Introduction
Bone homeostasis is maintained through osteocytes,
osteoclasts and osteoblasts that are present in bone tissues
(1,2). Osteoblasts, which develop from bone
marrow mesenchymal stem cells, promote bone formation and
mineralization. By contrast, osteoclasts are derived from
hematopoietic progenitor cells and stimulate bone resorption
(1,2). Bone marrow mesenchymal stem cells
(MSCs) are multipotent stromal cells that can differentiate into a
number of different cell types, including adipocytes, myoblasts,
osteoblasts and chondrocytes (3,4). MSC
differentiation is triggered by crosstalk between complex signaling
pathways involving numerous components, such as Wnt, delta/jagged,
bone morphogenic, Wnt and hedgehog proteins, as well as insulin,
insulin-like growth factors, fibroblastic growth factors, and
transcriptional regulators of adipocyte and osteoblast
differentiation, including peroxisome proliferators-activated
receptor-γ (PPARγ) and runt-related transcription factor 2
(5–7). Bone marrow MSC differentiation is
crucial for the homeostasis of bone remodeling.
Osteoporosis is associated with a deterioration of
bone mass through the suppression of osteoblastic osteogenesis and
promotion of osteoclastic bone resorption, and may result in bone
fractures (8). Osteoporosis is
widely recognized as a major public health problem worldwide and
the incidence is increasing in countries with ageing populations
(8). Fractures of the proximal femur
represent the most serious complication of this disease (8). Bone mass decrease in females is
primarily due to reduced secretion of estrogen following the
beginning of menopause (8); thus,
osteoporosis is an important cause of morbidity and mortality.
Furthermore, there is growing evidence that osteoporosis is
associated with obesity and diabetes, which are increasingly
becoming a major public health concern (9,10).
Notably, osteoporosis and obesity are implicated with a number of
features (3,4,11,12);
osteoblasts and adipocytes develop from bone marrow MSCs and there
is a reverse association between the differentiation of MSCs into
osteoblasts and adipocytes. Since MSCs differentiate osteoblasts,
the differentiation of osteoblasts into adipocytes is reduced
(3,4). In addition, a previous study identified
that obesity, diabetes and osteoporosis were associated with bone
marrow adiposity, which increases production of the inflammatory
cytokine tumor necrosis factor-α (TNF-α) (13). TNF-α has been shown to suppress
osteoblastogenesis and bone mineralization (14,15).
These previous findings suggest that agents inhibiting adipogenesis
and stimulating osteoblastogenesis will be useful in the prevention
and treatment of osteoporosis.
Numerous constituents of herbs are known to possess
anti-inflammatory and analgesic effects (16–19). The
sesquiterpene β-caryophyllene is present in various essential oils,
particularly in clove oil derived from the stems and flowers of
Syzygium aromaticum, rosemary oil from Rosmarinus
officinalis, hemp oil from Cannabis sativa, and in
cinnamon and hop oils (16–19). β-caryophyllene is found in numerous
edible plants that are ingested daily, and it is approved as a food
additive by the Food and Drug Administration (FDA). It is a
selective agonist of cannabinoid receptor type 2 (CB2) and exerts
anti-inflammatory effects in animals (17,19). In
addition, β-caryophyllene reduces acute and chronic pain associated
with inflammation (19–21). The anti-inflammatory effects of
β-caryophyllene have been implicated with reduced TNF-α and
interleukin (IL)-1β production, which is associated with opioid
receptors (22).
Plant-derived molecules that inhibit adipogenesis
and stimulate osteoblastic bone mineralization are poorly
understood. The present study aimed to determine whether
β-caryophyllene regulates the differentiation of bone marrow cells
that are associated with adipogenesis and osteoblastogenesis.
