Introduction
The activation or inactivation of the Wnt signaling
pathway is orderly regulated, and is important in the regulation of
physiological processes, including cell differentiation,
proliferation and apoptosis (1). The
development, morphology, and functional maintenance of the kidney
are dependent on the normal ‘open’ and timely ‘close’ of the Wnt
signaling pathway.
Inappropriate activation of the Wnt signaling
pathway may lead to a series of disorders, including developmental
malformations, carcinogenesis, tumorigenesis, osteoporosis,
Parkinson's disease and aging (1).
Previous findings have shown that sustained activation of the Wnt
signaling pathway is commonly associated with proteinuria and
glomerulosclerosis in diabetic nephropathy (2,3).
In the present study, we investigated the potential
mechanism of the Wnt signaling pathway affecting diabetic
nephropathy and the intervening effect of Shen'an granules on the
pathway.
Animals and methods
Mice
A total of 62 male 8-week-old BALB/c mice (n=62)
(purchased from Hubei Province Animal Research Center, Hubei,
China) of SPF grade, weighing 18–22 g, were selected for this
study. The mice were housed in a temperature-controlled room (21±
2°C) on a 12:12-h light/dark cycle (lights on at 06:00). The mice
had free access to water and food. The experimental protocol was
approved by the Animal Care and Use Committee of Huazhong
University of Science and Technology.
Drugs and reagents
Shen'an granule suspension (epimedium, astragalus
and rhubarb, ratio 2:2:1) was prepared by the Hubei Provincial
Hospital of Traditional Chinese Medicine (Hubei, China). Losartan
potassium tablets (50 mg/tablet) were provided by Hangzhou MSD
Pharmaceutical Co., Ltd. (Hangzhou, China), and 1 mg/ml suspension
was made using distilled water. Streptozotocin (STZ) was purchased
from Sigma (St. Louis, MO, USA). Horseradish peroxidase
(HRP)-labeled goat anti-rabbit polyclonal antibody and HRP-labeled
goat anti-rabbit polyclonal antibody were purchased from KPL, Inc.
(Gaithersburg, MD, USA).
Generation of the mouse model of
diabetic nephropathy
After 10 days of normal feeding, the animals were
fasted for 12 h prior to the intraperitoneal injection of STZ (150
mg/kg) dissolved in citrate buffer (pH 4.5). The injection was
administered once in all the groups of mice with the exception of
the normal control group. After 72 h, blood samples were collected
from the tails, to perform a blood sugar test (ACCU-CHEK blood
glucose meter; Roche Diagnostics, GmbH, Mannheim, Germany) and 24-h
microalbuminuria test. The induction of diabetes was considered
successful when blood sugar was >16.17 mmol/l. The same amount
of sterile citrate buffer was injected intraperitoneally in mice of
the control group. The mice were then fed with a high-fat diet for
one month, and the blood sugar test and 24-h microalbuminuria test
were repeated. The establishment of the mouse model of diabetic
nephropathy was confirmed with the blood sugar as >16.17 mmol/l
and the quantification of 24-h urinary albumin increased to
>150%.
Animal grouping and drug
administration
Sixty-two animals were randomly divided into five
groups: control (group A, n=12), model (group B, n=12), losartan
(group C, n=13), low-dose Shen'an granules (group D, n=13) and
high-dose Shen'an granules (group E, n=13). For drug
administration, mice in the control and model groups were given 10
ml/(kg·day) distilled water by gavage, mice in the Western medicine
group were administered 10 ml/(kg·day) losartan suspension by
gavage, and mice in the high- and low-dose Chinese medicine group
were administered 4 ml/(kg·day) and 8 ml/(kg·day) Shen'an granules
suspension, respectively. The mice were treated with drugs for 8
weeks.
