Introduction
Premature ovarian failure (POF) is defined as
ovarian failure which leads to amenorrhea before the age of 40
years. It is characterized by primary or secondary amenorrhea
accompanied by increased blood gonadotropin levels and reduced
levels of estrogen. The clinical features primarily include
symptoms related to low estrogen such as depression, facial
flushing, and low libido (1,2). Roussev et al examined 1,858
women with natural amenorrhea and demonstrated that the incidence
of POF was <1% before the age of 40 years, and <1/1,000 in
women aged under 30 years (3). In
Beijing, the incidence rate of POF is 1.8% (4).
Melatonin is an amine hormone produced primarily by
the pineal gland in mammals. It can regulate the reproductive
activity and the photoperiod determines its biosynthesis. Previous
findings showed there are many different melatonin receptors in the
ovary, which suggests that melatonin has a significant role in the
reproductive system (5). However,
the role of melatonin in POF is not clear.
By measuring the cell cycle of T lymphocytes and the
levels of reactive oxygen species (ROS) in the plasma of patients
with POF before and after treatment with melatonin, the aim of the
present study was to determine the mechanism of melatonin for
treating POF, and to provide a theoretical basis for the clinical
treatment of POF.
Patients and methods
Patients
From December 2014 to June 2015, 128 patients who
were diagnosed with POF in the Department of Gynaecology and
Obstetrics of Shandong Provincial Hospital were randomly divided
into the experimental and control groups. Patients in the
experimental group received melatonin tablets (1–3 mg/day), and
patients in the control group took the corresponding placebo
(similar in appearance to melatonin tablets). We measured the
levels of six sex hormones, cell cycle of T lymphocytes, and the
levels of ROS in plasma of patients 1 day before treatment, and at
1, 3 and 6 months after treatment. Data were collected and
analyzed. The inclusion criteria were: i) Patients were aged >18
years; and ii) diagnosis of POF by laboratory examination. The
exclusion criteria included: i) Ovarian tumors; ii) malignant
tumors of other tissues/organs; iii) diagnosis could not be made;
iv) cognitive impairment or suffering from mental illness; v) blood
samples could not be obtained from patients; vi) patients and their
families did not match the information they previously provided;
vii) patient quit the study; and viii) patients with poor general
condition, or unsuitable for diagnosis and treatment.
Blood collection
Patients fasted for 8 h and 3 ml blood from the
elbow vein was collected in 1.8 ml vacuum tubes containing 0.2 ml
3.8% sodium citrate. Specimens were collected within 1 h after
centrifugation (2,500 × g, 10 min). Serum or plasma was stored in
0.5 ml Eppendorf tubes and preserved at −30°C. Samples collected
were used within 1 month.
Reagents
DCFH-DA powder (Sigma-Aldrich, St. Louis, MO, USA),
sterile double-distilled water and phospho-TGF-β1 (p-TGF-β1)
antibody (1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA), β-actin antibody (1:5,000; Invitrogen, Carlsbad, CA, USA),
0.9% sterile saline (Otsuka Pharmaceutical Co., Ltd., Tokyo,
Japan), TRIzol (Invitrogen) and enzyme-linked immunosorbent assay
(ELISA) kit (Cayman Chemical Co., Ann Arbor, MI, USA) were
used.
Experimental instruments
Centrifuge and micropipettes (both from Eppendorf
AG, Hamburg, Germany), Haier ice machine, western blot
electrophoresis apparatus (Bio-Rad, Berkeley, CA, USA), −80°C
refrigerator (Thermo Fisher Scientific, Waltham, MA, USA); 10 ml
syringe, 5 ml syringe (HA well, Tianjin), experimental animal
special surgical instruments (Beijing Medical Instrument Factory,
Beijing, China), NanoDrop 2000 spectrophotometer (Thermo Fisher
Scientific, Wilmington, DE, USA), Eppendorf tubes (Eppendorf AG),
water bath (Beijing Medical Instrument Factory), pathological
tissue processor (Leica, Mannheim, Germany), and flow cytometer
(CyFlow; Partec, Münster, Germany) were used in the study.