β-caryophyllene was demonstrated to enhance osteoblastogenesis, and
suppress adipogenesis and osteoclastogenesis in mouse bone marrow
cells in vitro. To the best of our knowledge, this is the
first time that results concerning the role of β-caryophyllene in
the differentiation of bone marrow MSCs have been reported.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and
antibiotics (penicillin and streptomycin) were purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Fetal bovine
serum (FBS) was obtained from HyClone (GE Healthcare Life Sciences,
Logan, UT, USA). β-caryophyllene was purchased from Cayman Chemical
Company (Ann Arbor, MI, USA). TNF-α, tartrate-resistant acid
phosphatase (TRAP), insulin, dexamethasone,
3-isobutyl-1-methylxanthine (IBMX), Alizarin Red S, Oil Red O and
other reagents were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany). Insulin was dissolved in diluted acetic acid
solution and other reagents were dissolved in 100% ethanol.
Experimental animals and bone marrow
cell isolation
Female C57BL6 mice (n=8; age, 2 months; weight,
18–20 g), purchased from Charles River Laboratories (Wilmington,
MA, USA), were housed in a pathogen-free facility, with a 12 h
light/dark cycle, temperature/atomosphere and ad libitum
access to feed and water. All protocols used in the current study
were approved by the Institutional Animal Care and Use Committee at
Emory University School of Medicine (Atlanta, GA, USA). Tissue from
the femur and tibia was removed immediately following sacrifice
with exposure to CO2 in chamber box, and bone marrow
cells were isolated from these tissues with needle flush under
sterile conditions in safety cabinet (23,24).
In vitro adipogenesis assay
This experiment was based on the methods described
in our previous studies (23,24).
Bone marrow cells (1×106 cells/well; 2 ml medium added
per well using 12-well plates) were cultured in a water-saturated
atmosphere containing 5% CO2 and 95% air at 37°C for 3
days in culture medium, consisting of DMEM supplemented with 10%
FBS and 1% penicillin-streptomycin (10,000 units/l). The cells were
cultured in the presence or absence of differentiation medium (DM)
(23,24), which consisted of dexamethasone (1
µM/ml) and IBMX (0.5 mM/ml). Cells were treated with culture medium
only, DM plus ethanol (final concentration, 0.1%) or DM plus
β-caryophyllene (0.1–100 µM). Subsequently, the medium was replaced
with fresh culture medium containing insulin (10 µg/ml) without
dexamethasone and IBMX, and cells were cultured for a further 4
days in the presence or absence of β-caryophyllene (0.1–100 µM). In
other experimental groups, the cells were cultured in culture
medium only, DM plus vehicle (0.1% ethanol as a final
concentration) or DM plus β-caryophyllene (0.1–100 µM) for 3 days,
then the medium was replaced with fresh culture medium containing
insulin (10 µg/ml) and cultured for a further 4 days. The medium
was then removed, and adipocytes were stained with Oil Red O. Cell
numbers were counted under a light microscope (Olympus MTV-3;
Olympus, Tokyo, Japan) using a Hemocytometer plate (23). Quantification was performed by
extracting the dye with 0.2 ml of isopropanol for 1 min and
measuring the absorbance at 490 nm with a Spectracount microplate
photometer. Results are presented as the mean ± standard deviation
of 8 replicate samples per data set using different dishes and cell
preparations.
In vitro mineralization assay
Bone marrow cells (1×106 cells/ml/well)
were cultured in 12-well plates in the presence or absence of
DMEM-mineralization medium [DMEM-MM; culture medium plus ascorbic
acid (100 µg/ml) and β-glycerophosphate (4 mM)] along with the
vehicle or β-caryophyllene (0.1–100 µM) (23,24), for
7 or 18 days at 37°C and 5% CO2. The medium was changed
every 3 days. After 18 days of culture, the cells were washed with
phosphate-buffered saline (PBS) and stained with Alizarin Red S.
For quantification, the dye was eluted with 10% cetylpyridinium
chloride solution and the absorbance of the eluted solution at 570
nm was measured using a plate reader. Results were presented as the
mean ± standard deviation of 8 replicate samples per data set using
different dishes and cell preparation.
