Introduction
Diabetic nephropathy (DN), the leading cause of end
stage renal disease, is the major cause of mortality in type 1
diabetes mellitus (DM) and the second most severe complications in
type 2 diabetes (1,2). Deposition of extracellular matrix (ECM)
in mesangial areas is a feature of DN, and mesangial cells have
been proposed to be the determinant of ECM accumulation (3,4).
However, the mechanism underlying ECM accumulation in DN is not
fully clarified.
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent endopeptidases that can degrade numerous types of
ECM components (5,6). Among others, MMP-2 basally expresses
while MMP-9 is an inducible enzyme, both of which primarily degrade
types-I and -IV collagen and laminin, major components of ECM
(7–9). Generally, MMP-2 and MMP-9 are involved
in tumor metastasis (6,10). Furthermore, it has been shown that
insufficient MMP-2 and MMP-9 may be a contributor of ECM
accumulation in DN (11). However,
the expression levels of MMP-2 and MMP-9 in DN remain
controversial, and even short- and long-term hyperglycemia may
exert differential effects (8,12–14). As
an inducible enzyme, MMP-9 may be more easily affected in patients
with DN (15). Therefore, the
changes of MMP-2, and particularly MMP-9, for high glucose (HG)
stimulation require clarification.
C-peptide is the linker between the A-chain and
B-chain of insulin. Lack of C-peptide along with insulin is the
primary feature of type 1 DM and late stage of type 2 DM (16). C-peptide has been found to have
unique beneficial effects on DN, attenuating glomerular and tubular
injury (17–19). Physiological concentration of
C-peptide can reverse the fibrosis of glomerular and recover renal
function in DN (20–22). Various mechanisms have been reported
for the protective effects of C-peptide, such as binding to its
receptor on the cell membrane, transporting into the cytoplasm and
nucleus, and interacting with functional proteins to exhibit its
effect (23–25). In a prior study, we observed that
C-peptide could dynamically localize in the nucleus to serve its
functions in HG-stimulated mesangial cells, which provided an
impetus for further clarifying the intrinsic mechanism of its
unique reversal effect on DN (26).
Although it has been reported that C-peptide exerted little effect
on MMP-2 in diabetic rats (22), the
short- and long-term effects of C-peptide on MMP-9 and MMP-2 in
HG-treated mesangial cells remains unknown.
In the present study, rat mesangial cells were
cultured to investigate the short- and long-term effects of
C-peptide on HG-affected MMP-9 and MMP-2 expression levels. After
mesangial cells were treated, MMP-9 and MMP-2 mRNA expression
levels, MMP-9 protein content and secretion were evaluated using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), western blot and enzyme-linked immunosorbent assay
(ELISA) analyses.
Materials and methods
Cells and treatment
The rat mesangial cell line (HBZY-1) was obtained
from China Center for Type Culture Collection (Wuhan, China) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific Co., Ltd., Shanghai, China) containing 5 mM glucose and
10% fetal bovine serum (Thermo Fisher Scientific Co., Ltd.). Cells
were cultured in 20, 25, 30 and 35 mM HG (Thermo Fisher Scientific
Co., Ltd.) or control (5 mM) glucose for 24 h, then treated with 30
mM HG for 3, 6, 12, 24, 48 and 72 h. MMP-9 and MMP-2 mRNA
expression levels were subsequently evaluated. Subsequently, 0.1,
0.3, 0.5, 0.7 and 0.9 nM C-peptide (Shanghai Taishi Biotechnology
Co., Ltd., Shanghai, China) was used to treat the HG-stimulated
mesangial cells for 3, 6, 12 and 24 h, then 0.7 nM C-peptide
treatment expanded to 120 h, the MMP-9 and MMP-2 mRNA expression
levels were evaluated. Furthermore, MMP-9 protein content was
evaluated for 6, 72, 96 and 120 h C-peptide treatment. In addition,
the MMP-9 secretion for HG and C-peptide treatments were detected.
Low glucose (LG, 5 mM) was used as control.