Detection of ROS and VEGF levels
To determine ROS and vascular endothelial growth
factor (VEGF) levels, blood samples were centrifuged and the
supernatant was used for measurements. The human serum ROS and VEGF
kits were used according to the manufacturer's instructions.
T lymphocyte extraction
i) A proper amount of lymphocyte separation liquid
was added to appropriate tubes. ii) Heparin anticoagulated venous
blood and an equal volume of Hank's solution or RPMI-1640 were
uniformly mixed, and close attention was paid to maintain a clear
interface. Samples were centrifuged in a horizontal centrifuge
(1,600 × g, 20 min). iii) Samples in the tubes were resolved into
three layers; the upper layer was plasma and Hank's solution, and
the lower layer was primarily red blood cells and granular cells.
The middle layer was the lymphocyte separation solution. The
interface of the upper and the middle layers was the Buffy coat.
This layer also contained platelets. iv) The capillary was inserted
into the Buffy coat to aspirate cells. Cells were placed in another
short tube, and Hank's solution or RPMI-1640 was added. The samples
were then centrifuged (1,200 × g, 10 min) and cells were washed
twice. v) After the final centrifugation, RPMI-1640 with 10% fetal
bovine serum was added to the supernatant. A drop of cell
suspension and a drop of 0.2% trypan blue dye were mixed. The cells
were counted with a hemocytometer.
Melatonin treatment
The patients received 1 mg/night before bed service.
For patients aged 41–60 years, 1–3 mg were given every night.
Urinary oxidative stress test
After 6 weeks of melatonin treatment, urine was
collected and immediately stored at −80°C. The levels of urinary
8-OHdG and 8-isoPGF2α were determined by the ELISA kit.
Determination of NT levels in urine were by chemiluminescence kit
(Millipore Corp., Bedford, MA, USA).
Statistical analysis
SPSS 13.0 statistical software (Chicago, IL, USA)
was used for data analysis. Comparisons of mean values were
conducted using the t-test, and comparisons of rate were made using
the χ2 test. The correlation between the levels of
melatonin and ROS was determined by the correlation analysis and
multivariate linear regression analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Comparison of baseline clinical
characteristics
The baseline clinical characteristics of the two
groups including age, body mass index (BMI), and menstrual cycle
were similar. There were no statistically significant differences
between the two groups (P>0.05) (Table I).
 | Table I.Comparison of baseline clinical
characteristics. |
Table I.
Comparison of baseline clinical
characteristics.
| Groups | Patients (n) | Age (years) | BMI
(kg/m2) | Menstrual cycle
(days) |
|---|
| Experimental | 64 | 29.4±9.8 | 21.5±2.4 | 56.4±2.7 |
| Control | 64 | 31.2±8.3 | 20.3±1.7 | 61.5±1.2 |
| T-value | – | 0.22 | 0.27 | 0.98 |
| P-value | – | 0.38 | 0.71 | 0.11 |
Comparison of sex hormone levels
We treated the patients in the experimental group
with melatonin for 6 months. The control group was treated with
placebo. After 6 months of treatment, we found that the levels of
follicle-stimulating hormone (FSH) and luteinizing hormone (LH)
were significantly decreased in the experimental group, and the
levels of estradiol (E2) were significantly increased (P<0.05).
However, there was no significant difference in the menstrual cycle
(Table II).
 | Table II.Comparison of sex hormones
(pmol/l). |
Table II.