In vitro osteoclastogenesis assay
Bone marrow cells (2×105 cells/ml/well)
were plated in 24-well plates with culture media (1 ml/well) in an
atmosphere containing 5% CO2 at 37°C. Cells were
cultured in medium only (containing 0.1% ethanol as a final
concentration), β-caryophyllene (0.1–100 µM) only, TNF-α (5 ng/ml
medium) or TNF-α (5 ng/ml medium) plus β-caryophyllene (0.1–100 µM)
for 3 days. Next, 0.5 ml of the old medium was replaced with fresh
culture medium with or without TNF-α (5 ng/ml), and in the presence
or absence of β-caryophyllene (0.1–100 µM). The cultures were then
maintained for a further 4 days (25,26).
After a total of 7 days of culture, adherent cells were stained for
tartrate-resistant acid phosphatase (TRAP; Sigma-Aldrich; Merck
Millipore), a marker of osteoclasts (26). Briefly, cells were washed with PBS,
fixed with 10% neutralized formalin-phosphate (pH 7.2) for 10 min,
dried and then stained with acetate buffer (pH 5.0) containing
Naphthol AS-MX phosphate (Sigma-Aldrich; Merck Millipore) in the
presence of sodium tartrate (10 mM) for 90 min at room temperature.
TRAP-positive multinucleated cells (MNCs with ≥3 nuclei) were
considered to be osteoclast-like cells, and the cells were counted
using light microscopy. MNC scores are expressed as the mean ±
standard deviation of six cultures with 2 replicate wells per data
set using different dishes and cell preparation.
Statistical analysis
Statistical analysis was performed using GraphPad
InStat software (version 3; GraphPad Software, Inc., La Jolla, CA,
USA). Multiple comparisons were performed by one-way analysis of