RT-qPCR
The MMP-9 and MMP-2 transcription was evaluated by
RT-qPCR. Total RNA was isolated using TRIzol reagent (Takara Bio,
Inc., Otsu, Japan) and reverse transcribed into cDNA using
RevertAid First Strand cDNA synthesis Kit (Fermentas; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), followed by PCR amplification
using the specific primers (Sangon Biotech Co., Ltd., Shanghai,
China). Rat MMP-9 forward primer, 5′-AAACCCTGCGTATTTCCATTCATC-3′,
and reverse primer, 5′-CACATCTCTCCTGCCGAGTTGC-3′ with 185 bp
product; MMP-2 forward primer, 5′-TGGAAGCATCAAATCGGACTG-3′, and
reverse primer, 5′-CCACCCTCTTAAATCTGAAATCAC-3′ with 186 bp product.
A Rotor-Gene 3000 system (Corbett Life Science; Qiagen, Shenzhen,
China) was used to perform the PCR reaction, using aSYBR Premix Ex
Taq II (RR82LR; Takara Biotechnology Co., Ltd., Dalian, China) and
analyze the data. Actin primers were used as an internal
standard.
Western blot analysis
The protein content of MMP-9 was detected using the
protocol previously described, using an anti-MMP-9 antibody (1:500;
#3852; Cell Signaling Technology, Inc., Danvers, MA, USA) (27), and the horseradish peroxidase-labeled
secondary antibody (1:5,000; 074–1506; Kirkegaard & Perry Lab,
Inc., Gaithersburg, MA, USA). Band intensity was quantified and
calculated. Actin was routinely served as a loading control
(1:1,000; #4967; Cell Signaling Technology, Inc., Danvers, MA,
USA).
ELISA
Following treatment, the culture medium was
collected to analyze the MMP-9 secretion by ELISA (H146-4; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China), according to
