Introduction
Diabetes mellitus is a clinical disorder of
intermediary metabolism characterized by hyperglycemia and
glycosuria due to the inadequate secretion and/or utilization of
insulin, and previous investigation has demonstrated the potential
of islet transplantation for the cure for type 1 diabetes with
successful islet isolation and preparation (1). One crucial limitation of islets therapy
in laboratory research and clinic application is the shortage of
islets donations, which is due to the low efficiency of current
islet isolation method (2,3). Therefore, high yielding islet isolation
is a prerequisite of successful islet transplantation (4).
Over the past 40 years, various methods for the
isolation of rat islets have been introduced. Isolation of rat
pancreas islets was first described by Lacy and Kostianovsky
(5), and various subsequent
procedures have been reported for pancreatic islet isolation based
on this technique (6). However, the
majority of these methods were only able to obtain relatively low
islet yields, which resulted in a large number of wasted rat islets
(7). Following the application of
all of these methods in our laboratory, it was demonstrated that
the low isolation efficiency was predominantly due to the
inadequate digestion of pancreas tissue. To improve the digestion
efficiency, the rat pancreas was either perfused with enzyme
solution or cut into pieces before digestion (8,9).
However, the commonly used stationary method exhibited poor
tissue/enzyme interaction and resulted in inadequate digestion
(10,11). Islets were found to attach or become
trapped in undigested tissue, which led to a decrease in isolation
yield (12). The current
well-established stationary method, which involves cannulation
through the bile duct and Histopaque gradient centrifugation, is
able to harvest 500–1,000 islets from one rat, which is 50% of the
total amount of a rat pancreas (13,14).
In the present study, a mechanical shaking method
was established to solve the low efficiency of islet isolation and
improve the islet yield. By applying continuous shaking during
digestion, the pancreas tissue was adequately digested, which
resulted in an improved isolation yield of up to 1,500 islets per
rats (50% more than the well-established stationary method).
Notably, the majority of the isolated islets were morphologically
intact with a well-defined surface. Furthermore, optimization of
the shaking conditions was studied and characterization of the
islets demonstrated well-preserved function to control blood
glucose in vitro and in vivo. This facile mechanical
shaking method markedly improved the yield of islet isolation and
is likely to aid islet preparation and transplantation for the
treatment of type 1 diabetes.
Materials and methods
Animals
A total of 296 male Sprague-Dawley (SD) rats (8–12
weeks old), weighing 250–300 g, and 32 C57BL/6 mice (6–8 weeks
old), weighing 20–22 g were purchased from the Laboratory Animal
Services Center of Hubei University of Chinese Medicine (Wuhan,
China). Animals were maintained in a controlled environment (room
temperature, 20±1°C; relative humidity, 55±15%; 12-h light/dark
cycle) and permitted ad libitum access to food and water
throughout the experiment. All animals were treated in accordance
with international ethical guidelines and the National Institutes
of Health Guide concerning the Care and Use of Laboratory
Animals.
Facile mechanical shaking method
Male SD rats, weighing 250–300 g, were used.
Following CO2 euthanasia, 8 ml M199/HEPES buffer
digestion solution (Hyclone; GE Healthcare, Logan, UT, USA)
supplemented with 0.1% Liberase (Roche Diagnostics, Indianapolis,
IN, USA) was cannulated into the pancreas via the bile duct. The
pancreas was subsequently transferred to a 50-ml centrifuge tube
(non-cannulated pancreas was cut into 1 mm2 pieces using
surgical scissors) and maintained on ice for 30 min. Subsequently,
the tube was placed on a rotary shaker (500 rpm; VWR Scientific,
San Francisco, CA, USA) in a 37°C incubator for 5–35 min. Once all
the tissue was digested and the suspension was homogeneous 4°C PBS
was added to terminate digestion and the sample was centrifuged
twice in 4°C PBS to remove Liberase (15 min at 500 × g),.
The digested pancreas was filtered through a 450-µm sieve
(Sartorius AG, Goettingen, Germany) to remove large particles
(mostly non-cannulated pancreas) and the digested pancreas was
subsequently suspended in a Histopaque 1077/PBS media gradient
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), and was
centrifuged at ~1,000 × g for 30 min at room temperature.
Islets trapped on the sieve were obtained by washing the sieve
several times with PBS. All islets were purified by the selection
of complete and live islets by hand under a light microscope for
the following experiments under a light microscope (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Stationary method
Following cannulation, the pancreas was transferred
to a centrifuge tube, maintained on ice for 30 min and subsequently
incubated in a 37°C water bath for digestion. Following incubation,
the tube was shaken by hand to disrupt the pancreas until the
suspension was homogeneous. Subsequently, 4°C PBS was added to
terminate digestion and drastic shaking was applied 40 times to
completely disintegrate the large tissue particles. The remaining
procedure was the same as the mechanical shaking method.
Degrees of pancreas perfusion
To verify that the mechanical shaking method was
suitable for the pancreas, cannulation was performed in three
degrees, including fully-cannulated, half-cannulated and
non-cannulated. In the fully-cannulated group, 8 ml media was
applied and the pancreas was fully perfused; whereas in the
half-cannulated group, 4 ml media was applied and the pancreas was
just partially perfused. No media was cannulated into the pancreas
in the non-cannulated group.
