Introduction
In China, the incidence and mortality rates of
colorectal cancer (CRC) were 23.03/100,000 and 11.11/100,000,
respectively, in 2011, ranking only after lung cancer and gastric
cancer (1). As a result of early
stage CRC not displaying typical symptoms and signs associated with
the disease, patients are predominantly diagnosed at an advanced
stage, often accompanied by metastasis, thus missing the optimal
time-frame for effective treatment (2). In general, lymph node metastasis
represents the first step of tumor dissemination for CRC, and lymph
node micrometastasis is currently an accurate indicator for the
clinical staging, treatment and prognostic determination of CRC
(3). If the regional lymph nodes
were not appropriately treated, the traditional radical lumpectomy
of CRC alone may lead to inadequate or excessive chemotherapy,
which may cause unwanted damage to normal organs and tissues.
Therefore, lymph node metastasis in CRC has become an important
research focus for the elucidation of disease pathogenesis and
treatment, while the identification of the regulating factors
involved has also attracted increasing attention.
The pathogenesis and development of CRC have been
found to be regulated by various signaling pathways and other
factors. For instance, it has been shown that homolog 8 (CBX8) and
insulin-like growth factor-1 (IGF1) are closely associated with the
onset of CRC (4), and microRNA
(miRNA)-92 is a key oncogene in the development of the disease
(5). Conversely, the status of the
body's immune system is also important for tumor proliferation and
metastasis. The anti-tumor response is predominantly achieved by
cellular immunity, and natural killer (NK) cells and T-lymphocyte
subsets have been found to be implicated in tumor immune
surveillance (6).
Mitogen-activated protein kinase kinase kinase
kinase 4 (MAP4K4) is an upstream activator in the MAPK signaling
pathway, which has been demonstrated to promote the invasion,
metastasis and development of ovarian, breast and prostate cancer,
and malignant melanoma (7–11). MAP4K4 has been demonstrated to be
overexpressed in various tumors, accelerating tumor cell
transformation, promoting cell invasion and decreasing cell
adhesion (12). Furthermore, the
expression of MAP4K4 in CRC without lymph node metastasis is
significantly lower compared with lymph node metastasis, indicating
the role of MAP4K4 in promoting CRC proliferation, invasion and
metastasis (13). However, the
effect of MAP4K4 on the immune system in CRC has yet to be fully
elucidated.
In the present study, the role of miRNA-141 in the
pathogenesis of CRC, especially concerning its regulation of
MAP4K4, was investigated for the first time. The expression levels
of MAP4K4 and miRNA-141 in the tumor, lymph nodes and serum of CRC
patients were detected and analyzed, while the immune system in CRC
was also evaluated.
Patients and methods
Patients
In total, 58 patients with CRC were included in the
present study, who had been diagnosed and subjected to total
surgical resection prior to radiotherapy at The Fourth Hospital of
Hebei Medical University (Shijiazhuang, China) between January 2014
and December 2014. Disease diagnosis was based on the clinical
presentation, medical history and family history, physical
examination, laboratory tests, endoscopy, imaging detection, and
histopathological examination (14).
Of these patients, there were 5 cases of tubulovillous adenoma, 11
cases of papillary carcinoma, 21 cases of tubular adenocarcinoma,
13 cases of mucinous adenocarcinoma, 6 cases of signet ring cell
carcinoma and 2 cases of undifferentiated carcinoma. The occurrence
of lymph node metastasis was confirmed in 26 cases by postoperative
pathological examination on biopsy, and the remaining 32 cases were
free from lymph node metastasis. All patients were first-onset
cases and had not previously received hormone therapy, radiotherapy
or chemotherapy prior to surgery. In addition, 29 age- and
gender-matched healthy individuals were enrolled into the control
group. The CRC patients with lymph node metastasis included 11
males and 15 females, with ages of 34–85 years (median age, 61
years). CRC patients without lymph node metastasis included 17
males and 15 females, aged between 28–76 years (median age, 58
years). In the control group, there were 13 males and 16 females,
aged from 22–72 years (median age, 55 years). Prior written and
informed consent was obtained from each patient and the study was
approved by the Ethics Review Board of the Fourth Hospital of Hebei
Medical University.
Sample collection
The following specimens were collected from the
subjects in the present study: i) The excised tumor and adjacent
tissues, which were stored in liquid nitrogen; ii) the lymph nodes
in proximity to the surgical site, which were removed during
surgery and stored in liquid nitrogen; and iii) the peripheral
blood collected under fasting conditions in the morning, which was
mixed with EDTA anticoagulant (cat. no. 367525; BD Biosciences, San
Jose, CA, USA) and stored at −20°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of MAP4K4 and miRNA-141
were detected with the use of RT-qPCR. Briefly, for detection of
the expression levels in tumor and in lymph nodes, total RNA was
extracted with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The serum RNA was extracted
with the miRNeasy Serum/Plasma kit (Guangzhou Jianlun Biological
Technology Co., Ltd., Guangzhou, China). RT was performed to obtain
the cDNA with the RevertAid First Strand cDNA Synthesis kit
(Fermentas; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The 25 µl PCR system contained 2 liters
template, 1 liter each primer, 12.5 liters TransStart Top Green
qPCR SuperMix (Transgen Biotech, Inc., Beijing, China), and 8.5
liter double distilled H2O.