variance, followed by a post-hoc Tukey's range test for
parametric data as indicated. P<0.05 was considered to indicate
a a statistically significant difference.
Results
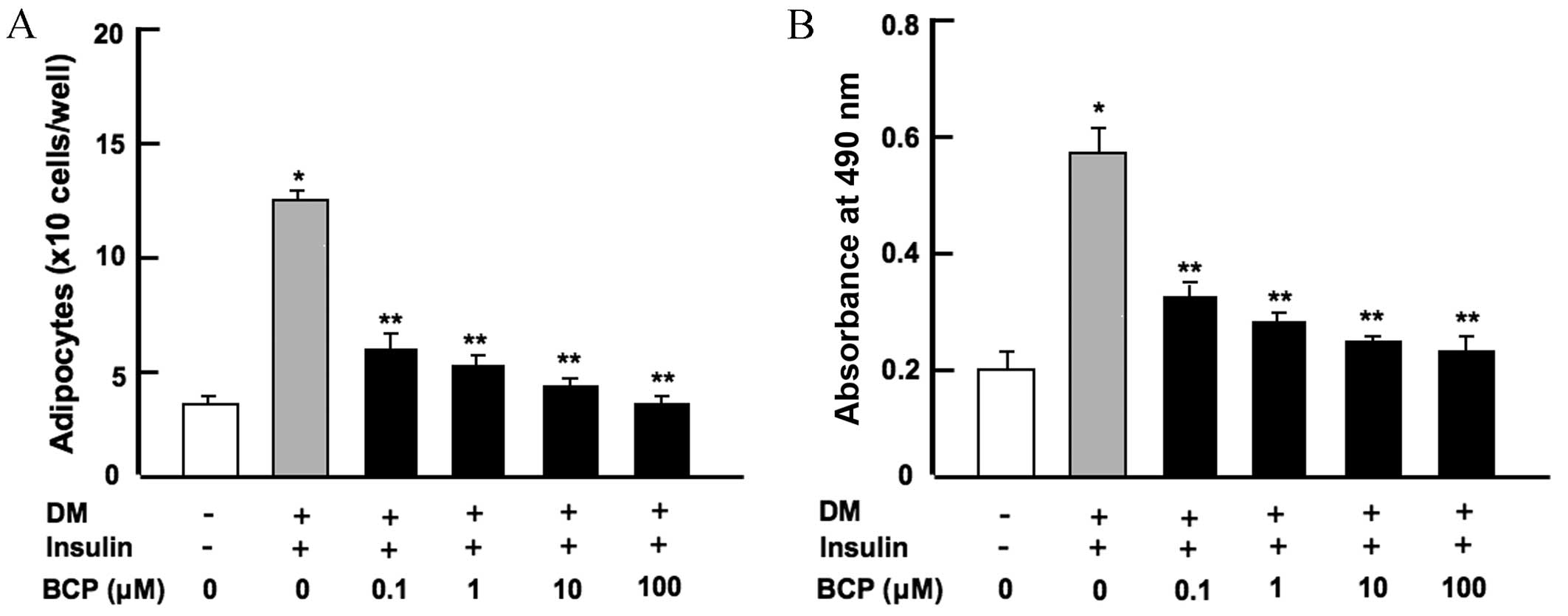
β-caryophyllene suppresses bone marrow
adipogenesis in vitro
Bone marrow MSCs are known to differentiate into
adipocytes (3,4). In order to determine the effect of
β-caryophyllene on bone marrow adipogenesis, mouse bone marrow
cells were cultured in either medium without DM and insulin or with
DM plus insulin, along with β-caryophyllene (0, 0.1, 1, 10 or 100
µM) for 7 days. Mouse bone marrow cells cultured without DM,
insulin or β-caryophyllene acted as a control. As shown in Fig. 1, cells treated with DM + insulin
alone significantly increased adipogenesis when compared with the
control group (without DM or insulin; P<0.001). The addition of
β-caryophyllene at any concentration was observed to significantly
suppress the differentiation of bone marrow cells into adipocytes
(P<0.001 vs. DM + insulin only) in a dose-dependent manner, as
determined by cell counting (Fig.
1A) and spectrophotometry (Fig.
1B) following Oil O Red staining. Furthermore, reduced
differentiation into adipocytes was found in bone marrow cells
cultured in media containing β-caryophyllene for 3 days and then in
media without β-caryophyllene for a further 4 days (data not
shown).
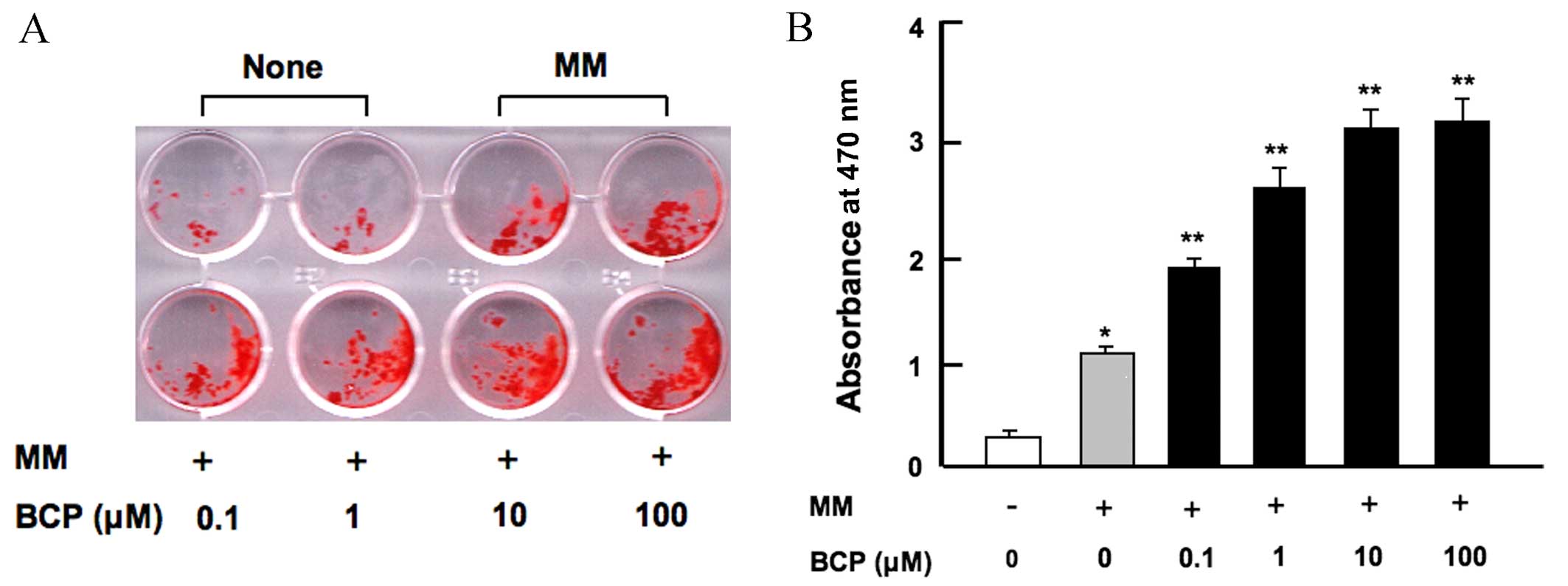
β-caryophyllene stimulates
osteoblastic mineralization in vitro
Osteoblasts develop from bone marrow MSCs (3,4). To
investigate the effect of β-caryophyllene on osteoblastogenesis and
mineralization in the bone marrow, cells were cultured in MM with