Addition of inhibitor of
caspase-activated DNase (ICAD)
Anti-rat ICAD was purchased from Medical and
Biological Laboratories, Co., Ltd., (Nagoya, Japan) (15). A total of 1 µg/ml ICAD was added to
the digestion media prior to shaking, and the formation of
DNA-crosslinking was monitored, as previously described (16).
Islet culture
Isolated islets were cultured in a non-treated Petri
dish with an islet density of 5 islets/cm2. The culture
media was RPMI-1640 (Hyclone; GE Healthcare) supplemented with 2.8
mmol glucose, 10% fetal bovine serum (FBS) and 1% penicillin and
streptomycin (all Gibco; Thermo Fisher Scientific, Inc.) at 37°C in
humidified air containing 5% CO2 (17). Prior to each test, the islets were
purified and complete and live islets were selected by hand under a
light microscope for use in subsequent experiments.
Morphology and size of isolated
islets
Following isolation, islet morphology was studied
under a light microscope (Thermo Fisher Scientific, Inc.). Islets
in good condition are brown in color with a well-identified surface
and no necrotic zone in the center. For diameter distribution
measurement, 200 islets were randomly chosen and analyzed under a
dissection microscope with a fluorescent illuminator equipped with
an ocular micrometer with an accuracy of 25 µm.
Glucose challenge
For the different isolation methods, 100 islets with
a diameter of 100–200 µm were selected. Islets were pre-incubated
for 1 h in 5 ml FBS-free RPMI-1640 medium containing low glucose
(2.8 mmol) in a 6-well plate at 37°C with 5% CO2. During
the following 3 h, RPMI-1640 medium with 2.8, 28 and 2.8 mmol
glucose was added consecutively at each hour, respectively.
Following incubation, the medium was collected and stored at −20°C
for subsequent insulin concentration determination.
Islet transplantation
Male C57BL/6 mice were utilized for transplantation.
To establish insulin-dependent diabetic mice models, normal C57BL/6
mice were treated with 50 mg/kg streptozotocin (STZ; Sigma-Aldrich;
Merck Millipore) via the tail veil for 5 consecutive days. The
blood glucose levels of all the mice were retested prior to
transplantation. Only mice with non-fasted blood glucose levels
>300 mg/dl for 2 consecutive days were considered diabetic and
underwent transplantation. Mice were anesthetized using 3%
isofluorane (AErrane; Baxter, Deerfield, IL, USA) in oxygen and
maintained at the same rate throughout the procedure. Prior to
transplantation, all islets were immersed in PBS three times to
completely remove the culture media. A total of 500 islets were
then suspended in 500 µl sterile PBS and transferred in a 1-ml
syringe. The abdomens of the mice were shaved and sterilized using
alcohol and the islets were subsequently injected into the
abdominal cavity. The blood glucose levels of treated mice were
monitored once every 2 days. A small drop of blood was collected
from the tail vein using a lancet and tested using a commercial
glucometer (Roche Diagnostics). Mice with blood glucose levels
<200 mg/dl were considered normoglycemic. Monitoring continued
until all mice had returned to a hyperglycemic state (blood glucose
levels >250 mg/dl).
Statistical analysis
All results are expressed as the mean ± standard
deviation of data obtained from triplicate experiments. All
analyses were performed using SPSS 17.0 statistical analysis
software (SPSS, Inc., Chicago, IL, USA). Two-tailed Student's
t-test was used for all statistical analyses, with P<0.05
considered to indicate a statistically significant difference.
Results
Islet yield is improved by the facile
mechanical shaking method
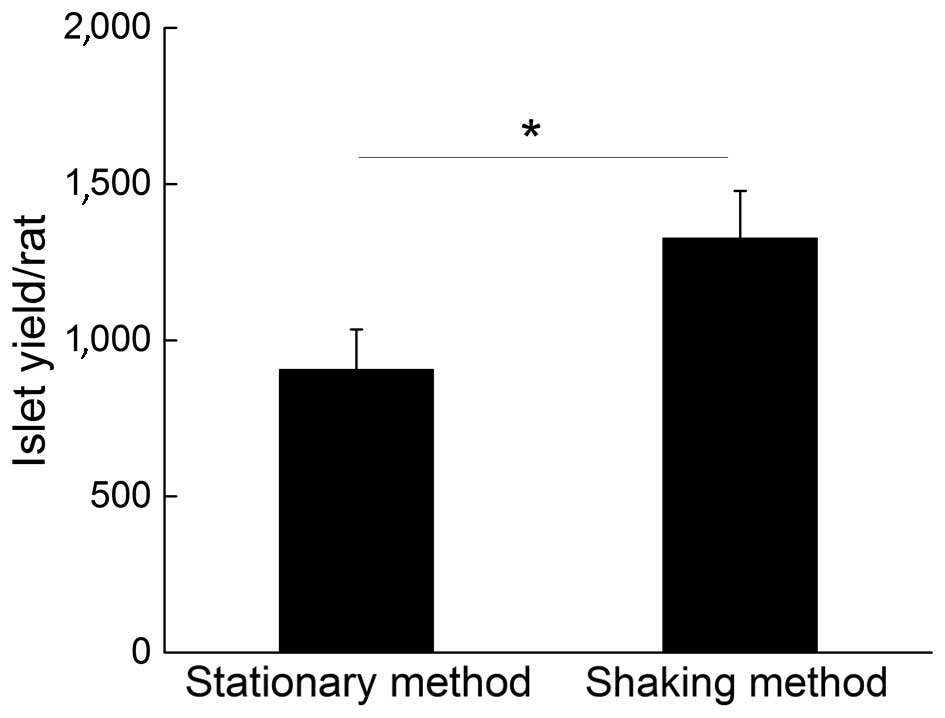
Fig. 1 demonstrates a
comparison of islet yields from a single rat using the common
stationary method and shaking methods. The results indicated that a
higher yield of isolated islets was obtained with the facile
mechanical shaking method (1,326±134), as compared with the common
stationary method (906±128), which was an improvement of ~50% on
the isolation yield.