For the detection of MAP4K4, the primer sequences
were as follows: MAP4K4 forward, 5′-AAGGAGAGAGCGGGAAGCTA-3′, and
reverse, 5′-TTGTTGCAACTGCCTCTGGA-3′; GAPDH forward,
5′-GTTGGAGGTCGGAGTCAACGGA-3′, and reverse,
5′-GAGGGATCTCGCTCCTGGAGGA-3′. The PCR conditions consisted of
denaturation at 94°C for 5 min, followed by 94°C for 30 sec, 60°C
for 30 sec and 72°C for 45 sec, for a total of 35 cycles. For the
detection of miRNA-141, the primer sequences were as follows:
miRNA-141, 5′-CCGGTAACACTGTCTGGTAA-3′, and U6,
5′-GCTTCGGCAGCACATATACTAAAAT-3′. The PCR conditions were as
follows: Denaturation at 95°C for 5 min, followed by 95°C for 15
sec, 58°C for 30 sec and 72°C for 30 sec, for 40 cycles. The
relative expression levels of target genes were calculated with the
2−ΔΔCq method (15).
Western blot analysis
Normal, tumor and lymph node metastasis tissues were
lysed on ice with lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), and the protein concentration was
determined with a BCA kit (cat. no. P0009; Beyotime Institute of
Biotechnology). Protein samples (20 mg) were subjected to 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then
electronically transferred onto a nitrocellulose membrane. The blot
was blocked with 5% non-fat milk at room temperature for 1 h, and
then incubated with rabbit anti-human anti-MAP4K4 polyclonal
primary antibody (1:1,000; cat. no. ab155583; Abcam, Cambridge, MA,
USA) or rabbit anti-human anti-GAPDH polyclonal primary antibody
(1:5,000; cat. no. ab9485; Abcam) at 4°C overnight. The membrane
was then incubated with goat anti-rabbit immunoglobulin G (1:3,000;
cat. no. ab6721; Abcam) at room temperature for 1 h. Subsequently,
the blot was developed using an enhanced chemiluminescence system
(cat. no. P0018A; Beyotime Institute of Biotechnology), and the
protein bands were analyzed with the Image Lab software version 3.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Serum MAP4K4 contents were detected with an ELISA
kit (cat. no. CSB-EL013439HU; Weijia Technology Co., Ltd.,
Guangzhou, China) according to the manufacturer's instructions.
Blood sample was centrifuged at 4°C at 800 × g for 10 min,
separating the serum from the blood cells. Sample (10 µl) was then
added into the 96-well plates in the ELISA microplate, followed by
the addition of 40 µl diluting solution. The experiment was
performed in triplicate. Subsequently, 100 µl horseradish
peroxidase-labeled antibody (contained within the ELISA kit) was
added into each well, and the plate was placed in an incubator for
1 h. After washing five times with ddH2O, 50 µl
substrate A and 50 µl substrate B were added into each well,
respectively, and the plate was incubated at 37°C for 15 min. The
reaction was stopped by adding 50 µl stopping solution, and the
optical density at 450 nm was read on a microplate reader within 15
min.
Flow cytometry
NK and T cells in the peripheral blood were detected
by flow cytometric analysis with CD3/CD4/CD8 and CD3/CD16+56 agents
(cat. nos. IM1650 and IM2076, respectively; Immunotech; Beckman
Coulter, Inc., Marseille, France), according to the manufacturer's
instructions. Briefly, 20 ml staining agent was added into the
anticoagulated whole blood at room temperature for 25 min, and 2 ml
hemolytic agent was then added at room temperature for 10 min.
Following centrifugation at 450 × g at 4°C for 5 min, the
supernatant was discarded. After washing with phosphate-buffered
saline twice, 500 µl 1% paraformaldehyde was added for fixation.
The sample was detected with a FACScan flow cytometer (BD
Biosciences), and the data were analyzed using Cellquest software
version 3.1 (BD Biosciences).
Bioinformatics analysis
To determine the target gene of miRNA-141,
bioinformatics analysis was performed with the following online
software and/or websites: miRanda (http://www.microrna.org/microrna/getExprForm.do),
TargetScan (www.targetscan.org), PiTa (http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PICTA (http://pictar.mdc-berlin.de/).
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. One-way analysis of variance was
performed for the comparison between the groups, along with the
least-significant difference and Student-Newman-Keuls tests (for
equal variance), or the Tamhane's T2 and Dunnett's T3 tests (when
equal variance was not assumed). P<0.05 was considered to
indicate a statistically significant difference.