the manufacturer's instructions. Standards and samples were added
to wells of the plate and incubated for 1 h. After the wells were
washed with the ELISA wash buffer, the conjugated antibody was
added and incubated for 1 h. Then the wells were washed with the
ELISA wash buffer. The substrate was added in the wells and
incubated for 15 min. The stop solution was added and absorption
was measured using an ELISA reader at 450 nm (Multiskan Spectrum:
Thermo Fisher Scientific, Inc.). All tests were performed in
duplicate.
Statistical analysis
Statistical analysis of the data was performed using
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons
between two groups were performed using Student's t-test.
All values are presented as the mean ± standard deviation.
P<0.05 were considered to indicate a statistically significant
difference.
Results
Early dual effects of C-peptide on
MMP-9 expression in HG-treated mesangial cells
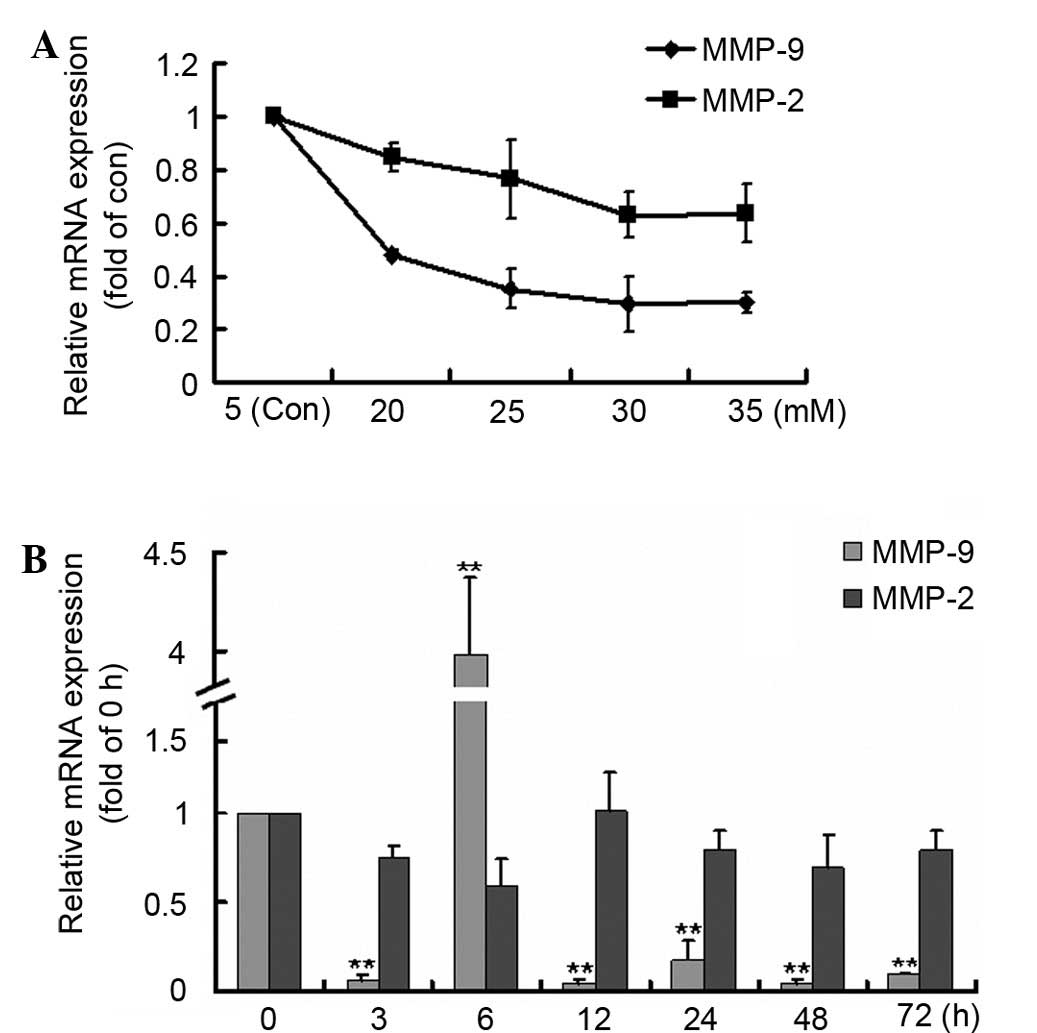
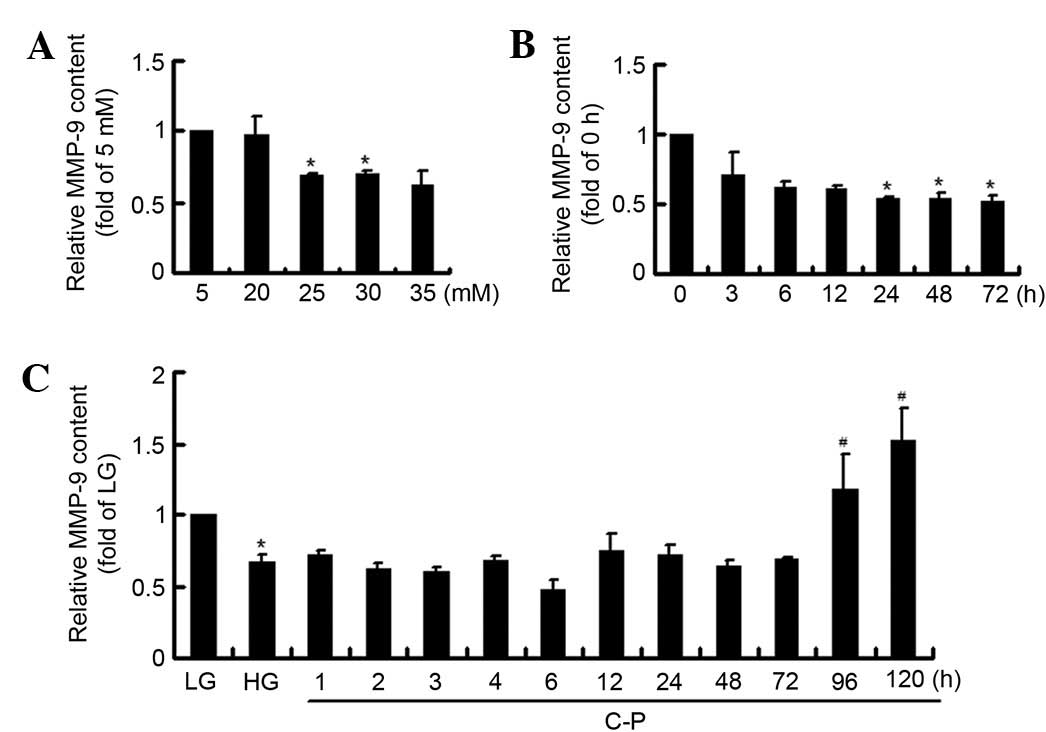
The concentration and time-dependent effects of HG
on MMP-9 and MMP-2 expression levels were detected. Both MMP-9 and
MMP-2 expression levels decreased following HG stimulation, most
markedly at 30 mM HG (Fig. 1A). Then
at 30 mM HG incubation, MMP-9 expression decreased significantly
compared with the 0 h group, except for an increase at 6 h;
however, MMP-2 expression showed no significant changes (Fig. 1B). The results confirmed that HG
suppressed MMP-9 expression in mesangial cells.