Fully cannulated treatment and the
presence of ICAD contributes to an increment of islet yield
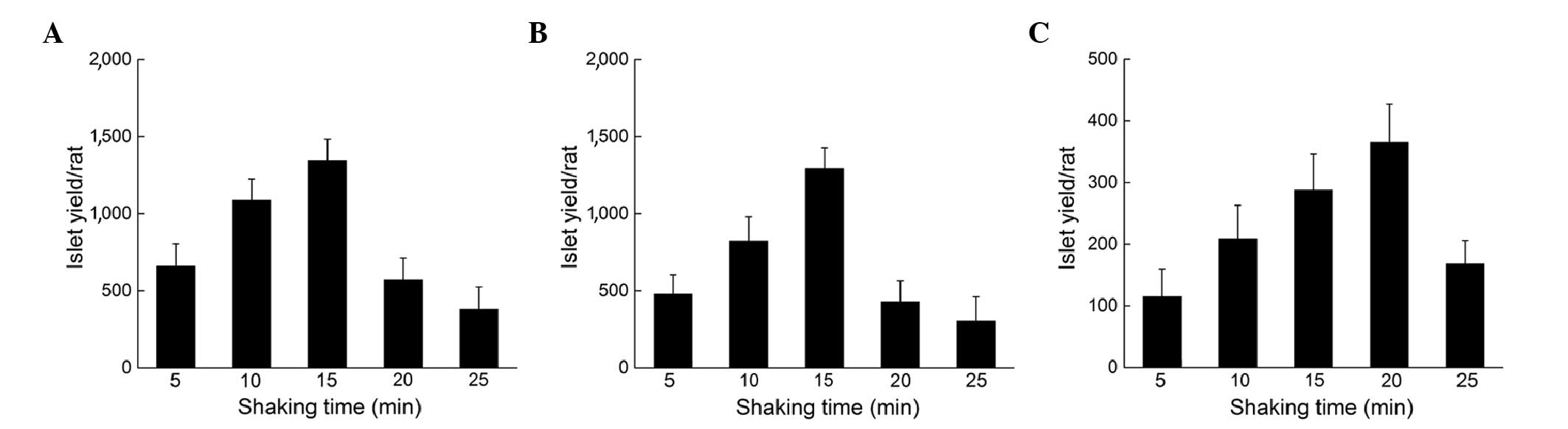
To study how shaking time may affect the isolation
yield, the pancreas was placed on the shaker for varying periods of
time and the different cannulation situations were studied
individually. For the fully-cannulated pancreas, as the shaking
time increased, the isolation yield exhibited an increasing trend
with a maximum yield of 1,346±137 when the shaking time was 15 min
(Fig. 2A). When the shaking time
increased further, the yield did not increase as expected; instead,
a marked reduction was detected due to the formation of
DNA-crosslinks. The half-cannulated pancreas exhibited a similar
trend to the fully-cannulated pancreas, with a maximum yield of
1,293±135 islets when shaken for 15 min (Fig. 2B). However, the non-cannulated
pancreas demonstrated a different trend, as the best shaking time
was delayed to ~20 min, with a maximum yield of 365±62 islets
(Fig. 2C). This may be due to larger
tissues requiring additional time to be digested. One phenomenon
noted in all the groups was that further shaking following the
maximum yield induced the formation of DNA-crosslinks, which
decreased the isolation yield markedly.