or without β-caryophyllene (0.1–100 µM) for 18 days. As shown in
Fig. 2, MM alone significantly
increased bone marrow mineralization compared with the control
group without MM (P<0.001). The addition of β-caryophyllene at
all doses significantly increased osteoblastic mineralization in a
dose-dependent manner when compared with cells treated with MM
alone (P<0.001; Fig. 2).
Furthermore, increased osteoblastic mineralization was observed
when bone marrow cells were cultured in the presence of
β-caryophyllene for 7 days (data not shown).
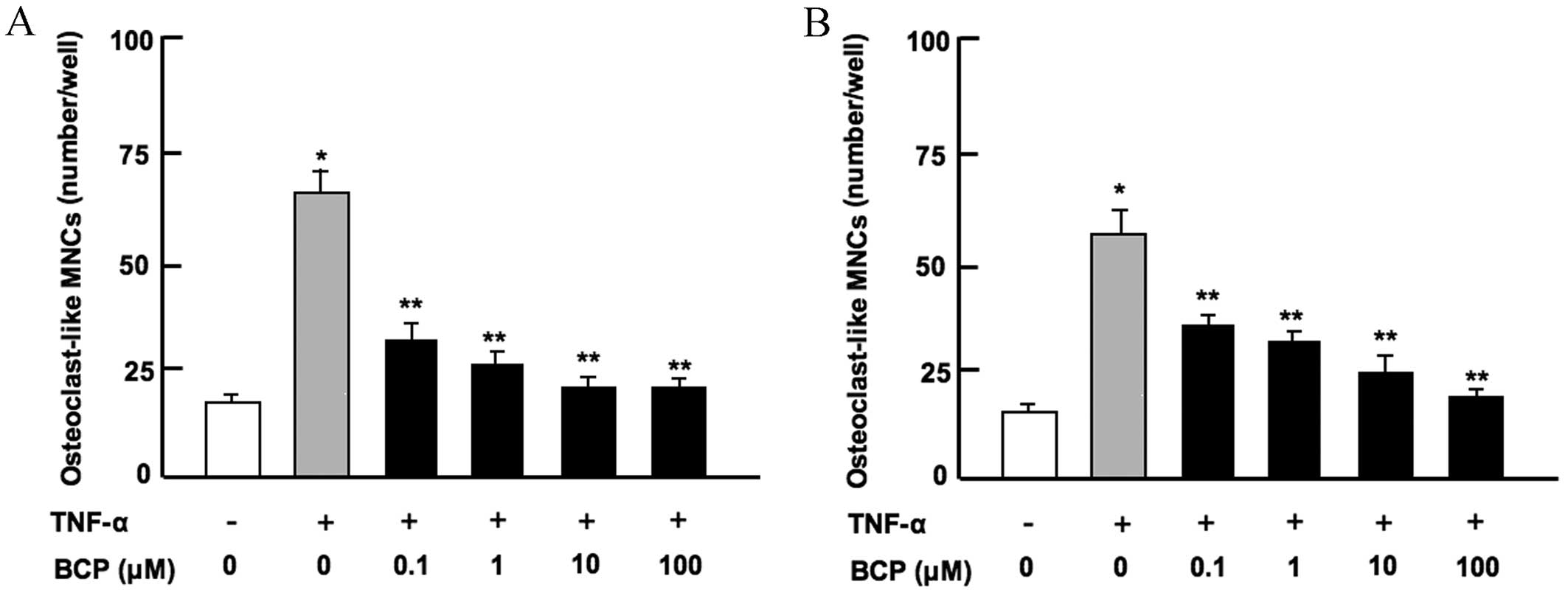
β-caryophyllene reduces
osteoclastogenesis in vitro
Osteoclasts are differentiated from monocytes and
macrophages in the bone marrow (26). To assess the effect of
β-caryophyllene on osteoclastogenesis in bone marrow in
vitro, bone marrow cells were cultured in the presence of
TNF-α, which stimulates osteoclastogenesis by activating nuclear
factor-κB (NF-κB) in preosteoclasts (13), and with or without β-caryophyllene
for 7 days. The results revealed that osteoclastogenesis was
significantly enhanced in the presence of TNF-α alone when compared
with the untreated control group (P<0.001; Fig. 3). This increase was significantly
suppressed following culture with β-caryophyllene at all doses for
7 days (P<0.001 vs. TNF-α only; Fig.
3A). Furthermore, β-caryophyllene (0.1–100 µM) did not have a
significant effect on osteoclastogenesis in the absence of TNF-α
(data not shown).
Discussion
In the present study, β-caryophyllene was
demonstrated to enhance osteoblastic mineralization, and to
suppress adipogenesis and osteoclastogenesis in mouse bone marrow