Results
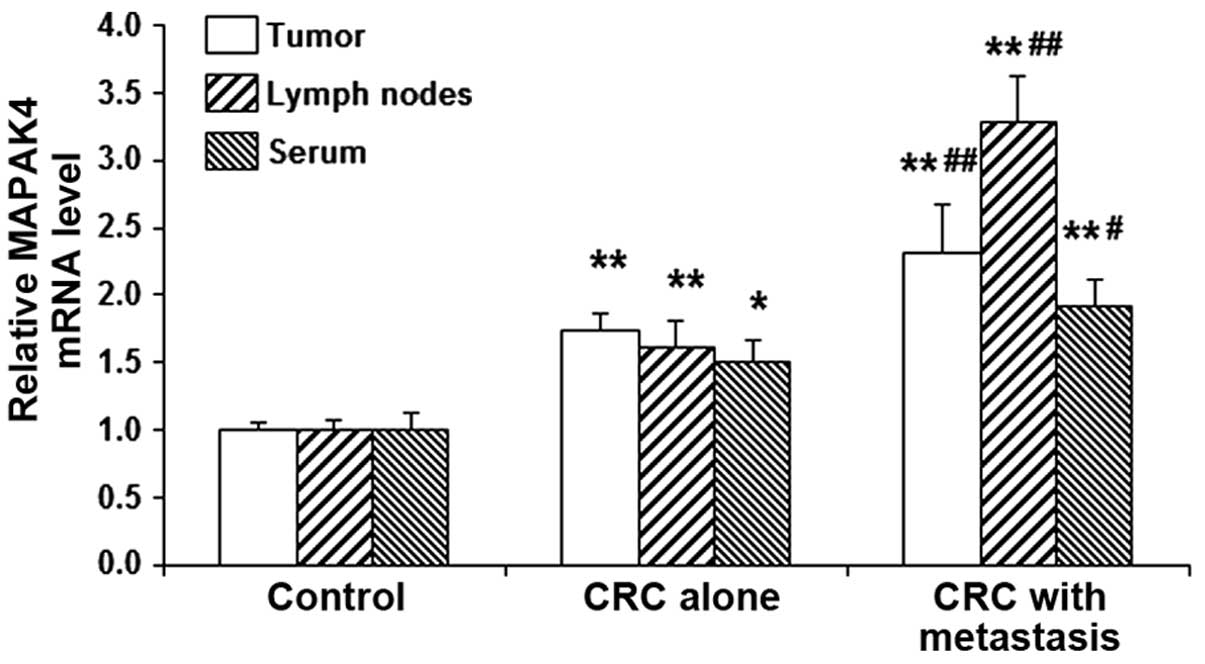
mRNA expression levels of MAP4K4 are
elevated in the tumor tissues, lymph nodes and serum in CRC
patients
In order to investigate the mRNA expression levels
of MAP4K4 in the tumor tissues, lymph nodes and serum in CRC
patients with or without lymph node metastasis, RT-qPCR was
performed. The present results indicated that, compared with the
control group, the mRNA expression levels of MAP4K4 were
significantly elevated in the tumor tissues, lymph nodes and serum
in patients with CRC (all P<0.05; Fig. 1). Furthermore, within the CRC
patients, the mRNA expression levels of MAP4K4 in the tumor
tissues, lymph nodes and serum in cases with lymph node metastasis
were all significantly higher compared with those in cases without
lymph node metastasis (P<0.05; Fig.
1). Thus, the results suggest that MAP4K4 may be upregulated in
CRC, particularly in cases with lymph node metastasis, and this may
contribute to the disease pathogenesis.