Sample collection
Prior to and after drug treatments, the blood
samples were collected from the retro-orbital plexus, and 24-h
urine samples were collected for biochemical indices. After the
drug treatment, kidneys without renal capsules were collected and
weighed on an analytical balance. The left kidney was rapidly
removed and a part of the renal cortex was cut into small sections
and fixed in 4% paraformaldehyde for pathological examinations,
while the right kidney was saved at −70°C for subsequent use.
Biochemical tests
Coomassie Brilliant Blue assay (Guge Biological
Technology Co., Hubei, China) was used for 24-h microalbuminuria.
An AU600 Biochemistry Analyzer (Olympus Corp., Tokyo, Japan) was
used to measure serum creatinine (SCr), blood urea nitrogen (BUN),
triglycerides (TG) and total cholesterol (CHOL).
Western blot assay for Wnt1 and
β-catenin protein
Total protein was extracted from a part of the renal
cortex and the protein concentration was determined using a
Bradford protein assay. SDS-acrylamide gel (Guge Biological
Technology Co.) was created and separated proteins were transferred
to a membrane. The membrane was blocked in 5% skim milk at 24°C for
1 h, incubated with primary polyclonal antibodies Wnt1 (Abcam,
Cambridge, MA, USA, cat no.: ab15251; dilution, 1:5000) and
β-catenin (Abcam, cat no.: ab32572; dilution, 1:5000) at 4°C
overnight followed by three washes in TBST for 5 min each. The
membrane was then incubated with the secondary polyclonal antibody
(Abcam, cat. no.: ab6721; dilution, 1:2000 for 30 min at 24°C,
washed with TBST 3 times for 5 min each and protein bands were
detected with DAB. The blot was scanned and optical density values
of the targeted bands were analyzed with Alpha software (Chicago,
IL, USA).
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed on paraffin-embedded mouse
renal cortex sections. In detail, total RNA was extracted by RNA
Isolation Kit (Biosharp, Hebei, China). Dnase I-treated total RNA
(10 µg) was used for reverse transcription with Superscript III
(Invitrogen, Carlsbad, CA, USA). Input RNA (100 ng) was amplified
by RT-PCR using the TaqMan PCR reagent kit and assay-on-demand gene
expression products. After reverse transcription, standard cDNA was
serially diluted to five standard solutions to prepare the
reference curve. RT-qPCR was carried out using the Rotor-Gene 3000
Real-Time PCR kit (Corbett Research, Sydney, Australia) with
SYBR-Green (1:1,000; Molecular Probes, Houston, TX, USA). After
reverse transcription, standard cDNA was serially diluted to five
standard solutions to prepare the standard curve. The relative
amount of cDNA in each sample was measured by interpolation using
the standard curve, and the relative ratio of β-catenin and Wnt1 to
β-actin expression was calculated for each sample. The primers are
as shown in Table I.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Gene | Primer sequences |
|---|
| β-catenin | F:
5′-AAAATGGCAGTGCGTTTAG 3′ |
|
| R:
5′-TTTGAAGGCAGTCTGTCGTA-3′ |
| Wnt1 | F:
5′-CAGAGCCACGAGTTTGGATGTT-3′ |
|
| R:
5′-GATTGGGTTGGGTTGGAGGTAA-3′ |
| β-actin | F:
5′-CCTGTACGCCAACACAGTGC-3′ |
|
| R:
5′-ATACTCCTGCTTGCTGATCC-3′ |
Statistical analysis
Data were analyzed using SPSS 17.0 statistical
software (Chicago, IL, USA) and presented as mean ± standard
deviation. Comparisons between groups were analyzed with ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Shen'an granules treatment decreases
urine albumin excretion
The urine albumin excretion of groups C-E was
significantly lower compared to group B (model group) (Table II; p<0.05). There were no
significant differences between group C and the lower (group D) or
higher (group E) Shen'an granules group (p>0.05). The results
suggested that the Shen'an granules treatment significantly
decreased urine albumin excretion, effects that were equal to those
of the losartan treatment (group C).
 | Table II.Measurements of 24-h urine albumin
excretion prior to and after the drug treatment (mean ± SD). |
Table II.