cell cultures in vitro. To the best of our knowledge, this
is the first time that these effects of β-caryophyllene are
reported. The effects of β-caryophyllene on adipogenesis and
osteoblastic mineralization were observed at an early stage of
culture following 7–8 days. This indicates that β-caryophyllene
strongly stimulates the differentiation of bone marrow MSCs into
osteoblasts, and strongly suppresses the differentiation to
adipocytes.
Bone marrow MSCs are multipotent cells that can
differentiate into adipocytes and osteoblasts (3,4). This
process is mediated through numerous complex signaling pathways,
including those involving PPARγ (5–7). For
instance, enhanced mitogen-activated protein kinase/extracellular
signal-regulated kinase signaling during adipogenesis potentiates
the activity of factors that regulate the expression of
CCAAT/enhancer-binding protein a and PPARγ (6,7).
Similarly, β-caryophyllene may exhibit a specific regulatory effect
on signaling pathways involved in the differentiation of bone
marrow MSCs to adipocytes.
Osteoclasts, which promote bone resorption, are
derived from hematopoietic progenitors (1,2,26). The current study demonstrated that
β-caryophyllene suppressed TNF-α-enhanced osteoclastogenesis in
mouse bone marrow in vitro, mediated through the activation
of NF-κB signaling in preosteoclasts. This suppressive effect was
observed following 7 days culture with bone marrow cells.
Therefore, the results of the present study suggest that
β-caryophyllene inhibited osteoclastogenesis at the stage of
differentiation into preosteoclasts in bone marrow culture.