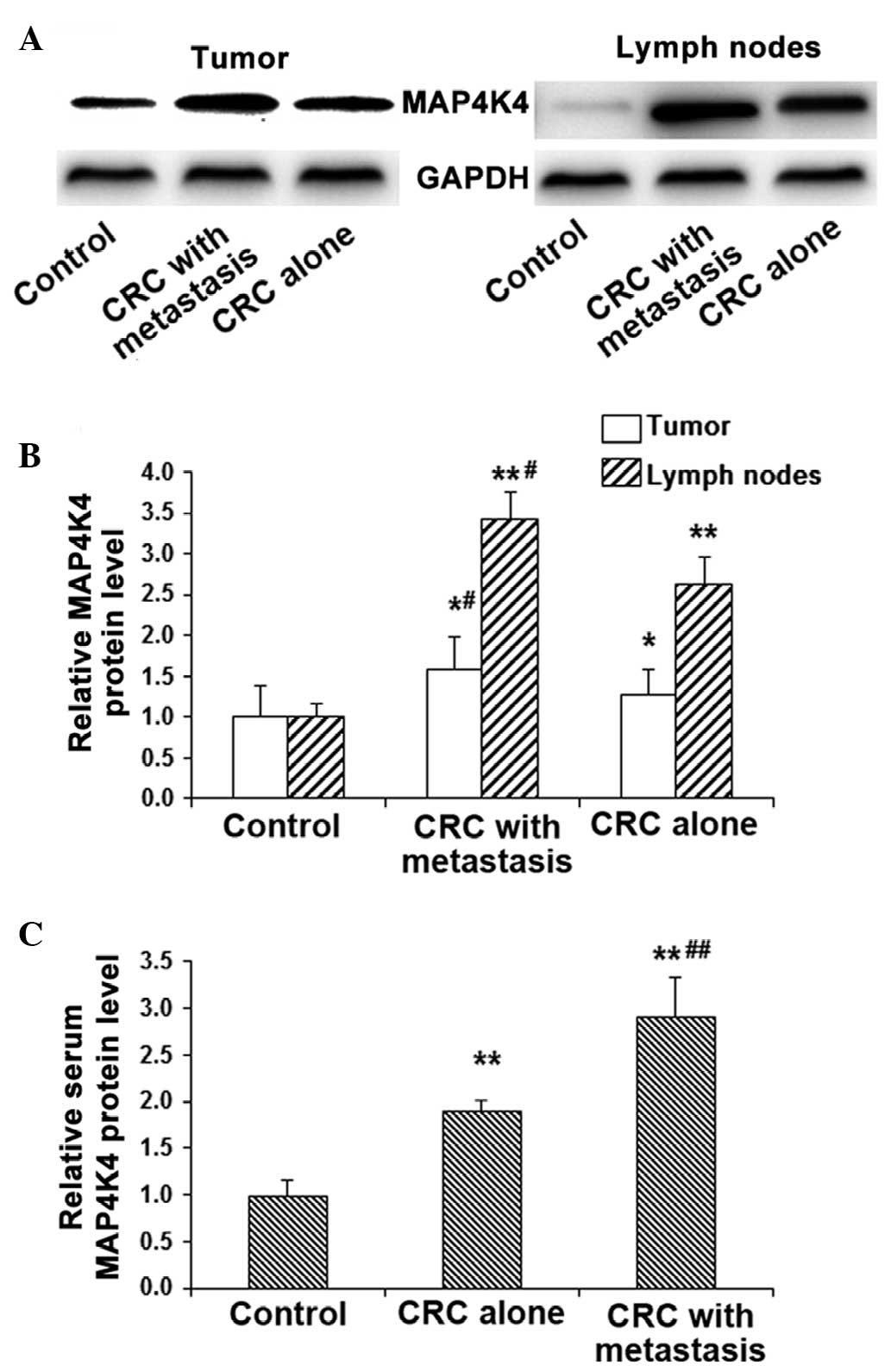
Protein expression levels of MAP4K4
are increased in the tumor tissues, lymph nodes and serum in CRC
patients
The protein expression levels of MAP4K4 in the tumor
tissues and lymph nodes were detected by western blot analysis,
while the serum expression was detected by ELISA. The results of
western blot analysis indicated that, compared with the control
group, the protein expression levels of MAP4K4 were significantly
increased in CRC tissues (P<0.05) and more evidently in the
lymph nodes (P<0.01; Fig. 2A and
B). In addition, the protein expression levels of MAP4K4 in the
tumor and lymph nodes in CRC patients with lymph node metastasis
were all significantly higher compared with those without lymph
node metastasis (P<0.05; Fig. 2A and
B).
Similar results were obtained for the protein
expression of MAP4K4 in the serum. ELISA analysis revealed that,
compared with the control group, the serum MAP4K4 expression was
significantly increased in CRC patients with and without lymph node
metastasis (P<0.01). However, the expression was elevated to a
greater extent in the cases with lymph node metastasis compared
with cases without metastasis (P<0.01; Fig. 2C). In accordance with the alteration
of the mRNA expression levels of MAP4K4, the aforementioned results
indicated that MAP4K4 may be involved in the pathogenesis of CRC,
particularly in the development of metastasis via the lymphatic and
blood circulation.