Comparison of sex hormones
(pmol/l).
| Test project | Groups | Patients (n) | Before | 1 month | 3 months | 6 months | T-value | P-value |
|---|
| FSH | Experimental | 64 | 45.3±3.6 | 33.3±1.6 | 20.4±1.6 | 15.49±2.3 | 24.37 | 0.004 |
|
| Control | 64 | 46.5±4.4 | 47.5±2.4 | 48.6±1.8 | 47.49±2.3 | 0.98 | 0.11 |
|
| T-value | – | 0.21 | 28.21 | 22.67 | 25.48 | – | – |
|
| P-value | – | 0.33 | 0.004 | 0.001 | 0.005 | – | – |
| LH | Experimental | 64 | 28.4±9.1 | 18.4±2.3 | 15.3±3.4 | 10.4±2.1 | 22.43 | 0.001 |
|
| Control | 64 | 28.7±6.4 | 27.8±2.1 | 28.2±3.3 | 27.9±0.42 | 0.58 | 0.41 |
|
| T-value | – | 0.87 | 10.22 | 39.38 | 42.48 | – | – |
|
| P-value | – | 0.24 | 0.012 | 0.004 | 0.013 | – | – |
| E2 | Experimental | 64 | 62.7±33.4 | 89.3±12.5 | 112.4±23.1 | 142.7±33.4 | 42.5 | 0.001 |
|
| Control | 64 | 72.4±15.8 | 72.4±12.8 | 79.4±35.8 | 76.4±21.8 | 0.48 | 0.25 |
|
| T-value | – | 0.47 | 12.9 | 24.9 | 31.3 | – | – |
|
| P-value | – | 0.52 | 0.04 | 0.02 | 0.006 | – | – |
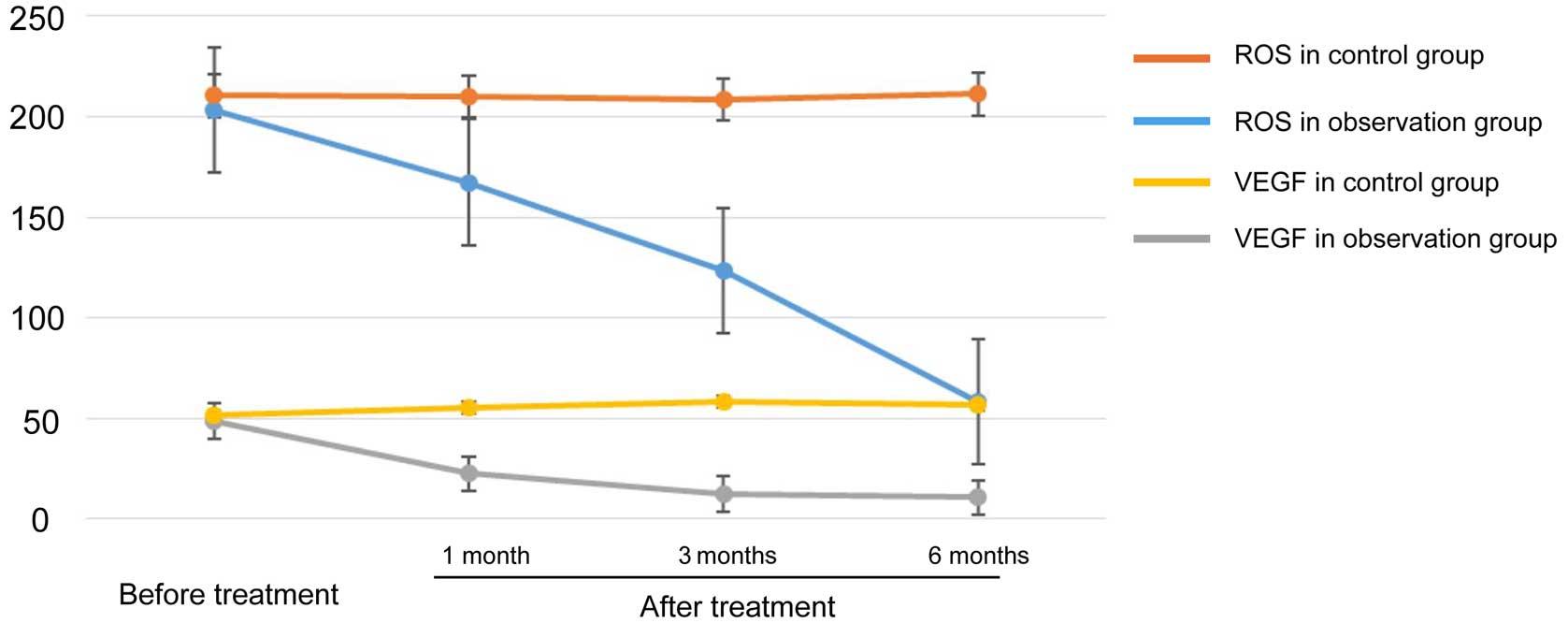
Comparison of plasma ROS and VEGF
levels
The levels of ROS and VEGF in plasma were
significantly lower in the experimental group compared with the
control group (P<0.05) (Table
III and Fig. 1).
