Introduction
Dental implants have become one of the most
effective ways to treat dental defects and repair dental system
function (1). The key to success of
dental implants and restoration is forming a stable
osseointegration between implants and bone interfaces, namely,
reliable physical and chemical bonding (2).
Titanium (Ti), with its excellent mechanical
properties and biocompatibility, is the most widely used implant
material, but it is biologically inert (3). After implantation, peripheral tissues
show a non-specific reaction and a random property (4). It needs a long healing period to form a
functional restoration. As a new restoration material, nano
tantalum (Ta) has been confirmed as optimal for the proliferation,
differentiation and mineralization of osteoblasts (5). Nano structure is better for the growth
of the osteoblasts than a micron structure (6). By analyzing the effects of Ta implants
on inducing osteoblast proliferation and differentiation, the
current study lays a theoretical foundation for clinical
application.
Materials and methods
Cell recovery, passage and
preservation
Supplies included MG-63 osteoblasts (Cell Bank of
Chinese Academy of Science, Shanghai, China), 0.25% trypsin (Gibco
Services, Inc., Amarillo, TX, USA) and Dulbecco's modified Eagle's
medium (DMEM) culture medium containing double-antibody, 10% calf
serum and NaHCO3 (Gibco Services, Inc.). We also used
micropipettes (Gilson, Inc., Villiers-le-Bel, France), an HB2460
super-clean bench, Micro Centaur desk centrifuge (MSE Limited,
London, England) and a phase contrast microscope (Nikon, Tokyo,
Japan). The cells were processed with conventional recovery,
passage and preservation.
Experimental grouping
Groups were divided into the osteoblast culture
group (the control group), osteoblast and Ti implant co-culture
group (Ti group), and osteoblast and Ta implant co-culture group
(Ta group). A grade 4 commercially pure Ti sheet of 10×1.5 mm
(Baoji Lihua Non-ferrous Metals Co., Ltd., Baoji, China) and a Ta
sheet of 10×1.5 mm (Zimmer Biomet, Warsaw, IN, USA) were used for
treatments. A pre-treatment and heat treatment of Ti and Ta sheets
were performed. Anodic oxidation under 10 V for 2 h was conducted.
A concentration of MG-63 osteoblasts was regulated to
2.5×104/ml. A cell suspension of 1 ml was added into
separate wells and placed in a culture plate with 12 wells. This
was then cultured with conventional fetal bovine serum DMEM culture
medium and incubated in a CO2 incubator. The culture
medium was changed every 2 days.
Cell growth on the material
surface
We used 25% glutaric acid to fix samples washed with
phosphate-buffered saline (PBS). These were then dehydrated with
gradient alcohol and desiccated to a critical point before being
sprayed on a vacuum surface. Growth, quantities and morphological
changes of cells on the material surface were observed through a
scanning electron microscope (SEM) (Olympus, Tokyo, Japan).
Cell proliferation at 1, 3 and 7 days
tested by 3-(4,5-dimethylthiazolyl-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) method
We added MTT solution into the well of the 12-well
plate (5 mg/ml) with 20 µl/well. The samples were incubated at 37°C
for 4 h, followed by terminating the culture. The supernatant was
discarded without destroying the blue crystal layer attached to the
material surface. Dimethyl sulfoxide of 150 µl was added into each
well and placed at 25°C. The material was oscillated for 20 min
until the crystals were fully dissolved. The material was placed
into a 96-well plate, with zero modulation in distilled water. A
selected wavelength of 490 nm on an ELISA reader (PerkinElmer,
Inc., Waltham, MA, USA) was used to determine the value of light
absorption [optical density (OD) value]. We measured 5 wells/group
and took the mean values of each one.
Alkaline phosphatase (ALP) level at 1,
3 and 7 days tested by ELISA method
We transferred samples of various groups into a new
24-well plate, using 0.01 mol/l PBS to wash 3 times and drain. We
then added 50 µl Triton X-100 (1 g/l) into each well, leaving it
overnight at 4°C after repeated pipetting. A substrate in ALP kits
was added at 100 µl/well. The material was placed in an incubator
for 30 min at 37°C before adding 0.2 moI/l NaOH of 50 µl to
terminate the reaction. A selected wavelength of A410 nm on an
ELISA reader was used to test the OD value and take the mean
value.