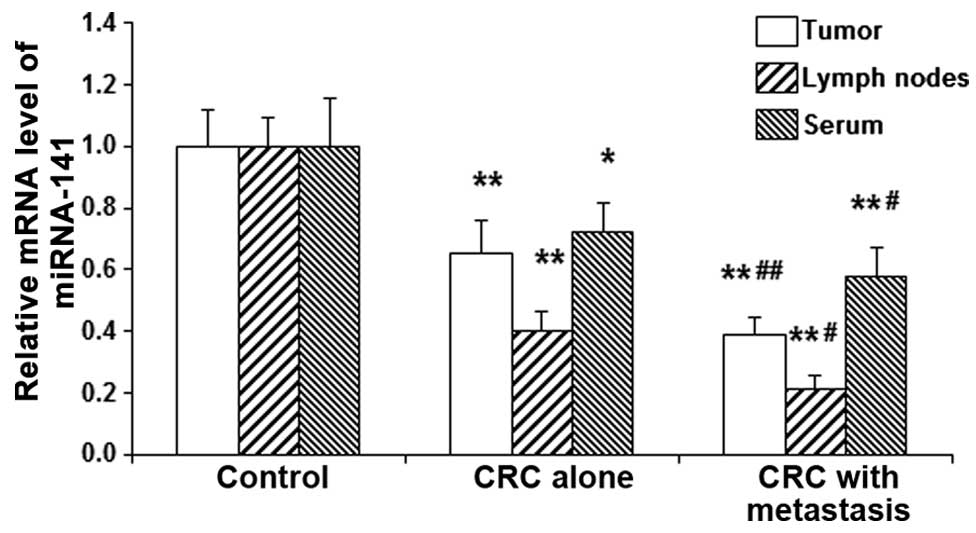
Expression levels of miRNA-141 are
reduced in the tumor tissues, lymph nodes and serum in CRC
patients
It has been reported that MAP4K4 may be regulated by
miRNA-141 in cases of pancreatic cancer (16). To further investigate the underlying
mechanisms through which MAP4K4 is modulated in CRC, bioinformatics
analysis was performed. Results from the miRanda, TargetSean, PiTa,
RNAhybrid, and PICTA analyses revealed that MAP4K4 may be the
target gene of miRNA-141 (Fig. 3),
and may be involved in the pathogenesis of CRC. RT-qPCR was then
performed to detect the expression levels of miRNA-141 in the tumor
tissues, lymph nodes and serum from CRC patients. The current
results demonstrated that, compared with the control group, the
expression levels of miRNA-141 in the tumor tissues, lymph nodes
and serum were significantly decreased in CRC patients (P<0.05;
Fig. 4). Furthermore, of the CRC
patients, the expression levels of miRNA-141 in the tumor tissues,
lymph nodes and serum in cases with lymph node metastasis decreased
to a significant extent when compared with those in patients
without lymph node metastasis (P<0.05; Fig. 4). The results suggest that miRNA-141
may participate in the pathogenesis and development of CRC, and
contribute to lymph node metastasis.
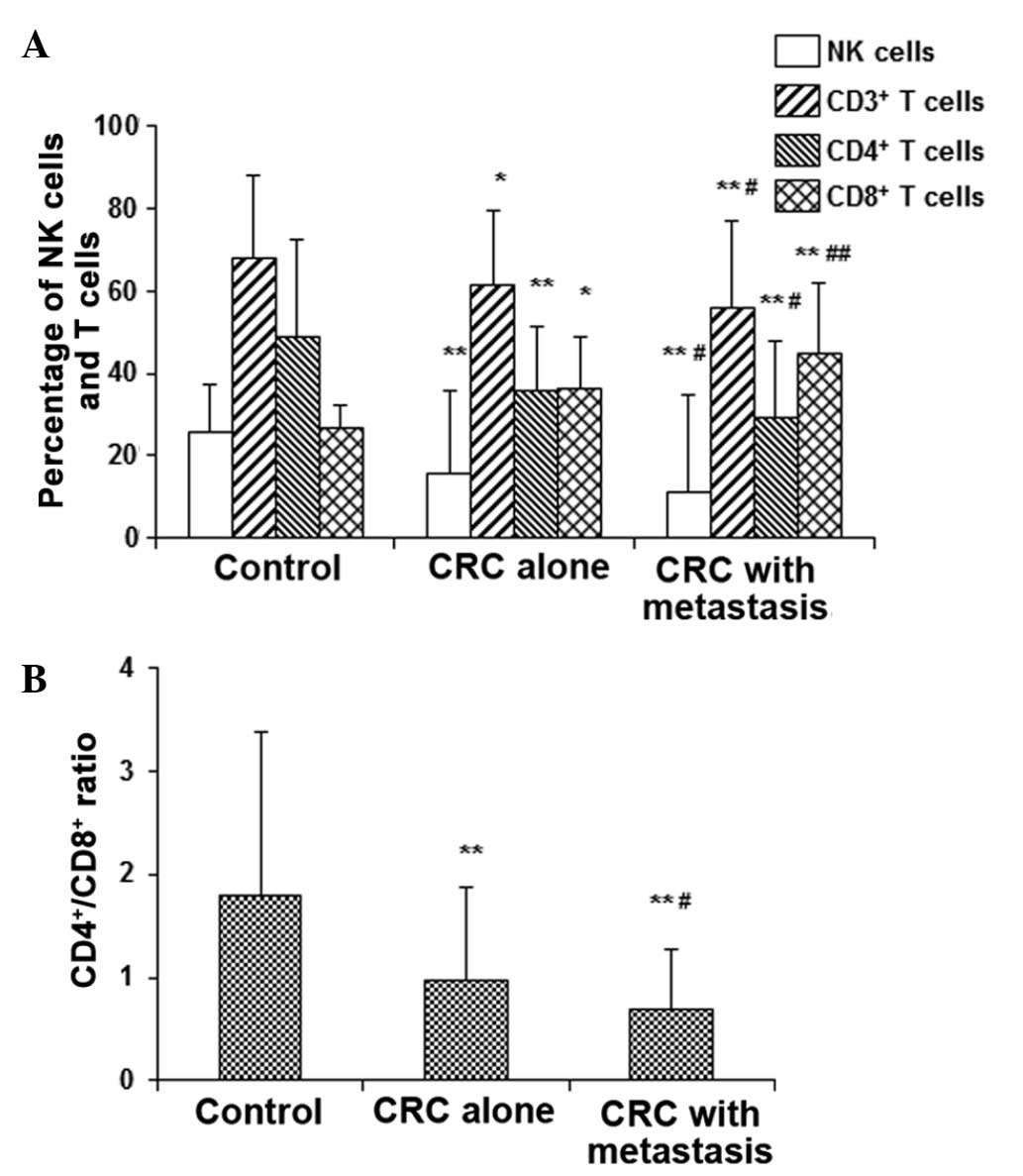
NK and T-cell subsets are changed in
peripheral blood in CRC
To investigate the anti-tumor response in CRC, the
NK cells and T-lymphocyte subsets were detected with flow
cytometry. The results revealed that, compared with the control
group, the percentage of NK, CD3+ T and CD4+
T cells in the peripheral blood of CRC patients were significantly
decreased, while the CD8+ T cells were significantly
increased (P<0.05 and P<0.01; Fig.