The sesquiterpene β-caryophyllene is present in
various essential oils, particularly in clove, hemp, rosemary and
hop oil (16–22). In addition, β-caryophyllene is found
in plants that are ingested daily and has been approved as a food
additive by the FDA (16–18). β-caryophyllene is a selective agonist
of CB2 and has been shown to have anti-inflammatory effects in
animals, which have been implicated with reduced TNF-α and IL-1β
production associated with opioid receptors (17,19,22).
Furthermore, this molecule reduces acute and chronic pain
associated with inflammation (19–21). In
the current study, β-caryophyllene was demonstrated to enhance
osteoblastic mineralization, and suppress adipogenesis and
osteoclastogenesis in mouse bone marrow cells in vitro.
These results indicate that β-caryophyllene stimulates osteoblastic
bone formation and suppresses osteoclastic bone resorption, which
may provide a means to prevent and treat osteoporosis.
Osteoporosis has been associated with obesity and
diabetes (9,10), which are increasingly prevalent
public health concerns. Osteoporosis and obesity share a number of
similar features (3,4,11,12);
bone marrow MSCs can differentiate into adipocytes and osteoblasts
and enhanced adipogenesis may suppress osteoblastogenesis in bone
marrow cells (3,4). In the present study, β-caryophyllene
was found to stimulate osteoblastogenesis and suppress adipogenesis
in mouse bone marrow cell cultures in vitro. Therefore,
β-caryophyllene may serve an important role in the prevention and
treatment of osteoporosis associated with obesity and diabetes.
In conclusion, the present study demonstrated that
β-caryophyllene promotes osteoblastic mineralization, and reduces
adipogenesis and osteoclastogenesis in mouse bone marrow cell
cultures in vitro. These results indicate that
β-caryophyllene may be a useful tool in the treatment of
osteoporosis. Further studies into the effects of β-caryophyllene
on bone remodeling should be performed to validate these effects in
an in vivo environment and in models of osteoporosis.
References
|
1
|
Raggatt LJ and Partridge NC: Cellular and
molecular mechanisms of bone modeling. J Biol Chem.
285:25103–25108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chambers TJ and Fuller K: How are
osteoclasts induced to resorb bone? Ann N Y Acad Sci. 1240:1–6.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Minguell JJ, Erices A and Conget P:
Mesenchymal stem cells. Exp Biol Med (Maywood). 226:507–520.
2001.PubMed/NCBI
|
|
4
|
Muruganandan S, Roman AA and Sinal CJ:
Adipocyte differentiation of bone marrow-derived mesenchymal stem
cells: Cross talk with the osteoblastogenesis program. Cell Mol
Life Sci. 66:236–253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laudes M: Role of WNT signaling in the
determination of human mesenchymal stem cells into preadipocytes. J
Mol Endocrinol. 46:R65–R72. 2011.PubMed/NCBI
|
|
6
|
Gharibi B, Abraham AA, Ham J and Evans BA:
Adenosine receptor subtype expression and activation influence the
differentiation of mesenchymal stem cells to osteoblasts and
adipocytes. J Bone Miner Res. 26:2112–2124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawai M and Rosen CJ: PPARγ: A circadian
transcription factor in adipogenesis and osteogenesis. Nat Rev
Endocrinol. 6:629–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weitzmann MN and Pacifici R: Estrogen
deficiency and bone loss: An inflammatory tale. J Clin Invest.