Expression level of type I collagen
protein (Col-1) and osteocalcin at 1, 3 and 7 days tested by
western blot analysis
We added RIPA lysate and PMSF liquid into cells and
set the material on ice for 10 min. Ultrasonic focalization
decomposition was performed for l min at 4°C with 12,000 × g of
liquid centrifuge for 15 min. A test concentration of protein
through Bradford colorimetry was attained and preserved at −20°C
for inspection. We added 5X SDS loading buffer into each protein
sample proportionally before heating for 5 min at 95°C and 12,000 ×
g centrifuging for 2 min. After absorbing the supernatant and
adding the gel-like substance to each well, we developed a
regulated protein concentration of 20 µl/well. We added 10 µl
pre-stained protein molecular weight marker into one of the wells
and applied electrophoresis for 30 min under 40 V with a vertical
protein electrophoresis apparatus (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A separation gel was added when reaching a dye
strip, and the voltage was changed to 60 V. Electrophoresis was
continued for 60 min under constant voltage until bromine phenol
blue rose to the bottom of the gel. At this point, we turned off
the power. Conventional transmembrane channels and the PVDF
membrane were sealed. Rabbit polyclonal anti-Col-1 antibody (cat.
no. sc-59772; dilution, 1:500), rabbit monoclonal osteocalcin
antibody (cat. no. sc-376726; dilution, 1:500) and rabbit
polyclonal β-actin (cat. no. sc-47778; dilution, 1:500) (all from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were added
into a hybrid bag. The material was oscillated and incubated
overnight at 4°C, then washed with PBS for 10 min × 5 times. A
diluted secondary antibody labeled with HRP with blocking solution
in proportion of 1:5,000 was added and oscillated for 1 h before
being washed with PBS for 10 min × 5 times Pierce ECL kits (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and Image-Pro Plus
software (Media Cybernetics, Rockville, MD, USA) were used to
develop, expose, photograph and analyze the material.
Statistical analysis
SPSS 20.0 (IBM SPSS, Armonk, NY, USA) was used for
statistical analysis. Measurement data are expressed as mean ±
standard deviation, with comparisons among groups analyzed by a
one-way ANOVA comparison through LSD between two groups. A
comparison within groups was analyzed by variance of repeated
measurement data. p<0.05 was considered to indicate a
statistically significant difference.
Results
Cell growth on the material
surface
Cells on the material surface of the control group
were insignificant 7 days after culture. The cells bulged and were
cord-like. Many cells stretched on the Ti and Ta sheet surfaces and
presented a flat shape, with many pseudopodia. However, more cells
were produced on the Ta sheet (Table
I).
 | Table I.Cell proliferation (OD value). |
Table I.
Cell proliferation (OD value).
| Groups | 1 day | 3 days | 7 days | F-value | P-value |
|---|
| Control | 0.09±0.02 | 0.10±0.03 | 0.10±0.04 | 0.532 | 0.685 |
| Ti | 0.25±0.06 | 0.36±0.08 | 0.42±0.10 | 5.324 | 0.036 |
| Ta | 0.33±0.09 | 0.42±0.12 | 0.48±0.13 | 5.865 | 0.030 |
| F-value | 5.754 | 5.968 | 6.230 |
|
|
| P-value | 0.031 | 0.028 | 0.025 |
|
|
Cell proliferation
The proliferation of cells in the Ti and Ta groups
increased as time progressed. Furthermore, the cell proliferation
rate of the Ta group increased over time when compared with the Ti
group. The difference was statistically significant (p<0.05)
(Table I).
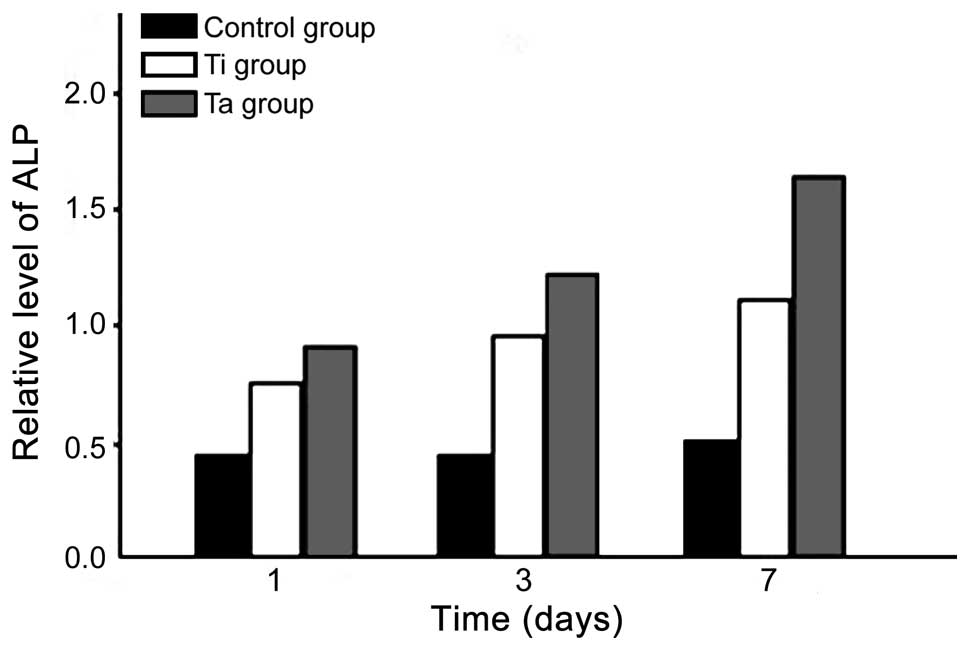
ALP level
The ALP levels of the Ti and Ta groups increased as
time progressed. The level in the Ta group was higher at various
times when compared with the Ti group. The difference was
statistically significant (p<0.05) (Fig. 2).