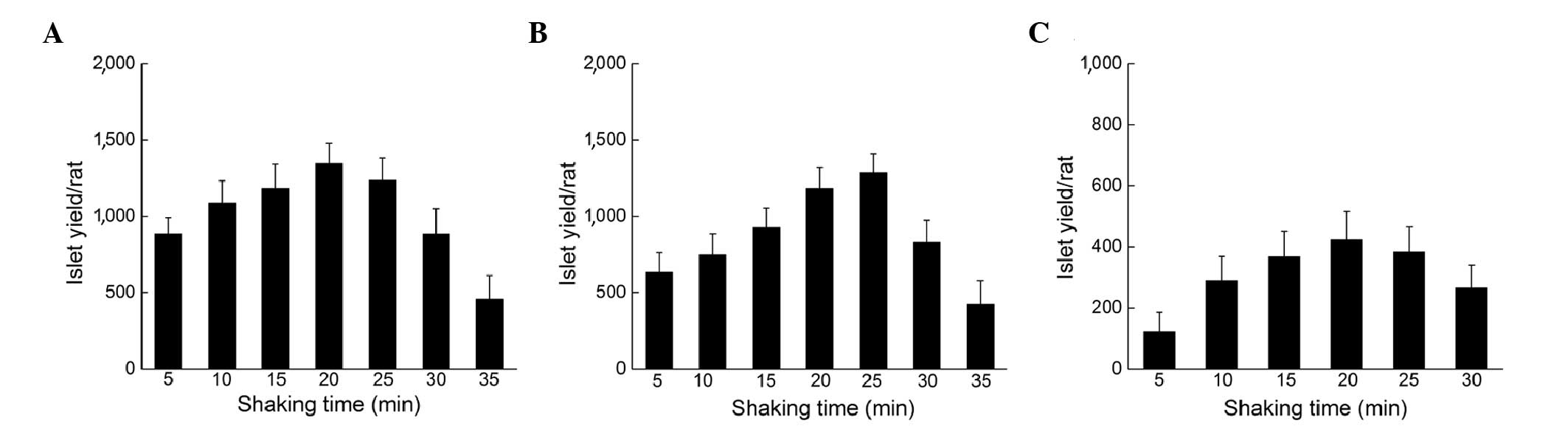
To avoid the formation of DNA-crosslinks, ICAD was
added to the digest media. Fig 3
shows the improvement of islet yield following the addition of
ICAD. For both the fully cannulated and half-cannulated pancreas,
the optimal shaking time was shifted to 20 min with a further
increased yield of 1,344±134 and 1,286±124 islets (Fig. 3A and B). One crucial improvement was
that, following the optimal shaking time, the yield gradually
decreased over a longer period of time; therefore, the addition of
ICAD made the shaking procedure more controllable. For the
non-cannulated pancreas, the effect of ICAD addition was not
obvious; the optimal shake time remained at 20 min with 423±92
islets (Fig. 3C).
Shaking procedures retain the
biological activity and function of islets
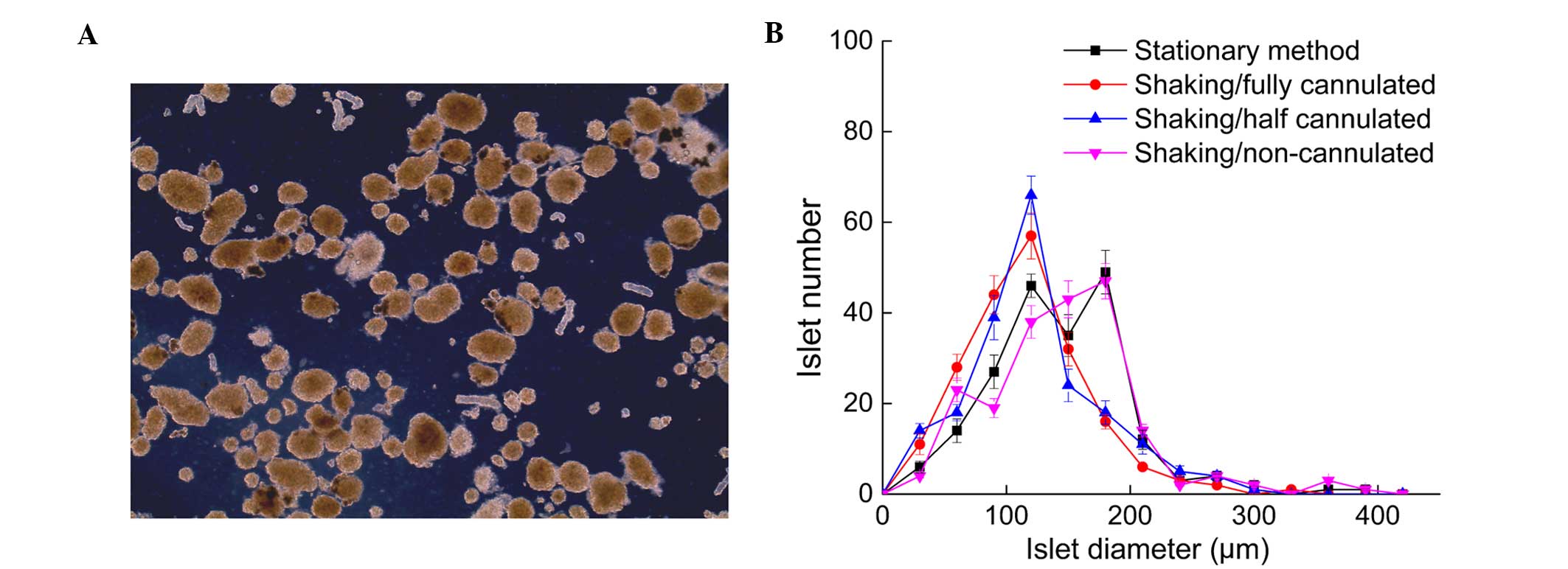
Fresh islets were obtained following the shaking
method. The majority of isolated islets were morphological intact
with a well-defined surface, indicating the shaking procedure did
not break the islets apart (Fig. 4).
Almost none of the isolated islets exhibited a central necrotic
zone, which suggested the islet condition in the shaking method
group was consistent with the stationary method. Islets size
distribution was also calculated and the findings indicated that
the islets isolated via the stationary method has the same size
distribution as the non-cannulated group, which had more larger
islets than the fully-cannulated and half-cannulated groups
subjected to the shaking method (Fig.