Measurements of 24-h urine albumin
excretion prior to and after the drug treatment (mean ± SD).
|
|
| Measurements of 24-h
urine albumin excretion (µg) |
|---|
|
|
|
|
|---|
| Group | n | Before | After |
|---|
| A | 12 | 26.46±8.45 | 25.00±3.39 |
| B | 10 |
272.80±70.45a | 312.00±8.37 |
| C | 11 |
244.00±32.86a |
183.20±12.44b |
| D | 13 |
238.00±37.68a |
185.40±15.65b |
| E | 12 |
244.00±33.62a |
178.00±14.83b |
Shen'an granules treatment decreases
the BUN and SCr level
Prior to the drug treatment, the levels of BUN and
SCr in groups C-E were significantly higher than those in group A
(Table III; p<0.05). However,
no difference was found in groups B-E (p>0.05). The levels of
BUN and SCr following the Shen'an granules treatment (groups D and
E) were significantly lower compared with levels prior to the drug
treatment (group B, p<0.05). Furthermore, the BUN and SCr levels
were similar between the losartan treatment group (group B) and the
Shen'an granules treatment group (Table III; p>0.05).
 | Table III.Measurements of BUN and SCr values
before and after the drug treatment (mean ± SD). |
Table III.
Measurements of BUN and SCr values
before and after the drug treatment (mean ± SD).
|
|
| BUN (mmol/l) | SCr (µmol/l) |
|---|
|
|
|
|
|
|---|
| Group | n | Before | After | Before | After |
|---|
| A | 12 | 10.57±1.64 | 12.80±0.84 | 12.07±1.38 | 14.20±1.48 |
| B | 12 |
24.56±1.89a | 30.98±6.77 |
45.41±7.31a | 52.80±3.96 |
| C | 12 |
20.86±2.76a |
15.78±0.90b |
48.20±2.86a |
26.20±1.10b |
| D | 13 |
20.20±1.92a |
15.62±1.06b |
46.52±2.86a |
26.40±2.30b |
| E | 13 |
19.78±1.81a |
13.80±1.30b |
44.80±2.39a |
22.80±1.79b |
Shen'an granules treatment increases
TG level and decreases the CHOL level
To investigate the blood lipid levels in blood the
TG and CHOL levels were also examined. The results showed that the
Shen'an granules treatment (groups D and E) significantly increased
the TG level compared to group B (model group) (Table IV; p<0.05). Furthermore, the
Shen'an granules treatment also significantly decreased the CHOL
level compared to group B. Notably, the effects of Shen'an granules
could even achieve the level of losartan (Table IV).
 | Table IV.Measurements of CHOL and TG values
before and after the drug treatment (mean ± SD). |
Table IV.
Measurements of CHOL and TG values
before and after the drug treatment (mean ± SD).