5A), resulting in significantly decreased
CD4+/CD8+ ratios (P<0.01; Fig. 5B). Furthermore, the alterations were
significantly more marked in the CRC patients with lymph node
metastasis compared with those without lymph node metastasis
(P<0.05). Thus, the results reveal declined anti-tumor responses
in CRC patients, which is worse in cases with lymph node metastasis
compared with those without metastasis.
Discussion
In the present study, the expression levels of
MAP4K4 and the upstream miRNA-141 in the tumor tissues, lymph nodes
and serum in patients with CRC with or without lymph node
metastasis were investigated. In addition, the anti-tumor responses
(as indicated by the NK cells and T-lymphocyte subsets) were also
detected. The underlying mechanisms for the lymph node metastasis
of CRC and the changes in the immune system, particularly those
concerning the regulation of MAP4K4 by miRNA-141, were also
discussed.
In 2011, >12 million cases were diagnosed with
new-onset CRC worldwide, ranking third in terms of incidence in
cases of malignant tumors in males, and only second to breast
cancer in females (17,18). The primary treatment for CRC is
surgical resection, combined with radiotherapy and chemotherapy, as
well as molecular targeted therapy. However, the surgical cure
rates and the postoperative survival rates are not satisfactory
(19,20). The 5-year survival rate of CRC is
associated with the local infiltration and regional lymph node
metastasis. According to the American Joint Committee on Cancer
(AJCC), CRC may be divided into stages II and III, based on the
presence or absence of regional lymph node metastasis (21). Dukes' staging (stages B and C) is
also associated with the status of regional lymph node metastasis
(22). Therefore, it is of great
importance to screen for lymph node metastasis and to investigate
the early-diagnosis molecular indicators for CRC.
The role of MAP4K4 in the pathogenesis of CRC was
investigated in the present study. Dhillon et al (23) reported that MAP4K4 was able to
activate p38 stress-activated protein kinase to enhance tumor
proliferation. Wright et al (12) indicated that MAP4K4 was able to
promote the malignant transformation, the colony formation and
increase cell invasion and metastasis. In addition, using siRNA
technology, Collins et al (11) demonstrated that the knockdown of
MAP4K4 was able to inhibit the invasion of ovarian cancer cells. In
accordance with the aforementioned findings, the results of the
current study showed that the expression levels of MAP4K4 were
significantly elevated in the tumor and lymph nodes in CRC,
indicating that MAP4K4 may promote the pathogenesis and development
of tumors, and regulate tumor proliferation and invasion.
Furthermore, the mRNA and protein expression levels of MAP4K4 in
the serum were also significantly elevated in CRC patients. As the
blood circulation is an important factor for tumor metastasis, the
current results suggest that MAP4K4 may contribute to the
metastasis of CRC via the blood circulation.
miRNA is able to regulate the mRNAs of target genes
that may serve an important role in the development and progression
of tumors (24,25). It has been reported that a class of
endogenous, small non-coding miRNAs may act upon the mRNA of MAP4K4
and inhibit its translation (26).
To further evaluate the regulating mechanisms of MAP4K4 in CRC,
bioinformatics analysis was performed. The results revealed that
miRNA-141, which has previously been confirmed to serve a role in
the occurrence and development of pancreatic cancer (16), may be the upstream regulator for
MAP4K4 in CRC. Furthermore, compared with CRC patients without
lymph node metastasis, the expression levels of miRNA-141 were
significantly higher in the tumor tissues, lymph nodes, and serum
in cases with lymph node metastasis. These results suggest that the
downregulation of miRNA-141 may be associated with the
proliferation, infiltration and metastasis of CRC. According to the
present results concerning MAP4K4 in CRC, the upregulation of
MAP4K4 may be associated with the downregulation of miRNA-141 in
the development and progression of CRC. Considering the association
between miRNA-141 and MAP4K4, as well as the important role of
MAP4K4 in tumorigenesis, miRNA-141 expression (particularly in the
serum) may be used as an indicator of CRC metastasis. The regional
lymph node metastasis of CRC is known to result from the local
tumor invasion; in addition, distant metastasis and/or metastasis
into other organs is also observed (27,28).