Expression level of Col-1 and
osteocalcin
The expression level of Col-1 and osteocalcin of the
Ti and Ta groups increased over time. The level in the Ta group was
higher at various times. The difference was statistically
significant (p<0.05) (Table
II).
 | Table II.Expression level of Col-1 and
osteocalcin. |
Table II.
Expression level of Col-1 and
osteocalcin.
|
| Col-1 | Osteocalcin |
|---|
|
|
|
|
|---|
| Groups | 1 day | 3 days | 7 days | F-value | P-value | 1 day | 3 days | 7 days | F-value | P-value |
|---|
| Control | 0.06±0.02 | 0.05±0.02 | 0.07±0.03 | 0.234 | 0.852 | 0.04±0.02 | 0.03±0.02 | 0.05±0.02 | 0.316 | 0.765 |
| Ti | 0.25±0.06 | 0.28±0.10 | 0.32±0.12 | 5.233 | 0.034 | 0.26±0.09 | 0.30±0.12 | 0.34±0.14 | 5.326 | 0.032 |
| Ta | 0.36±0.08 | 0.40±0.12 | 0.43±0.14 | 5.754 | 0.030 | 0.35±0.07 | 0.42±0.13 | 0.46±0.15 | 5.869 | 0.027 |
| F-value | 7.521 | 7.123 | 7.627 |
|
| 6.569 | 6.857 | 7.105 |
|
|
| P-value | 0.013 | 0.015 | 0.013 |
|
| 0.025 | 0.021 | 0.013 |
|
|
Discussion
Osteoblasts are the most important functional cells
in the bone remodeling process and are among the most active cells
during the formation of the implant-bone interface. Maturely
differentiated osteoblasts secrete collagen and non-collagenous
proteins in bone matrix, and the cytoplasm is basophilic (7). Those secretions can be synthesized as
membrane-bound ALP, which are related to mineralization (8). Basophilic cytoplasm can be released by
phospholipid phosphatidylinositol-specific phospholipase C, which
may be related to transmembrane signal pathways (9). Osteocalcin is the main non-collagenous
protein in the bone matrix and is now considered as a mark of the
latest expression in the osteoblasts (10).
Findlay et al compared the effects of Ta, Ti,
cobalt chrome and plastic products for culturing use on osteoblasts
(11). On day 3 of our study,
osteoblast proliferation in the plastic products was slightly
faster than that in Ta. On day 7, the Ta group was superior to
other material groups in terms of either the absolute number or
division number of cells, but there was no difference in mRNA
expression, osteoblast activity and mineralization extent. This
suggested that Ta was a good substance for attachment, development
and differentiation of osteoblasts. It also was suitable for the
proliferation of osteoblasts, not significantly different from the
plastic articles (which are usually taken as a BenchMark). Sakai
et al tested the influence of 14 kinds of metals and a
non-metallic substance on the in vitro viability of
osteoblast-like cells (12). The
study found that the influence of cells on particles depended on
the direct action between particles and cells, indirect action of
dissolved ions and the type of particle elements. The cytotoxicity
of Ta particles (<44 µm) and their extractives were low. When
cells were co-cultured with Ta and other metal particles for 3
days, the cytotoxicity was lower than that of the control group.
However, it was similar to the control group on day 6, which
illustrated that cells could be restored and proliferated after the
first damage (13). Recent studies
show that nanoparticles in the body can induce autophagy, a
double-edged sword. Nanoparticles in normal cells could induce
cytotoxicity, which should be avoided. Conversely, nanoparticles
can be used in the treatment of disease in specific cells, such as
for neurodegenerative diseases, including Parkinson's disease
(14).