|
|
| CHOL (mmol/l) | TG (mmol/l) |
|---|
|
|
|
|
|
|---|
| Group | n | Before | After | Before | After |
|---|
| A | 12 | 2.98±0.21 | 2.88±0.03 | 1.34±0.19 | 1.42±0.12 |
| B | 12 |
5.90±0.26a | 6.50±0.16 |
3.38±0.09a | 4.50±0.17 |
| C | 12 |
5.83±0.16a |
5.21±0.02b |
3.37±0.06a |
4.20±0.08b |
| D | 13 |
5.88±0.18a |
5.14±0.09b |
3.42±0.09a |
3.94±0.08b |
| E | 13 |
5.96±0.11a |
5.03±0.10b |
3.37±0.10a |
3.87±0.20b |
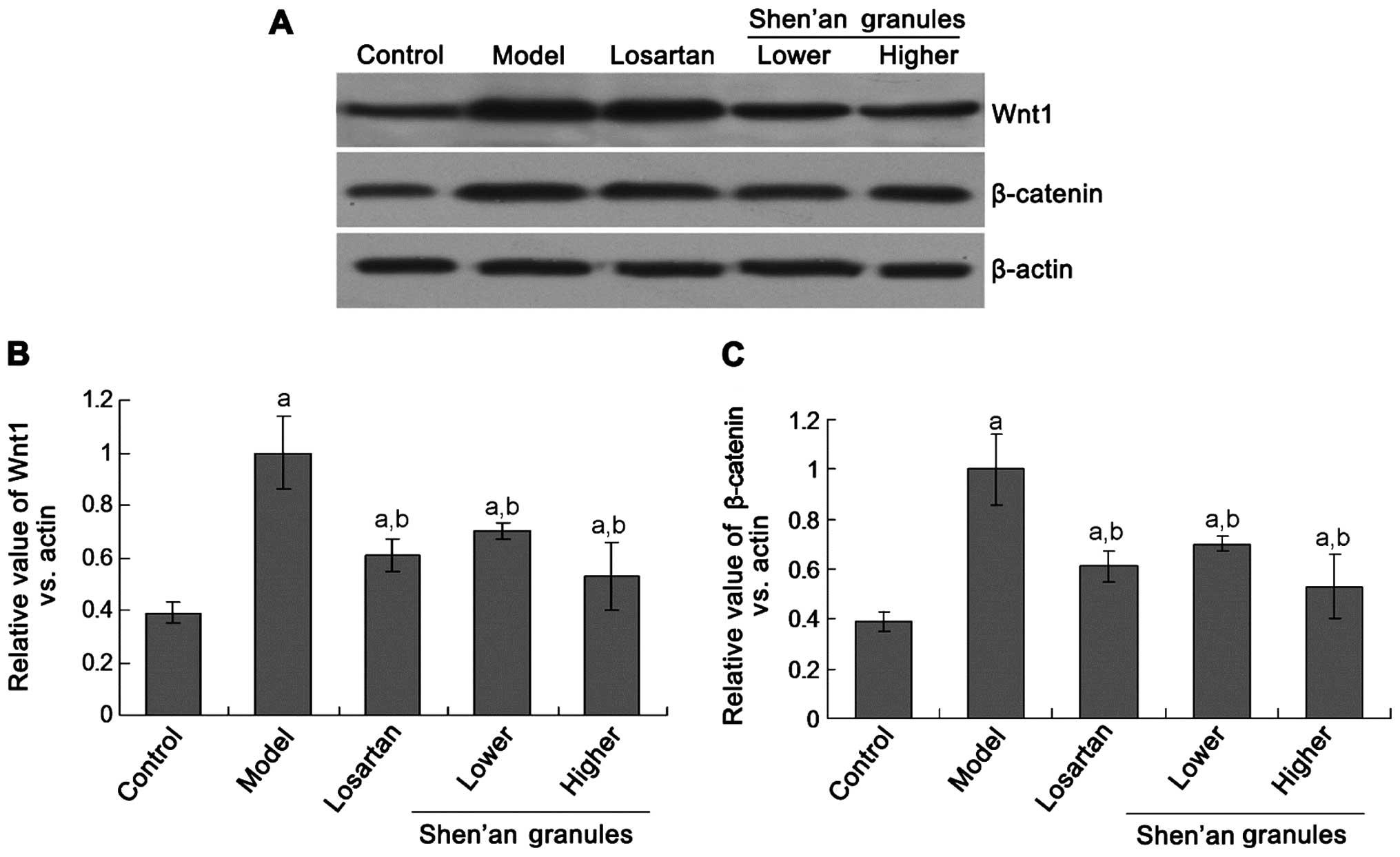
Shen'an granules inhibits the Wnt1 and
β-catenin protein expression
To investigate the mechanism of the effects of
Shen'an granules treatment, the Wnt pathway molecule was detected.