Accordingly, the downregulation of miRNA-141 may also contribute to
the tumor dissemination into other organs and tissues.
In order to investigate the effects of miRNA-141 on
the immune system, the NK and T cells in the peripheral blood of
CRC patients were detected. The results demonstrated that the
percentages of CD3+ and CD4+ T cells were
significantly decreased, whereas the percentage of CD8+
T cells was significantly increased in CRC patients, resulting in a
decreased CD4+/CD8+ ratio. Changes in the
immune system were more evident in cases with lymph node
metastasis, compared with the CRC patients without lymph node
metastasis. NK cells and T lymphocytes represent the initial
defense against tumors, thus, their dysfunction would weaken tumor
resistance. The elevation in CD8 may result from the T lymphocytes
induced by the tumor-secreted immunosuppressive factors (29,30),
which may disturb the immune surveillance and facilitate the
proliferation and metastasis of tumors. Therefore, these results
suggest that the alterations in the immune system may be associated
with the changes in miRNA-141 and MAP4K4 expression levels, which
may contribute to the pathogenesis of CRC. Considering the various
factors associated with the pathogenesis of CRC (31–33), and
due to the limited number of subjects enrolled in the present
study, further in-depth studies are required to investigate the
detailed mechanisms through which miRNA-141 and MAP4K4 are involved
in the development of CRC.
In conclusion, the current results showed that the
mRNA and protein expression levels of MAP4K4 were elevated in the
tumor tissues, lymph nodes and serum of CRC patients. The
expression levels of MAP4K4 were higher in CRC patients with lymph
node metastasis compared with those in patients without metastasis.
Furthermore, the expression levels of miRNA-141 were reduced in the
tumor tissues, lymph nodes and serum in CRC, with a more evident
decline observed in cases with lymph node metastasis. In addition,
the percentage of NK, CD3+ T, and CD4+ T
cells was significantly decreased and that of CD8+ T
cells was significantly increased in the peripheral blood of the
CRC patients, resulting in significantly decreased
CD4+/CD8+ ratios. The aforementioned findings
may contribute to an improved understanding of the pathogenesis of
CRC and provide evidence for the application of therapies involving
miRNA-141.
Acknowledgements
The present study was supported by the Key Projects
of Medical Sciences from the Hebei Provincial Department of Health
(grant no. 20150769).
References
|
1
|
Wan DS: Epidemiological trend and control
strategy of colorectal cancer in China. Zhong Hua Zhong Liu Za Zhi.
33:481–483. 2011.(In Chinese).
|
|
2
|
Thosani N, Guha S and Singh H: Colonoscopy
and colorectal cancer incidence and mortality. Gastroenterol Clin
North Am. 42:619–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Chou WP, Shen CJ, Wang XY and Luo
J: Risk factors of postoperative recurrence after radical resection
of colon cancer. Dalian Yi Ke Da Xue Xue Bao. 35:65–67. 2013.(In
Chinese).
|
|
4
|
Yang S, Liu W, Li M, Wen J, Zhu M and Xu
S: Insulin-like growth factor-1 modulates polycomb Cbx8 expression
and inhibits colon cancer cell apoptosis. Cell Biochem Biophys. Nov
15–2014.(Epub ahead of print).
|
|
5
|
Tsuchida A, Ohno S, Wu W, Borjigin N,
Fujita K, Aoki T, Ueda S, Takanashi M and Kuroda M: miR-92 is a key
oncogenic component of the miR-17-92 cluster in colon cancer.
Cancer Sci. 102:2264–2271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Väyrynen JP, Kantola T, Väyrynen SA,
Klintrup K, Bloigu R, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ,
Tuomisto A and Mäkinen MJ: The relationships between serum cytokine
levels and tumor infiltrating immune cells and their clinical
significance in colorectal cancer. Int J Cancer. 139:112–121. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JM, Cho SJ, Oh YK, Jung HY, Kim YJ and
Kim N: Nuclear factor-kappa B activation pathway in intestinal
epithelial cells is a major regulator of chemokine gene expression
and neutrophil migration induced by Bacteroides fragilis
enterotoxin. Clin Exp Immunol. 130:59–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin SH, Kim TI, Han DS, Shin SK and Kim
WH: Thalidomide suppresses the interleukin 1beta-induced NFkappaB
signaling pathway in colon cancer cells. Ann N Y Acad Sci.
973:414–418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Royuela M, Rodríguez-Berriguete G, Fraile
B and Paniagua R: TNF-alpha/IL-1/NF-kappaB transduction pathway in
human cancer prostate. Histol Histopathol. 23:1279–1290.