4).
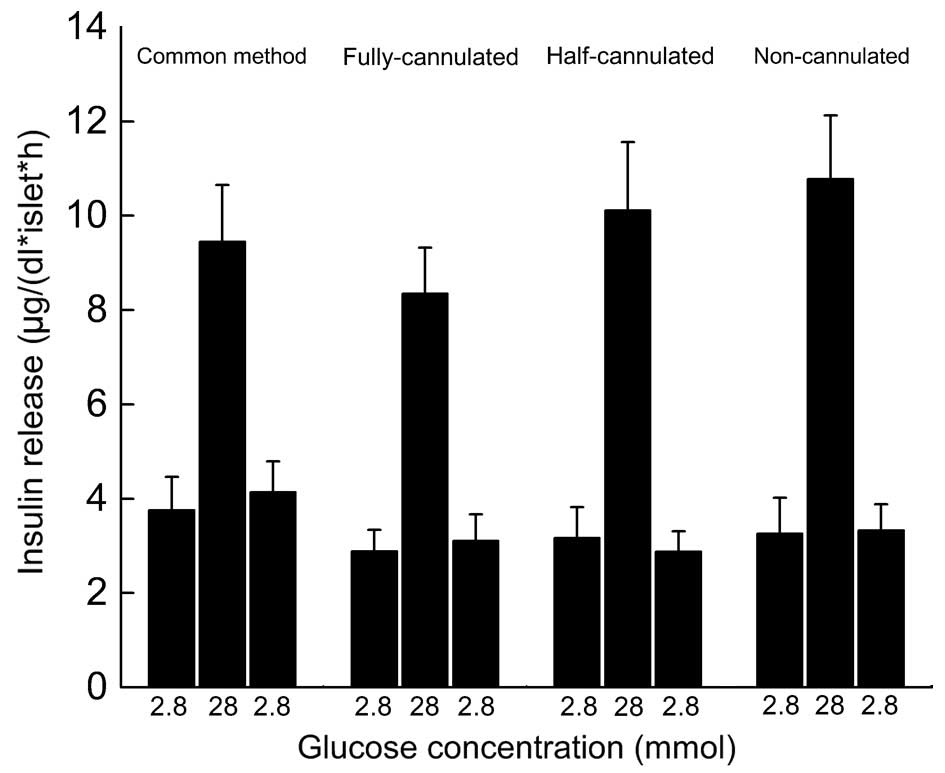
Glucose challenge is an effective method for the
study of islet functions in vitro prior to transplantation.
The refraction index (high value/low value) represents insulin
secretion ability via the islets' response to changes in blood
glucose levels. As Fig 5
demonstrates, the refraction indices of all groups were >2.5,
which indicated the well-preserved function of the isolated
islets.
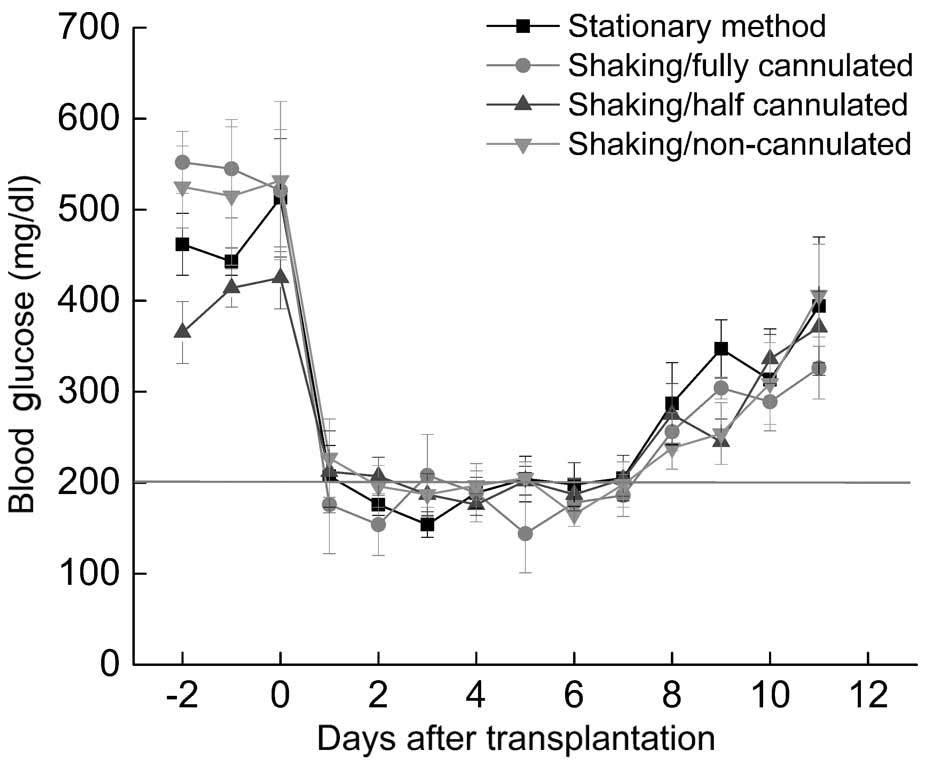
Transplantation of isolated islets
with mechanical shaking successfully decreases the increased blood
glucose levels of diabetic mice
Transplanted islets exhibited therapeutic effects
after 1 day of transplantation and all diabetic mice became
normoglycemic (Fig. 6). All the
groups exhibited the same trend at the initial stage, but failed to
control blood glucose level after ~7 days of transplantation.