The western blot assay showed that Wnt1 protein expression in
groups D and E was significantly decreased compared to group B
(Fig. 1; p<0.05). Additionally,
there was no significant differences between the Shen'an granules
treatment group (groups D and E) and the losartan treatment group
(group C) (p>0.05). There were also no significant differences
between the lower (group D) and higher (group E) Shen'an granules
treatment group (p>0.05).
The β-catenin protein expression was significantly
decreased in groups D and E compared to group B (Fig. 1; p<0.05). There were no
significant differences between groups D and E, and group C
(p>0.05). Furthermore, there were no significant differences
between groups D and E (p>0.05).
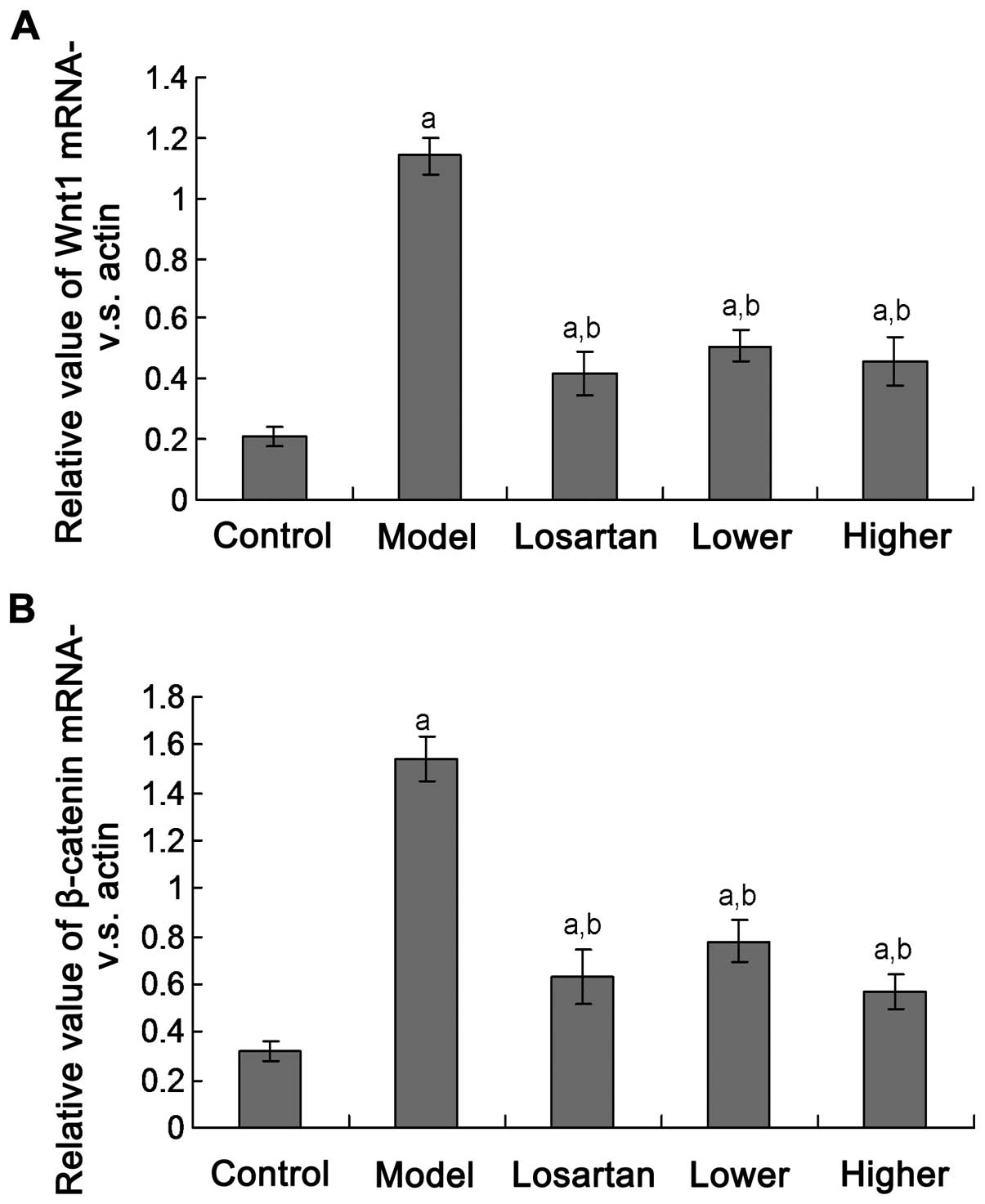
Shen'an granules suppress Wnt1 and
β-catenin protein expression
Wnt1 and β-catenin mRNA transcription were examined
using the PCR assay. The results indicated that the Wnt1 and
β-catenin mRNA expression levels were significantly decreased in
groups D and E compared to group B (Fig.
