DT, Parkman
HP, Thomas RM and Ryan JP: Acute experimental colitis decreases
colonic circular smooth muscle contractility in rats. Am J Physiol.
273:G928–G936. 1997.PubMed/NCBI
|
|
26
|
Pedersen J, LaCasse EC, Seidelin JB,
Coskun M and Nielsen OH: Inhibitors of apoptosis (IAPs) regulate
intestinal immunity and inflammatory bowel disease (IBD)
inflammation. Trends Mol Med. 20:652–665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Becker C, Watson AJ and Neurath MF:
Complex roles of caspases in the pathogenesis of inflammatory bowel
disease. Gastroenterology. 144:283–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weimann BI and Hermann D: Studies on wound
healing: Effects of calcium D-pantothenate on the migration,
proliferation and protein synthesis of human dermal fibroblasts in
culture. Int J Vitam Nutr Res. 69:113–119. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halliwell B and Chirico S: Lipid
peroxidation: Its mechanism, measurement, and significance. Am J
Clin Nutr. 57(5 Suppl): 715S–724S, 725S. 1993.PubMed/NCBI
|
|
31
|
Kaya E, Gür ES, Ozgüç H, Bayer A and
Tokyay R: L-glutamine enemas attenuate mucosal injury in
experimental colitis. Dis Colon Rectum. 42:1209–1215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Southorn P and Powıs G: Free radicals in
medicine. I. Chemical nature and biologic reactions. Mayo Clin
Proc. 63:381–389. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cetinkaya A, Bulbuloglu E, Kurutas EB,
Ciralik H, Kantarceken B and Buyukbese MA: Beneficial effects of
N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J
Exp Med. 206:131–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alzoghaibi MA, Al Mofleh IA and Al-Jebreen
AM: Lipid peroxides in patients with inflammatory bowel disease.
Saudi J Gastroenterol. 13:187–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Byrav DSP, Medhi B, Prakash A, Chakrabarti
A, Vaiphei K and Khanduja KL: Comparative evaluation of different
doses of PPAR-γ agonist alone and in combination with sulfasalazine
in experimentally induced inflammatory bowel disease in rats.
Pharmacol Rep. 65:951–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gul M, Kayhan B, Elbe H, Dogan Z and Otlu
A: Histological and biochemical effects of dexmedetomidine on liver
during an inflammatory bowel disease. Ultrastruct Pathol. 39:6–12.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thomas MJ: The role of free radicals and
antioxidants: How do we know that they are working? Crit Rev Food
Sci Nutr. 35:21–39. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Flora G, Gupta D and Tiwari A: Toxicity of
lead: A review with recent updates. Interdiscip Toxicol. 5:47–58.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krzystek-Korpacka M, Neubauer K, Berdowska
I, Zielinski B, Paradowski L and Gamian A: Impaired erythrocyte
antioxidant defense in active inflammatory bowel disease: Impact of
anemia and treatment. Inflamm Bowel Dis. 16:1467–1475. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
D'Odorico A, Bortolan S, Cardin R, D'Inca'
R, Martines D, Ferronato A and Sturniolo GC: Reduced plasma
antioxidant concentrations and increased oxidative DNA damage in
inflammatory bowel disease. Scand J Gastroenterol. 36:1289–1294.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kruidenier L, Kuiper I, van Duijn W,
Marklund SL, van Hogezand RA, Lamers CB and Verspaget HW:
Differential mucosal expression of three superoxide dismutase
isoforms in inflammatory bowel disease. J Pathol. 201:7–16. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nieto N, Torres MI, Fernández MI, Girón
MD, Ríos A, Suárez MD and Gil A: Experimental ulcerative colitis
impairs antioxidant defense system in rat intestine. Dig Dis Sci.
45:1820–1827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
El-Beltagi HS and Mohamed HI: Reactive
oxygen species, lipid peroxidation and antioxidative defense
mechanism. Notulae Botanicae Horti Agrobotanici Cluj-Napoca.
41:44–57. 2013.
|
|
44
|
Hagar HH, El-Medany A, El-Eter E and Arafa
M: Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid
induced colitis in rats. Eur J Pharmacol. 554:69–77. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ermis H, Parlakpinar H, Gulbas G, Vardi N,
Polat A, Cetin A, Kilic T and Aytemur ZA: Protective effect of
dexpanthenol on bleomycin-induced pulmonary fibrosis in rats.
Naunyn Schmiedebergs Arch Pharmacol. 386:1103–1110. 2013.
View Article : Google Scholar : PubMed/NCBI
|