Foreign body reactions or an immune response to xenografts may
result in the death of transplanted islets. The present results
indicated that greater numbers of islets can be isolated with the
facile mechanical shaking method, and these islets also exhibited
the normal function of decreasing blood glucose levels.
Discussion
The yield of islets isolation is crucial for islet
transplantation in vivo (18). Though islets isolation methods have
been extensively studied, the yield remains relatively low compared
with the total islets isolated from a rat pancreas (19,20). In
the present study, a facile mechanical shaking method was
established, which improved the isolation yield to up to 1,600
islets per rat. Moreover, the islets exhibited well-preserved
function to control blood glucose in vitro and in
vivo. The present results provide an improved alternative
isolation approach for islet preparation and transplantation in
diabetes treatment.
Cannulation through the bile duct is paramount for
islets isolation due to more comprehensive interaction between the
pancreas tissue and digest media. However, successful cannulation
required good cutting off of the ampulla site and careful handling
of the syringe, as a minor error may cause enzyme media leakage to
the surrounding tissue or duodenum, leading to the half-cannulated
state (8). When cannulation was not
adequate, particularly when the pancreas was removed without
cannulation, it was difficult to harvest a large number of islets
via the stationary method. Furthermore, large sections of tissue
were not well-digested when the stationary technique was utilized.
By studying the islets yield following various shaking times, the
optimal procedure for different cannulation situations was
elucidated. In addition, the present findings also indicated that
the optimal shaking time for the non-cannulated pancreas was
relatively longer than with the fully and half cannulated pancreas,
which may be explained by the increased time required by the enzyme
to digest the larger sections of tissue. Notably, a singular
optimal shaking time point was not elucidated, as the pancreas
states prior to digestion varied each time. The key point
demonstrated by the present findings was that digestion should be
monitored until the majority of the large sections of tissue have
been digested into tiny sections, which ensure that they are able
to precipitate at the bottom like sand.
In the present study, only 500–800 islets were
successfully isolated from one rat pancreas via the
well-established stationary method. Following the stationary
digestion process, numerous islets were found to be attached to
large sections of tissue. By drastically shaking the tissue in the
tube, some of the islets were detached from the tissue. However,
violent shaking may also cause islets to disintegrate (21). To solve this problem, instead of
shaking after digestion, a continuous shaking protocol was applied
during enzyme digestion. The results demonstrated that the shaking
method resulted in an islet yield that was ~2 times greater than
that of the stationary method. We hypothesize that the following
aspects may contribute to this higher yield of rat islets: i) The
digested enzyme had more comprehensive interaction with the
pancreas tissue in the tube when shaking was applied, which means
the enzyme can digest deeper into the tissue and disintegrate it
more effectively, resulting in much smaller tissue sections
compared with the stationary method (22); or ii) shaking during digestion
provided a continuous and relatively gentle shear force to those
islets exposed in the media (23).
Islets may gradually detach from the tissue instead of becoming
trapped deep into the undigested tissue, thus they are able to
maintain their morphological integration.
Notably, obvious differences were observed for the
different situations in which digestion occurred, when shaking was
conducted for too long a time period. For example, tiny tissue
sections were further digested, causing cell rupture and DNA
release (data not shown) (24). Tiny
tissue sections may undergo DNA crosslinking and become flocculent,
which results in the tissue being unable to precipitate to the
bottom of the sample being treated, inducing a huge decrease in the
isolation yield (25). However, the
same phenomenon was not observed in the non-cannulated pancreas
since the digestion efficiency was relatively lower and individual
cells did not rupture before the large tissue sections had been
digested. Flocculent tissue forms due to the release of DNA and
caspase-activated DNase subsequently breaks the DNA, which then
acts as a cross-linker, and islets becomes trapped in the
cross-linked tissue. A previous study has shown that the addition
of the ICAD may help to avoid the effect of broken DNA (26). Shaking in the presence of ICAD
attenuated the formation of cross-linked tissue. During the full
and half cannulation protocols that required a more controlled
shaking time, the addition of ICAD had a marked effect in that
there was no flocculent tissue formed even following extended
shaking times and instead, the tissue was further digested. This
not only made the shaking procedure easier to control but also
further increased the islet yields in the cannulated groups.
However, for the non-cannulated groups, the islet yield was lower
even in the presence of ICAD since the tissue size was larger and
the enzyme may not have been able to reach the central tissue to
rupture individual cells. Quantity and quality are usually mutually
exclusive.