2; p<0.05). There were no significant differences between
groups D and E, and group C (p>0.05). There were also no
significant differences between groups D and E (p>0.05).
Discussion
Diabetic nephropathy is a glomerular sclerosis
caused by the abnormal metabolism of diabetes mellitus, and
constitutes an important component of systemic microangiopathy
(4). In China, diabetic nephropathy
is second only to glomerular disease as the leading cause of
end-stage renal disease (ESRD), and constitutes an important cause
for mortality in diabetes. Compared with non-diabetic nephropathy,
renal insufficiency in diabetic nephropathy features higher
proteinuria, and more severe edema. These features may be
associated with podocyte injury and damage of the glomerular
filtration barrier, leading to large amounts of protein leaking
into urine, decreased body protein level and reduced plasma colloid
osmotic pressure, which result in gradual aggravation of edema
(5).
In diabetic nephropathy, the canonical Wnt signaling
pathway is abnormally activated (6–8). In
microdissected human kidney samples collected from patients with
diabetic nephropathy, FSGS, and IgA nephropathy, microarray
analysis has shown that the Wnt/β-catenin pathway is a continuously
upregulated pathway (9). The
sustained activation of this pathway in podocytes leads to the
development of proteinuria and glomerular sclerosis. Dai et
al successfully induced the accumulation of β-catenin in
podocytes by intravenous injection of a Wnt1-expressing plasmid in
mice, and a transient proteinuria and podocyte foot process
effacement occurred shortly after the stable expression of
β-catenin (10). Kato et al
found that at 20 weeks, the heterozygous mice
(NPHS2cre/Ctnnb1FloxE3/WT) with a stable β-catenin expression in
podocytes exhibited mild mesangial proliferation and proteinuria,
as well as diffuse and irregular thickening of the glomerular
basement membrane observed by electron microscopy (3). By contrast, the homozygous mice with
stable β-catenin expression exhibited prominent thickening of the
glomerular basement membrane and a large amount of proteinuria and
glomerular sclerosis.
Previous findings showed that a sustained high
expression of β-catenin causes podocyte epithelial-mesenchymal
transition (EMT), increased expression of Snail, inhibition of
E-cadherin, and a decreased expression of the podocyte marker
nephrin in vitro and in vivo (3,10,11).
Mouse models and podocytes treated with a Wnt signaling pathway
inhibitor exhibited improved cell survival, decreased cell-matrix
adhesion, increased mobility, and reduced migration. In a previous
study, mouse urine samples were tested and large numbers of shedded
podocytes were found, supporting that the abnormal activation of
Wnt/β-catenin signaling pathway reduces podocyte adhesion,
resulting in proteinuria and glomerular sclerosis (3). At the time, when the expression of
podocyte marker nephrin is altered, the cells obtain the expression
of mesenchymal cell markers, such as matrix metalloproteinase-7
(MMP-7), and the process of EMT leads to an increased mobility of
podocytes and to a certain degree, damages the structural integrity
of the filtration barrier, and eventually results in the leakage of
large amounts of protein into urine (12). The mechanism may be associated with
the interaction between β-catenin and integrin β1,
calmodulin-dependent protein kinase II, or angiotensin II (13). These phenotypes are similar to those
exhibited in podocyte-specific Ilk, Ddr1,
Itgb1 and Itga3 gene-knockout animal models,
suggesting that these genes may be components of the same signaling
pathway (14,15).