116:1186–1194. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leslie WD, Rubin MR, Schwartz AV and Kanis
JA: Type 2 diabetes and bone. J Bone Miner Res. 27:2231–2237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nielson CM, Srikanth P and Orwoll ES:
Obesity and fracture in men and women: An epidemiologic
perspective. J Bone Miner Res. 27:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gharibi B, Abraham AA, Ham J and Evans BA:
Adenosine receptor subtype expression and activation influence the
differentiation of mesenchymal stem cells to osteoblasts and
adipocytes. J Bone Miner Res. 26:2112–2124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pirih F, Lu J, Ye F, Bezouglaia O, Atti E,
Ascenzi MG, Tetradis S, Demer L, Aghaloo T and Tintut Y: Adverse
effects of hyperlipidemia on bone regeneration and strength. J Bone
Miner Res. 27:309–318. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Li A, Strait K, Zhang H, Nanes MS
and Weitzmann MN: Endogenous TNFalpha lowers maximum peak bone mass
and inhibits osteoblastic Smad activation through NF-kappaB. J Bone
Miner Res. 22:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang J, Wang Z, Tang E, Fan Z, McCauley
L, Franceschi R, Guan K, Krebsbach PH and Wang CY: Inhibition of
osteoblastic bone formation by nuclear factor-kappaB. Nat Med.
15:682–689. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cortez M, Carmo LS, Rogero MM, Borelli P
and Fock RA: A high-fat diet increases IL-1, IL-6, and TNF-α
production by increasing NF-κB and attenuating PPAR-γ expression in
bone marrow mesenchymal stem cells. Inflammation. 36:379–386. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghelardini C, Galeotti N, Di Cesare
Mannelli L, Mazzanti G and Bartolini A: Local anaesthetic activity
of beta-caryophyllene. Farmaco. 56:387–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gertsch J, Leonti M, Raduner S, Racz I,
Chen JZ, Xie XQ, Altmann KH, Karsak M and Zimmer A:
Beta-caryophyllene is a dietary cannabinoid. Proc Nat Acad Sci USA.
105:9099–9104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ormeño E, Baldy V, Ballini C and Fernandez
C: Production and diversity of volatile terpenes from plants on
calcareous and siliceous soils: Effect of soil nutrients. J Chem
Ecol. 34:1219–1229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katsuyama S, Mizoguchi H, Kuwahata H,
Komatsu T, Nagaoka K, Nakamura H, Bagetta G, Sakurada T and
Sakurada S: Involvement of peripheral cannabinoid and opioid
receptors in β-caryophyllene-induced antinociception. Eur J Pain.
17:664–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paula-Freile LI, Andersen ML, Gama VS,
Molska GR and Carlini EL: The oral administration of
trans-caryophyllene attenuates acute and chronic pain in mice.
Phytomedicine. 21:356–362. 2015. View Article : Google Scholar
|
|
21
|
Chavan MJ, Wakte PS and Shinde DB:
Analgestic and anti-inflammatory activity of caryophyllene oxide
from Annona squamosa L. Bark. Phytomedicine. 17:149–151. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martinez RM, Zarpelon AC, Cardoso RD,
Vicentini FT, Georgetti SR, Baracat MM, Andrel CC, Moreira IC,
Verri WA Jr and Casagrande R: Tephrosia sinapou ethyl acetate
extract inhibits inflammatory pain in mice: Opioid receptor
dependent inhibition of TNFα and IL-1β production. Pharm Biol.
51:1262–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamaguchi M, Weitzmann MN, Baile CA and
Murata T: Exogenous regucalcin suppresses osteoblastogenesis and
stimulates adipogenesis in mouse bone marrow culture. Integr Biol
(Camb). 4:1215–1222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi M, Zhu S, Zhang S, Wu D, Moore
TM, Snyder JP and Shoji M: Curcumin analogue UBS109 prevents bone
loss in breast cancer bone metastasis mouse model: Involvement in
osteoblastogenesis and osteoclastogenesis. Cell Tissue Res.
357:245–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minkin C: Bone acid phosphatase:
Tartrate-resistant acid phosphatase as a marker of osteoclast
function. Calcif Tissue Int. 34:285–290. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zaidi M, Blair HC, Moonga BS, Abe E and
Huang CL: Osteoclastogenesis, bone resorption, and osteoblast-based
therapeutics. J Bone Miner Res. 18:599–609. 2003. View Article : Google Scholar : PubMed/NCBI
|