During the isolation progress, analysis of islet
size distribution was conducted to determine if the continuous
shear force was able to break islets apart. The present results
indicated that there was a slight difference between the stationary
technique and the non-cannulated method, which experienced less
shear force in shaking and resulted in more large islets. Under a
light microscope, the differences were difficult to recognize and
islets from all methods were morphologically in good condition with
a well-defined surface. Furthermore, it should be noted that bigger
islets are not necessarily better, as previous culture and
transplantation studies found that larger islets had a large
necrotic zone when beta cells in the center experienced a low
oxygen transport rate (27,28). Glucose challenge was not only used to
verify the viability of islets by studying the capability of islets
to secret insulin response to various glucose levels, but it is
also a critical in vitro experiment to predict the
therapeutic efficiency of islets transplantation (29). The present results showed minimal
differences between the islets isolated via the shaking and
stationary methods, respectively, which have already been proven to
exhibit good therapeutic efficiency when transplanted. The high
reflection index indicated a sufficient amount of insulin would be
secreted by islets isolated via the shaking method when blood
glucose level raise. Islet transplantation has been demonstrated to
be the most promising approach to elucidating a cure for type 1
diabetics and it may also be a direct way to verify the in
vivo activity of isolated islets (30). The intraperitoneal cavity has been
shown to be one of best transplantation sites, where nutrition and
oxygen are abundant (31). Blood
glucose levels exhibited the same trend for all the protocols, and
xenograft islets were able to control the blood glucose levels of
diabetic mice following transplantation. Viability persisted for
approximately one week, which was consistent with the findings of
previous studies (32,33).
The present study described a facile mechanical
shaking method for the high isolation efficiency of rat islets. In
the presence of rat ICAD, the isolation yield could reach as high
as 1,600 islets per rat, which was 55% more than the current
well-established stationary method. In vitro tests
demonstrated that isolated islets were morphologically intact and
had well-preserved function in response to blood glucose
alterations, and the transplantation of isolated islets
successfully reversed the blood glucose levels of diabetic mice for
a week. With this facile mechanical shaking method, islet
transplantation studies conducted with rats in the laboratory
should be more effective. In addition, this method may be applied
in human islet isolation and could potentially improve the
efficiency of clinical islet therapy.
References
|
1
|
Lakey JR, Burridge PW and Shapiro AM:
Technical aspects of islet preparation and transplantation. Transpl
Int. 16:613–632. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsumoto S, Okitsu T, Iwanaga Y, Noguchi
H, Nagata H, Yonekawa Y, Yamada Y, Fukuda K, Tsukiyama K, Suzuki H,
et al: Insulin independence after living-donor distal
pancreatectomy and islet allotransplantation. Lancet.
365:1642–1644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsumoto S, Okitsu T, Iwanaga Y, Noguchi
H, Nagata H, Yonekawa Y, Yamada Y, Fukuda K, Shibata T, Kasai Y, et
al: Successful islet transplantation from nonheartbeating donor
pancreata using modified Ricordi islet isolation method.
Transplantation. 82:460–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan KM, Desai CS, Kalb B, Patel C,
Grigsby BM, Jie T, Gruessner RW and Rodriguez-Rilo H: MRI
prediction of islet yield for autologous transplantation after
total pancreatectomy for chronic pancreatitis. Dig Dis Sci.
58:1116–1124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lacy PE and Kostianovsky M: Method for the
isolation of intact islets of Langerhans from the rat pancreas.
Diabetes. 16:35–39. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyazaki J, Araki K, Yamato E, Ikegami H,
Asano T, Shibasaki Y, Oka Y and Yamamura K: Establishment of a
pancreatic beta cell line that retains glucose-inducible insulin
secretion: Special reference to expression of glucose transporter
isoforms. Endocrinology. 127:126–132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sutherland DE, Radosevich DM, Bellin MD,
Hering BJ, Beilman GJ, Dunn TB, Chinnakotla S, Vickers SM, Bland B,
Balamurugan AN, et al: Total pancreatectomy and islet
autotransplantation for chronic pancreatitis. J Am Coll Surg.
214:409–424; discussion 424–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li DS, Yuan YH, Tu HJ, Liang QL and Dai
LJ: A protocol for islet isolation from mouse pancreas. Nat Protoc.
4:1649–1652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soltani SM, O'Brien TD, Loganathan G,
Bellin MD, Anazawa T, Tiwari M, Papas KK, Vickers SM, Kumaravel V,
Hering BJ, et al: Severely fibrotic pancreases from young patients
with chronic pancreatitis: Evidence for a ductal origin of islet
neogenesis. Acta Diabetol. 50:807–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin SM, Oh SH, Kim SK, Jung HS, Choi SH,
Jang KT, Lee KT, Kim JH, Lee MS, Lee MK and Kim KW: Diabetes-free
survival in patients who underwent islet autotransplantation after
50% to 60% distal partial pancreatectomy for benign pancreatic
tumors. Transplantation. 95:1396–1403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goto T, Tanioka Y, Sakai T, Terai S,
Kamoda Y, Li S, Tanaka T, Tsujimura T, Matsumoto I, Fujino Y, et
al: Application of the two-layer method on pancreas digestion
results in improved islet yield and maintained viability of
isolated islets. Transplantation. 83:754–758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Machida T, Tanemura M, Ohmura Y, Tanida T,
Wada H, Kobayashi S, Marubashi S, Eguchi H, Ito T, Nagano H, et al:
Significant improvement in islet yield and survival with modified
ET-Kyoto solution: ET-Kyoto/Neutrophil elastase inhibitor. Cell
Transplant. 22:159–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito T, Chen D, Chang CW, Kenmochi T, Saito
T, Suzuki S and Takemoto JY: Mesobiliverdin IXα enhances rat
pancreatic islet yield and function. Front Pharmacol. 4:502013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Haan BJ, Faas MM, Spijker H, van
Willigen JW, de Haan A and de Vos P: Factors influencing isolation
of functional pancreatic rat islets. Pancreas. 29:e15–e22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enari M, Sakahira H, Yokoyama H, Okawa K,
Iwamatsu A and Nagata S: A caspase-activated DNase that degrades
DNA during apoptosis, and its inhibitor ICAD. Nature. 391:43–50.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J and Xu M: Apoptotic DNA
fragmentation and tissue homeostasis. Trends Cell Biol. 12:84–89.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YH, Wee YM, Choi MY, Lim DG, Kim SC
and Han DJ: Interleukin (IL)-10 induced by CD11b(+) cells and
IL-10-activated regulatory T cells play a role in immune modulation
of mesenchymal stem cells in rat islet allografts. Mol Med.