 | Table III.Comparison of levels of ROS and
VEGF. |
Table III.
Comparison of levels of ROS and
VEGF.
| Test projects | Groups | Patients | Before
treatment | 1 month | 3 months | 6 months | T-value | P-value |
|---|
| ROS (ng/ml) | Experimental | 64 | 203.4±21.2 | 167.4±12.1 | 123.2±13.5 | 58.5±22.7 | 44.37 | 0.004 |
|
| Control | 64 | 210.4±18.3 | 209.8±15.6 | 208.3±19.4 | 211.2±13.3 | 87.4 | 0.001 |
|
| T-value | – | 0.21 | 20.35 | 44.72 | 50.53 | – | – |
|
| P-value | – | 0.33 | 0.01 | 0.001 | 0.006 | – | – |
| VEGF (ng/ml) | Experimental | 64 | 48.5±12.6 | 22.5±10.7 | 12.3±2.6 | 10.7±9.4 | 24.3 | 0.002 |
|
| Control | 64 | 51.3±10.9 | 55.2±9.3 | 58.4±11.5 | 56.5±12.1 | 0.67 | 0.33 |
|
| T-value | – | 0.29 | 28.37 | 39.42 | 33.51 | – | – |
|
| P-value | – | 0.77 | 0.009 | 0.005 | 0.006 | – | – |
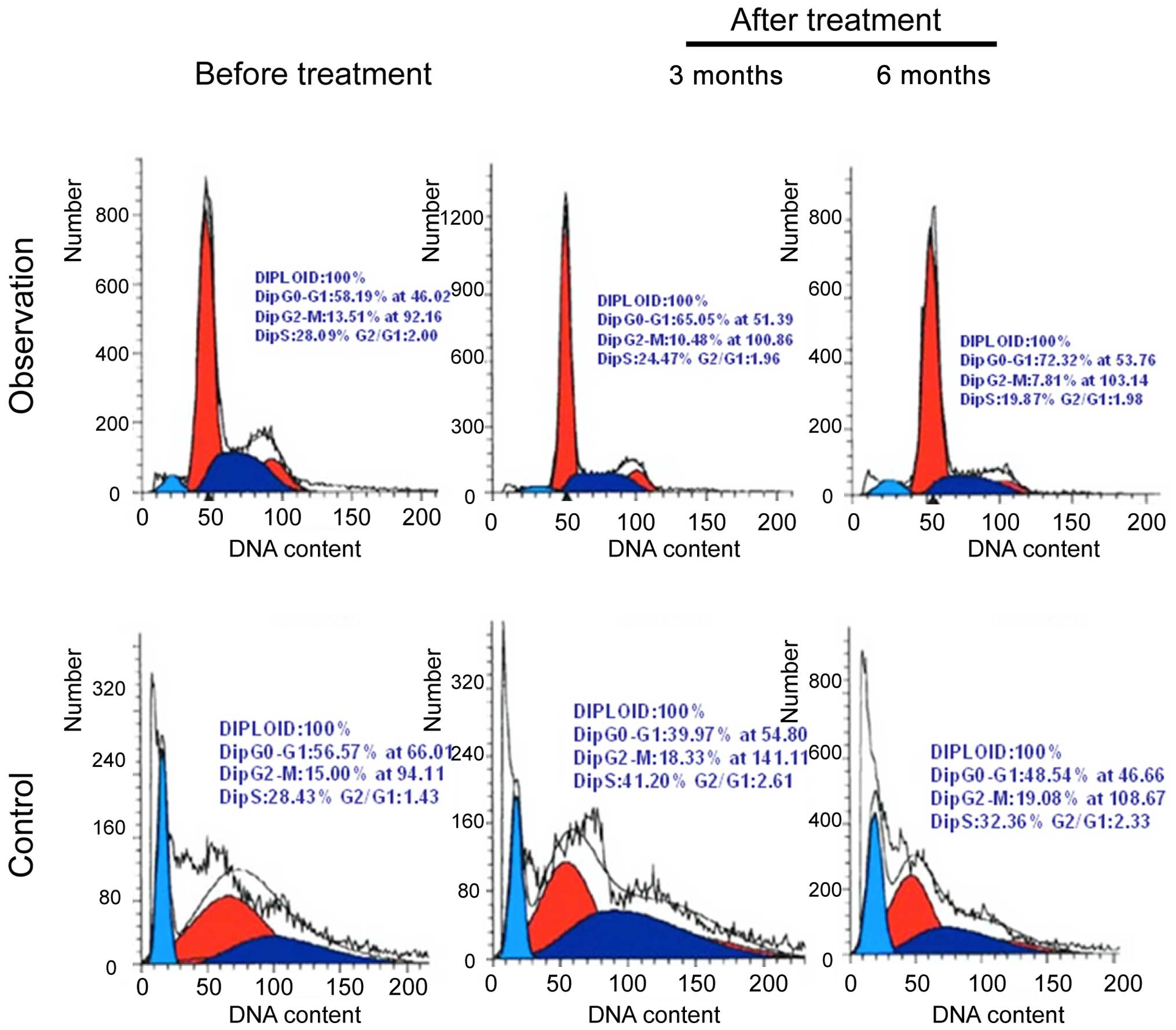
Determination of T lymphocyte cell
cycle
Peripheral blood T lymphocytes were extracted from
the two groups of patients before treatment, and after 1, 3 and 6
months of treatment. The T lymphocyte cell cycle was measured. The
number of cells in the G1/M phase in the experimental
group was less than that in the control group, and the difference
was statistically significant (P<0.05) (Table IV and Fig. 2).
 | Table IV.Comparison of percentage of T
lymphocytes in G1/M phase (%). |
Table IV.
Comparison of percentage of T
lymphocytes in G1/M phase (%).
| Groups | Patients | Before
treatment | 1 month | 3 months | 6 months | T-value | P-value |
|---|
| Experimental | 64 | 33.7±3.4 | 22.5±1.6 | 18.3±1.4 | 15.5±2.5 | 44.37 | 0.004 |
| Control | 64 | 38.4±2.7 | 37.3±2.4 | 38.6±2.7 | 37.6±3.2 | 0.44 | 0.507 |
| T-value | – | 0.21 | 22.7 | 31.5 | 34.7 | – | – |
| P-value | – | 0.33 | 0.01 | 0.008 | 0.007 | – | – |
Urinary oxidative stress test
Urinary oxidative stress levels were measured before
and after treatment in the two groups. The results showed that
after treatment, the levels of urinary 8-OHdG and 8-isoPGF2α were
significantly lower in the experimental group compared with the
control group (P<0.05) (Table
V).
 | Table V.Measurement of urinary oxidative
stress. |
Table V.