The basic pathogenesis of diabetic nephropathy is
congenital insufficiency of kidney essence, and spleen-kidney dual
deficiency. Dysfunction of kidney for the activation of Qi,
hypofunction of ascending lucidity caused by spleen deficiency, and
non-consolidation of essences lead to large amounts of proteins
while other essences leak into urine (16). Spleen-kidney yang deficiency causes
failure of moist evaporation and water transportation, internal
stagnation and diffusion of fluid-dampness, which eventually result
in edema. Long-term stagnation of fluid-dampness may produce phlegm
and stasis, and the accumulation of phlegm turbid in turn further
affects the ascending and descending of vital Qi, aggravating the
leakage of protein and other essences (17). Shen'an granules are composed of
astragalus, epimedium, and rhubarb at a ratio of 2:2:1. Although
the formula only contains three ingredients, it is established
through long-term clinical practice and has definite efficacy. In
the formula, astragalus can strengthen the spleen and ascend the
clear, which induce diuresis in order to alleviate edema; epimedium
can warm kidney and protect semen, thus improving Qi transformation
and inducing diuresis (18). The
combination of the two herbs replenishes both inborn and acquired
deficiency, and replenishes, and consolidates vital essences,
leading to the recovery of the function of spleen and kidney on the
regulation of fluid-dampness. Furthermore, the wine-processed
rhubarb of only half the amount of astragalus and epimedium can
eliminate side effects and maintain beneficial effects, resolve
stasis and turbidity without damaging the vital Qi, and also clears
the stasis and turbidity from excrement (19,20). The
combination of the three herbs can eliminate side effects and
maintain beneficial effects, nourish the basics of spleen-kidney,
recover their functions, consolidate the essences, remove stasis
and turbidity, and replenish the vital essence, leading to the
effective relief of symptoms, such as proteinuria and edema, in
patients with diabetic nephropathy.
The results of the present study have shown that the
expression levels of Wnt1 and β-catenin proteins in the model group
were significantly lower than those in the control group, and the
expression was positively correlated with SCr, urea nitrogen, CHOL,
and TG. Different concentrations of Shen'an granules can inhibit
the expression of Wnt1 and β-catenin proteins, and decrease levels
of 24-h urine albumin, SCr, BUN, CHOL, and TG, thereby reducing
urinary protein, improving renal function, and regulating lipid
metabolism. Experiments demonstrated that Shen'an granules reduce
urinary protein, improve renal function, and regulate lipid
metabolism by inhibiting the abnormal activation of the Wnt signal
transduction pathway, thereby delaying the progression of diabetic
nephropathy. The study provided experimental basis for Wnt signal
transduction pathway as a new target for the treatment of diabetic
nephropathy using Chinese traditional medicines.
Acknowledgements
The present study was granted by the Natural Science
Foundation of Hubei Province (grant no. 2010CDA033).
References
|
1
|
Chen C, Lu Y, Liu J, Li L, Zhao N and Lin
B: Genome-wide ChIP-seq analysis of TCF4 binding regions in
colorectal cancer cells. Int J Clin Exp Med. 7:4253–4259.
2014.PubMed/NCBI
|
|
2
|
Kawakami T, Ren S and Duffield JS: Wnt
signalling in kidney diseases: dual roles in renal injury and
repair. J Pathol. 229:221–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato H, Gruenwald A, Suh JH, Miner JH,
Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB and
Susztak K: Wnt/β-catenin pathway in podocytes integrates cell
adhesion, differentiation, and survival. J Biol Chem.