17:697–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Najjar M, Manzoli V, Abreu M, Villa C,
Martino MM, Molano RD, Torrente Y, Pileggi A, Inverardi L, Ricordi
C, et al: Fibrin gels engineered with pro-angiogenic growth factors
promote engraftment of pancreatic islets in extrahepatic sites in
mice. Biotechnol Bioeng. 112:1916–1926. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attia AA: Histological and electron
microscopic studies of the effect of beta-carotene on the pancreas
of streptozotocin (STZ)-induced diabetic rats. Pak J Biol Sci.
12:301–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Hong F, Chen H, Fan RF, Zhang XL,
Zhang Y and Zhu JX: Distinctive expression and cellular
distribution of dopamine receptors in the pancreatic islets of
rats. Cell Tissue Res. 357:597–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klaffschenkel RA, Waidmann M, Northoff H,
Mahmoud AA and Lembert N: PK11195, a specific ligand of the
peripheral benzodiazepine receptor, may protect pancreatic
beta-cells from cytokine-induced cell death. Artif Cells Blood
Substit Immobil Biotechnol. 40:56–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimoda M, Noguchi H, Fujita Y, Takita M,
Ikemoto T, Chujo D, Naziruddin B, Levy MF, Kobayashi N, Grayburn PA
and Matsumoto S: Islet purification method using large bottles
effectively achieves high islet yield from pig pancreas. Cell
Transplantat. 21:501–508. 2012. View Article : Google Scholar
|
|
23
|
Silva PN, Green BJ, Altamentova SM and
Rocheleau JV: A microfluidic device designed to induce media flow
throughout pancreatic islets while limiting shear-induced damage.
Lab Chip. 13:4374–4384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Itoh T, Takita M, SoRelle JA, Shimoda M,
Sugimoto K, Chujo D, Qin H, Naziruddin B, Levy MF and Matsumoto S:
Correlation of released HMGB1 levels with the degree of islet
damage in mice and humans and with the outcomes of islet
transplantation in mice. Cell Transplant. 21:1371–1381. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deans AJ and West SC: DNA interstrand
crosslink repair and cancer. Nat Rev Cancer. 11:467–480. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto-Tanaka M, Makino T, Motoyama A,
Miyai M, Tsuboi R and Hibino T: Multiple pathways are involved in
DNA degradation during keratinocyte terminal differentiation. Cell
Death Dis. 5:e11812014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barkai U, Weir GC, Colton CK, Ludwig B,
Bornstein SR, Brendel MD, Neufeld T, Bremer C, Leon A, Evron Y, et
al: Enhanced oxygen supply improves islet viability in a new
bioartificial pancreas. Cell Transplantat. 22:1463–1476. 2013.
View Article : Google Scholar
|
|
28
|
Henriksnäs J, Lau J, Zang G, Berggren PO,
Köhler M and Carlsson PO: Markedly decreased blood perfusion of
pancreatic islets transplanted intraportally into the liver:
Disruption of islet integrity necessary for islet
revascularization. Diabetes. 61:665–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szkudelski T:
Streptozotocin-nicotinamide-induced diabetes in the rat.
Characteristics of the experimental model. Exp Biol Med (Maywood).
237:481–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakata N, Sumi S, Yoshimatsu G, Goto M,
Egawa S and Unno M: Encapsulated islets transplantation: Past,
present and future. World J Gastrointest Pathophysiol. 3:19–26.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bruni A, Gala-Lopez B, Pepper AR,
Abualhassan NS and Shapiro AJ: Islet cell transplantation for the
treatment of type 1 diabetes: Recent advances and future
challenges. Diabetes Metab Syndr Obes. 7:211–23. 2014.PubMed/NCBI
|
|
32
|
Caballero F, Siniakowicz K, Hollister-Lock
J, Duran L, Katsuta H, Yamada T, Lei J, Deng S, Westermark GT,
Markmann J, et al: Birth and death of human β-cells in pancreases
from cadaver donors, autopsies, surgical specimens, and islets
transplanted into mice. Cell Transplant. 23:139–151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang SB, Lee HY, Young DM, Tien AC,
Rowson-Baldwin A, Shu YY, Jan YN and Jan LY: Rapamycin induces
glucose intolerance in mice by reducing islet mass, insulin content
and insulin sensitivity. J Mol Med (Berl). 90:575–585. 2012.
View Article : Google Scholar : PubMed/NCBI
|