Measurement of urinary oxidative
stress.
|
|
| 8-OHdG | 8-isoPGF2α |
|---|
|
|
|
|
|
|---|
| Groups | Patients (n) | Before
treatment | After
treatment | Before
treatment | After
treatment |
|---|
| Experimental | 64 | 15.7±3.4 |
11.5±1.6a | 14.3±1.4 |
11.5±2.5b |
| Control | 64 | 15.4±2.7 | 16.3±2.4 | 14.6±2.7 | 15.6±3.2 |
| T-value | – | 0.11 | 21.7 | 36.5 | 34.2 |
| P-value | – | 0.83 | 0.01 | 0.008 | 0.007 |
Comparison of ovulation
We compared ovulation in the two groups of patients
after treatment and found that compared with the control group,
there were significantly more patients with ovulation in the
experimental group after treatment (P<0.05) (Table VI).
 | Table VI.Comparison of ovulation. |
Table VI.
Comparison of ovulation.
| Groups | Patients | Positive | Negative |
|---|
| Experimental | 64 | 18 | 46 |
| Control | 64 | 0 | 64 |
| χ2
value | – | 12.83 |
|
| P-value | – |
0.011 |
|
Correlation analysis of serum
melatonin and ROS
We performed a correlation analysis of melatonin and
the levels of ROS. We found a negative correlation between serum
melatonin level and the levels of ROS, and the difference was
statistically significant (P<0.05), correlation coefficient
rs=−0.481 (Tables VII and VIII).
 | Table VII.Correlation analysis between ROS
level and clinical characteristics of patients. |
Table VII.
Correlation analysis between ROS
level and clinical characteristics of patients.
| Melatonin | Gender | Age (years) | FSH | LH | E2 | Periods | ROS | BMI
(kg/m2) |
|---|
| r |
0.021 |
0.351 |
0.172 |
0.151 |
0.064 |
0.382 |
−0.481 |
0.141 |
| P-value | >0.05 | >0.05 | >0.05 | >0.05 | <0.05 | >0.05 | <0.05 | <0.05 |
 | Table VIII.Multivariate linear regression
analysis. |
Table VIII.
Multivariate linear regression
analysis.
|
| (95% CI) |
|---|
|
|
|
|---|
| Variables | β | SE | β' | t | P-value | Upper | lower |
|---|
| BMI | 0.531 | 0.14 | 0.764 | 0.412 | >0.05 | 0.26 | 0.81 |
| E2 | 0.581 | 0.10 | 0.642 | 0.652 | >0.05 | 0.39 | 0.78 |
| ROS | 0.768 | 0.08 | 0.871 | 0.981 | <0.05 | 0.61 | 0.92 |
Discussion
Melatonin (N-acetyl-5-methoxytryptamine, MT) is a
neuroendocrine hormone secreted by the pineal gland. Recent studies
have indicated that it plays a significant role in the reproductive
process (6–9). Human sinus follicular fluid contains a
high concentration of MT. The surface of ovarian tissue granulosa
cells also express the MT receptor, which indicates that MT can
directly regulate ovarian function through a variety of ways.
Findings have shown that in many patients with POF, ROS participate
in normal follicular development at each physiological stage
including: Folliculogenesis and atresia, ovulation, oocyte
maturation, and corpus luteum formation (10). MT and its metabolites are considered
to be potent antioxidants and free radical scavengers which prevent
POF. The pathogenesis of POF is unclear, although genetic,
physical, chemical, and immune factors can cause abnormal ovarian
function (11–13).
In this study, we found that ovarian secretion of
hormones was significantly reduced in patients with POF. In
addition, the percentage of peripheral blood T lymphocytes in the
G1/M period was significantly decreased (P<0.05). The
above results are consistent with those of the study by Xie et
al (14), which analyzed healthy
women, women with POF, and women with ovarian reserve dysfunction.
Their study demonstrated that the proportion of Treg cells in the
blood of patients with POF was decreased (P<0.01). Furthermore,
the levels of IFN-α in peripheral blood were significantly
increased.