286:26003–26015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Auinger M, Edlinger R, Prischl F,
Kautzky-Willer A, Prager R, Rosenkranz AR, Roden M, Saemann M,
Clodi M and Schernthaner G: [Diabetic nephropathy - update 2012].
Wien Klin Wochenschr. 124:Suppl 2. 42–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalla VM, Masiero A, Roiter AM, Saller A,
Crepaldi G and Fioretto P: Is podocyte injury relevant in diabetic
nephropathy? Studies in patients with type 2 diabetes. Diabetes.
52:1031–1035. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou T, He X, Cheng R, Zhang B, Zhang RR,
Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, et al: Implication of
dysregulation of the canonical wingless-type MMTV integration site
(WNT) pathway in diabetic nephropathy. Diabetologia. 55:255–266.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He W, Dai C, Li Y, Zeng G, Monga SP and
Liu Y: Wnt/β-catenin signaling promotes renal interstitial
fibrosis. J Am Soc Nephrol. 20:765–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He W, Kang YS, Dai C and Liu Y: Blockade
of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria
and kidney injury. J Am Soc Nephrol. 22:90–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woroniecka KI, Park AS, Mohtat D, Thomas
DB, Pullman JM and Susztak K: Transcriptome analysis of human
diabetic kidney disease. Diabetes. 60:2354–2369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai C, Stolz DB, Kiss LP, Monga SP,
Holzman LB and Liu Y: Wnt/beta-catenin signaling promotes podocyte
dysfunction and albuminuria. J Am Soc Nephrol. 20:1997–2008. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iglesias DM, Hueber PA, Chu L, Campbell R,
Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D and
Goodyer PR: Canonical WNT signaling during kidney development. Am J
Physiol Renal Physiol. 293:F494–F500. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF
and Liu Y: Matrix metalloproteinase-7 as a surrogate marker
predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol.
23:294–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, Xu L, Song Y, Li J, Mao J, Zhao
AZ, He W, Yang J and Dai C: Calmodulin-dependent protein
kinaseII/cAMP response element-binding protein/Wnt/beta-catenin
signaling cascade regulates angiotensin II-induced podocyte injury
and albuminuria. J Biol Chem. 288:23368–23379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pozzi A, Jarad G, Moeckel GW, Coffa S,
Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, et al:
Beta1 integrin expression by podocytes is required to maintain
glomerular structural integrity. Dev Biol. 316:288–301. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin Y, Wang L, Duan Q, Gong Z, Yang F and
Song Y: Differential expression of 5-HT-related genes in
symptomatic pulmonary embolism patients. Int J Clin Exp Med.
8:512–518. 2015.PubMed/NCBI
|
|
16
|
Lv J, Wang YX and Liu YN: Exploration of
heat treatment for diabetic nephropathy from the inflammation
pathogenesis. Chin J Integr Trad Western Nephrol. 15:60–61.
2014.(In Chinese).
|
|
17
|
Wang YH, Pan Z and Wang YX: Molecular
pathological basis ofabdominal mass in Shen collaterals. J Beijing
Univ Trad Chin Med. 29:301–303. 2006.(In Chinese).
|
|
18
|
Chen LJ, Wang XQ and Ping AY: Influence of
kidney-qi-nourishing therapy on T lymphocyte subgroup of spleen in
natural abortion model mice. Chin Archives Trad. Chin Med.
2012.
|
|
19
|
Qian H, Yang JJ, Pan DY, Tang WT, Xu KJ
and Qi MY: [Protective effectof total flavonoids of epimedium on
the kidney in experimental diabetic rats]. Zhongguo Ying Yong Sheng
Li Xue Za Zhi. 30:314–317. 2014.(In Chinese). PubMed/NCBI
|
|
20
|
Ye D, Wu Z, Zhang T and Yue Y:
Experimental study of rhubarb and epimedium decoction in
counteracting hearing impairment due to chronic renal failure. Acta
Univ Trad Med Sin Pharmacol Shanghai. 1998.
|