There was abundant cell spreading on the surfaces of
the Ti and Ta sheets after 7 days, with flat cells and many
pseudopodia. Additionally, there were more cell components in the
Ta group. Concurrently, cell proliferation in the Ti and Ta groups
increased. The level of ALP and the expression level of Col-1 and
osteocalcin also increased over time. The indexes of the Ta group
were more apparent than those of the Ti group at each time-point,
and the differences were statistically significant (p<0.05). In
conclusion, the nano Ti implants resulted in improved proliferation
and differentiation of osteoblasts.
Acknowledgements
The present study was supported by the Scientific
Research Project for Middle-aged and Young Scientists of Shandong
Province (no. BS2013SW041) and the Natural Science Foundation of
China (no. 81271105).
References
|
1
|
Mantripragada VP, Lecka-Czernik B,
Ebraheim NA and Jayasuriya AC: An overview of recent advances in
designing orthopedic and craniofacial implants. J Biomed Mater Res
A. 101:3349–3364. 2013.PubMed/NCBI
|
|
2
|
Velasco-Ortega E, Alfonso-Rodríguez CA,
Monsalve-Guil L, España-López A, Jiménez-Guerra A, Garzón I,
Alaminos M and Gil FJ: Relevant aspects in the surface properties
in titanium dental implants for the cellular viability. Mater Sci
Eng C. 64:1–10. 2016. View Article : Google Scholar
|
|
3
|
Hotchkiss KM, Ayad NB, Hyzy SL, Boyan BD
and Olivares-Navarrete R: Dental implant surface chemistry and
energy alter macrophage activation in vitro. Clin Oral
Implants Res. 3:12–13. 2016.
|
|
4
|
Sollazzo V, Pezzetti F, Massari L,
Palmieri A, Brunelli G, Zollino I, Lucchese A, Caruso G and Carinci
F: Evaluation of gene expression in MG63 human osteoblastlike cells
exposed to tantalum powder by microarray technology. Int J
Periodontics Restorative Dent. 31:e17–e28. 2011.PubMed/NCBI
|
|
5
|
Chang YY, Huang HL, Chen YC, Hsu JT, Shieh
TM and Tsai MT: Biological characteristics of the MG-63 human
osteosarcoma cells on composite tantalum carbide/amorphous carbon
films. PLoS One. 9:e955902014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng J and Wang Y: The metal tantalum in
orthopedic applications. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi.
28:419–422. 2011.(In Chinese). PubMed/NCBI
|
|
7
|
Frandsen CJ, Brammer KS, Noh K, Johnston G
and Jin S: Tantalum coating on TiO2 nanotubes induces
superior rate of matrix mineralization and osteofunctionality in
human osteoblasts. Mater Sci Eng C. 37:332–341. 2014. View Article : Google Scholar
|
|
8
|
Ninomiya JT, Struve JA, Krolikowski J,
Hawkins M and Weihrauch D: Porous ongrowth surfaces alter
osteoblast maturation and mineralization. J Biomed Mater Res A.
103:276–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sagomonyants KB, Hakim-Zargar M, Jhaveri
A, Aronow MS and Gronowicz G: Porous tantalum stimulates the
proliferation and osteogenesis of osteoblasts from elderly female
patients. J Orthop Res. 29:609–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balla VK, Bodhak S, Bose S and
Bandyopadhyay A: Porous tantalum structures for bone implants:
fabrication, mechanical and in vitro biological properties.
Acta Biomater. 6:3349–3359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Findlay DM, Welldon K, Atkins GJ, Howie
DW, Zannettino AC and Bobyn D: The proliferation and phenotypic
expression of human osteoblasts on tantalum metal. Biomaterials.
25:2215–2227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakai T, Takeda S and Nakamura M: The
effects of particulate metals on cell viability of osteoblast-like
cells in vitro. Dent Mater J. 21:133–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha SW, Weitzmann MN and Beck GR Jr:
Bioactive silica nanoparticles promote osteoblast differentiation
through stimulation of autophagy and direct association with LC3
and p62. ACS Nano. 8:5898–5910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Howe JLC, Yu Z, Leong DT, Chu JJ,
Loo JS and Ng KW: Exposure to titanium dioxide nanoparticles
induces autophagy in primary human keratinocytes. Small. 9:387–392.
2013. View Article : Google Scholar : PubMed/NCBI
|