ROS refer to a series of oxygen free radicals
including: O2−, H2O2,
HO2 and OH. Middle and high concentrations of ROS caused
cell apoptosis and necrosis induced by oxidative stress (15). With the progression of free radical
biology, ROS have been shown to regulate the apoptosis and
proliferation of certain tumor cells. In addition, our study found
that patients in the experimental group (received melatonin
treatment) had significantly decreased levels of plasma ROS and
VEGF, whereas in the control group, there were no changes. Since it
is structurally stable as a non-enzymatic antioxidant, MT is less
prone to auto-oxidation. It can clear hydroxyl (−OH) groups, and
different types of ROS, and plays a role in antioxidation.
Melatonin reduced the production of ROS. With both
in vivo and in vitro experiments, previous studies
reported that melatonin can inhibit hypoxia inducible factor-1
(HIF-1). HIF-1 is a transcription factor responsible for the
expression of VEGF. Melatonin can reduce hypoxia induced
angiogenesis. It was found that the ability of MT to clear -OH is
more than 5 times than that of glutathione (16,17). MT
also has an indirect antioxidant effect, in that it can promote the
expression of antioxidant defense enzymes by nuclear receptor
mediated mechanisms, and enhance the activity of antioxidant
enzymes (18–20).
Since anti-ROS mechanisms are involved in various
pathophysiological processes, which generally require different
inflammatory factors and multiple signaling pathways together to
function, the results of the present study require further
validation (19–21). The change in VEGF alone cannot
explain the mechanism between POF and ROS. Further investigation is
required to prove that decreasing ROS levels result in improved
ovarian function (22).
Based on our results and those of other authors, we
believe that reducing the levels of ROS with melatonin can regulate
the T lymphocyte cycle and increase sex hormone levels in patients
with POF. Melatonin may have clinical significance for the
treatment of POF.
References
|
1
|
Blumenfeld Z and Evron A: Endocrine
prevention of chemotherapy-induced ovarian failure. Curr Opin
Obstet Gynecol. 28:223–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mishra B, Ortiz L and Luderer U: Charged
iron particles, components of space radiation, destroy ovarian
follicles. Hum Reprod. 31:1816–1826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roussev RG, Kaider BD, Price DE and Coulam
CB: Laboratory evaluation of women experiencing reproductive
failure. Am J Reprod Immunol. 35:415–420. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu X, Cai H, Kallianpur A, Li H, Yang G,
Gao J, Xiang YB, Ji BT, Yu-Tang, Zheng W and Shu XO: Impact of
premature ovarian failure on mortality and morbidity among Chinese
women. PLoS One. 2014.9:e89597 View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamura H, Nakamura Y, Korkmaz A,
Manchester LC, Tan DX, Sugino N and Reiter RJ: Melatonin and the
ovary: physiological and pathophysiological implications. Fertil
Steril. 92:328–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang H, Lee OH, Lee Y, Yoon H, Chang EM,
Park M, Lee JW, Hong K, Kim JO, Kim NK, et al: Melatonin prevents
cisplatin-induced primordial follicle loss via suppression of
PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal
Res. 60:336–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He H, Jiang DM, Kang B, Ma R, Bai L, Wang
X and Zhao L: Gene expression profiling of melatonin receptor
subtypes in the ovarian hierarchical follicles of the Sichuan white
goose. Anim Reprod Sci. 145:62–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Q, Zhang T, Zhang P and Wang ZY:
Melatonin attenuates postharvest physiological deterioration of
cassava storage roots. J Pineal Res. 60:424–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurer-Orhan H, Karaaslan C, Ozcan S,
Firuzi O, Tavakkoli M, Saso L and Suzen S: Novel indole-based
melatonin analogues: evaluation of antioxidant activity and
protective effect against amyloid β-induced damage. Bioorg Med
Chem. 24:1658–1664. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lohana CK and Samir N: Risk management of
osteoporosis in postmenopausal women; a study of women in a
teaching hospital. Glob J Health Sci. 8:555052016.