2008.PubMed/NCBI
|
|
10
|
Rangaswami H and Kundu GC: Osteopontin
stimulates melanoma growth and lung metastasis through
NIK/MEKK1-dependent MMP-9 activation pathways. Oncol Rep.
18:909–915. 2007.PubMed/NCBI
|
|
11
|
Collins CS, Hong J, Sapinoso L, Zhou Y,
Liu Z, Micklash K, Schultz PG and Hampton GM: A small interfering
RNA screen for modulators of tumor cell motility identifies MAP4K4
as a promigratory kinase. Proc Natl Acad Sci USA. 103:3775–3780.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wright JH, Wang X, Manning G, LaMere BJ,
Le P, Zhu S, Khatry D, Flanagan PM, Buckley SD, Whyte DB, et al:
The STE20 kinase HGK is broadly expressed in human tumor cells and
can modulate cellular transformation, invasion and adhesion. Mol
Cell Biol. 23:2068–2082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang B, Shen ZL, Gao ZD, Zhao G, Wang CY,
Yang Y, Zhang JZ, Yan YC, Shen C, Jiang KW, et al: MiR-194,
commonly repressed in colorectal cancer, suppresses tumor growth by
regulating the MAP4K4/c-Jun/MDM2 signaling pathway. Cell cycle.
14:1046–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bureau of Medical Administration, National
Health; Oncology Branch of Chinese Medical Association, .
Standardization of diagnosis and treatment for colorectal cancer in
China (2015 edition). Zhonghua Xiaohua Waike Zazhi. 14:783–799.
2015.(In Chinese).
|
|
15
|
Grimholt RM, Urdal P, Klingenberg O and
Piehler AP: Rapid and reliable detection of α-globin copy number
variations by quantitative real-time PCR. BMC Hematol. 14:42014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao G, Wang B, Liu Y, Zhang JG, Deng SC,
Qin Q, Tian K, Li X, Zhu S, Niu Y, et al: miRNA-141, downregulated
in pancreatic cancer, inhibits cell proliferation and invasion by
directly targeting MAP4K4. Mol Cancer Ther. 12:2569–2580. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franko J, Shi Q, Goldman CD, Pockaj BA,
Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR and Sargent
DJ: Treatment of colorectal peritoneal carcinomatosis with systemic
chemotherapy: A pooled analysis of north central cancer treatment
group phase III trials N9741 and N9841. J Clin Oncol. 30:263–267.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akkoca AN, Yanik S, Ozdemir ZT, Cihan FG,
Sayar S, Cincin TG, Cam A and Ozer C: TNM and modified dukes
staging along with the demographic characteristics of patients with
colorectal carcinoma. Int J Clin Exp Med. 7:2828–2835.
2014.PubMed/NCBI
|
|
23
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Gen. 8:93–103. 2007. View
Article : Google Scholar
|
|
25
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao X, Mohan R, Özcan S and Tang X:
MicroRNA-30d induces insulin transcription factor MafA and insulin
production by targeting mitogen-activated protein 4 kinase 4
(MAP4K4) in pancreatic β-cells. J Biol Chem. 287:31155–31164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iguchi N, Takeda Y, Sato N, Ukichi K,
Katakura A, Ueda K, Narushima T, Higuchi S and Ogasawara K: The
antihistamine olopatadine regulates T cell activation in palladium
allergy. Int Immunopharmacol. 35:70–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quan H, Fang L, Pan H, Deng Z, Gao S, Liu
O, Wang Y, Hu Y, Fang X, Yao Z, et al: An adaptive immune response
driven by mature, antigen-experienced T and B cells within the
microenvironment of oral squamous cell carcinoma. Int J Cancer.
138:2952–2962. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin B: Cell and molecular immunology.
World Book Publishing Company; Beijing: 1995
|
|
30
|
Bi A: Medical immunology. People's
Publishing House; Beijing: 1996
|
|
31
|
Wan LY, Deng J, Xiang XJ, Zhang L, Yu F,
Chen J, Sun Z, Feng M and Xiong JP: miR-320 enhances the
sensitivity of human colon cancer cells to chemoradiotherapy in
vitro by targeting FOXM1. Biochem Biophys Res Commun. 457:125–132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Xing X, Liu Q, Wang Z, Xin Y, Zhang
P, Hu C and Liu Y: Hypoxia-induced autophagy reduces
radiosensitivity by the HIF-1α/miR-210/Bcl-2 pathway in colon
cancer cells. Int J Oncol. 46:750–756. 2015.PubMed/NCBI
|
|
33
|
Tian Y, Pan Q, Shang Y, Zhu R, Ye J, Liu
Y, Zhong X, Li S, He Y, Chen L, et al: MicroRNA-200 (miR-200)
Cluster Regulation by Achaete Scute-like 2 (Ascl2) impact on the
epithelial-mesenchymal transition in colon cancer cells. J Biol
Chem. 289:36101–36115. 2014. View Article : Google Scholar : PubMed/NCBI
|