|
|
11
|
Vriend J and Reiter RJ: Melatonin and the
von Hippel-Lindau/HIF-1 oxygen sensing mechanism: a review. Biochim
Biophys Acta. 1865:176–183. 2016.PubMed/NCBI
|
|
12
|
Xu Y, Wang S, Jiang L, Wang H, Yang Y, Li
M, Wang X, Zhao X and Xie K: Identify melatonin as a novel
therapeutic reagent in the treatment of 1-bromopropane(1-BP)
intoxication. Medicine (Baltimore). 95:e22032016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rhee YH and Ahn JC: Melatonin attenuated
adipogenesis through reduction of the CCAAT/enhancer binding
protein beta by regulating the glycogen synthase 3 beta in human
mesenchymal stem cells. J Physiol Biochem. 72:145–155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie JY, He W, Zhao LM, Chen M and Liang
ZQ: The changes of CD4− CD25− regulatory T
cells and changes of interferon gamma and expression of
transforming growth factor beta 1 in patients with premature
ovarian failure. West China Med J. 3:377–379. 2013.(In
Chinese).
|
|
15
|
Bodas M, Van Westphal C,
Carpenter-Thompson R, Mohanty KD and Vij N: Nicotine exposure
induces bronchial epithelial cell apoptosis and senescence via ROS
mediated autophagy-impairment. Free Radic Biol Med. 97:441–453.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mehrzadi S, Kamrava SK, Dormanesh B,
Motevalian M, Hosseinzadeh A, Tabatabaei SM Hosseini and Ghaznavi
H: Melatonin synergistically enhances protective effect of
atorvastatin against gentamicin-induced nephrotoxicity in rat
kidney. Can J Physiol Pharmacol. 94:265–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Check JH, Wilson C, DiAntonio G and
DiAntonio A: In vitro fertilization (IVF) outcome in women in overt
menopause attempting to induce follicular maturation by follicle
stimulating hormone (FSH) receptor down-regulation. Clin Exp Obstet
Gynecol. 43:181–183. 2016.PubMed/NCBI
|
|
18
|
Sundaresan NR, Leo MD Marcus, Subramani J,
Anish D, Sudhagar M, Ahmed KA, Saxena M, Tyagi JS, Sastry KV and
Saxena VK: Expression analysis of melatonin receptor subtypes in
the ovary of domestic chicken. Vet Res Commun. 33:49–56. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu T, Li Q, Wang S, Chen C and Zheng J:
Transplantation of ovarian granulosa-like cells derived from human
induced pluripotent stem cells for the treatment of murine
premature ovarian failure. Mol Med Rep. 13:5053–5058.
2016.PubMed/NCBI
|
|
20
|
Kleszczyñski K, Zillikens D and Fischer
TW: Melatonin enhances mitochondrial ATP synthesis, reduces ROS
formation and mediates translocation of the nuclear erythroid
2-related factor 2 resulting in activation of phase-2 antioxidant
enzymes (ã-GCS, HO-1, NQO1) in UVR-treated normal human epidermal
keratinocytes (NHEK). J Pineal Res. (In press). 2016. View Article : Google Scholar
|
|
21
|
Benedict C, Thom B, N Friedman D,
Diotallevi D, M Pottenger E, Raghunathan JN and Kelvin JF: Young
adult female cancer survivors' unmet information needs and
reproductive concerns contribute to decisional conflict regarding
posttreatment fertility preservation. Cancer. 122:2101–2109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Laisk-Podar T, Lindgren CM, Peters M,
Tapanainen JS, Lambalk CB, Salumets A and Mägi R: Ovarian
physiology and GWAS: biobanks, biology, and beyond. Trends
Endocrinol Metab. 27:516–528. 2016. View Article : Google Scholar : PubMed/NCBI
|