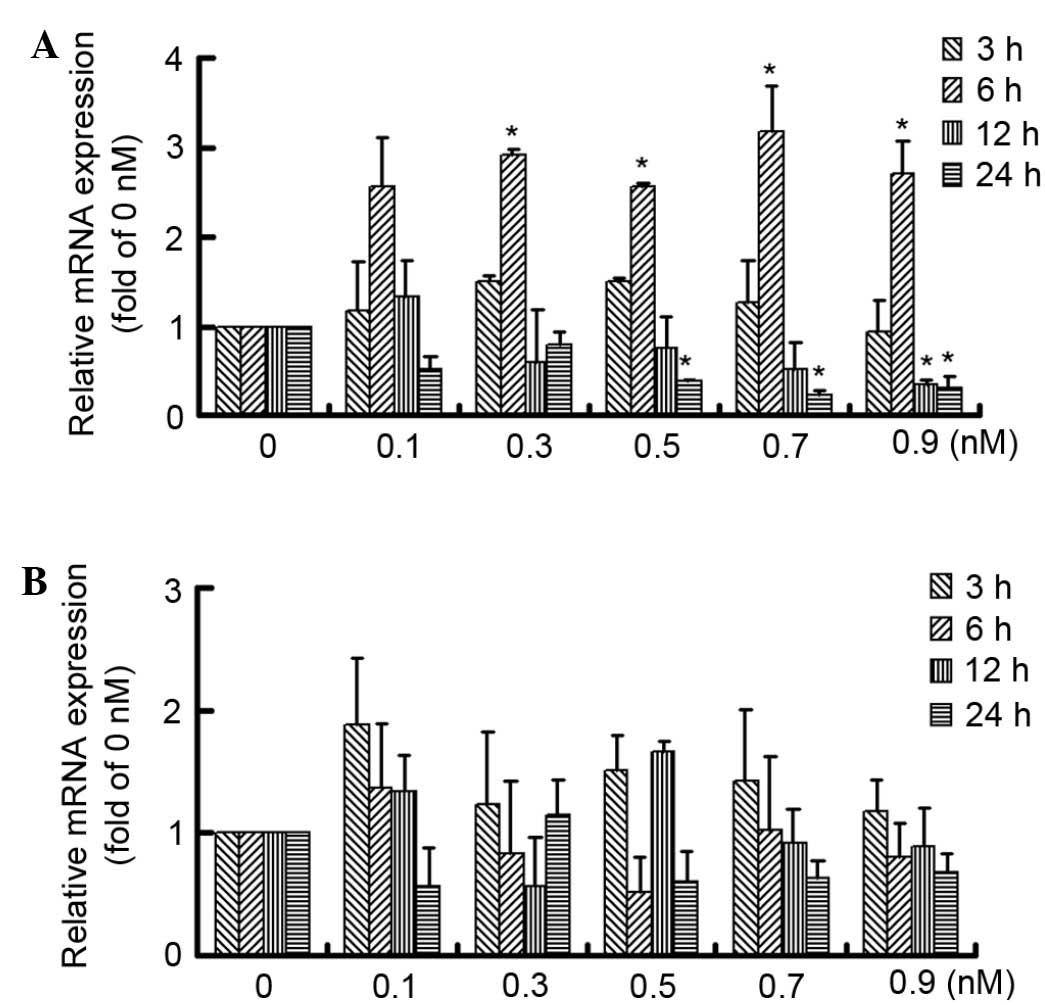
After pretreatment with HG for 24 h, the early
effects of C-peptide on MMP-9 and MMP-2 expression were
investigated. Although C-peptide treatment induced an increase in
MMP-9 expression at 6 h, MMP-9 expression decreased over time,
particularly at 24 h. Furthermore, 0.5, 0.7 and 0.9 nM C-peptide
produced a similar effect on MMP-9 expression (Fig. 2A). However, no significant difference
in MMP-2 expression was observed among groups (Fig. 2B). The results showed that C-peptide
exhibited an early dual effect on MMP-9 expression within 24 h
treatment in HG-treated mesangial cells.
Late induction effect of C-peptide on
MMP-9 in HG-treated mesangial cells
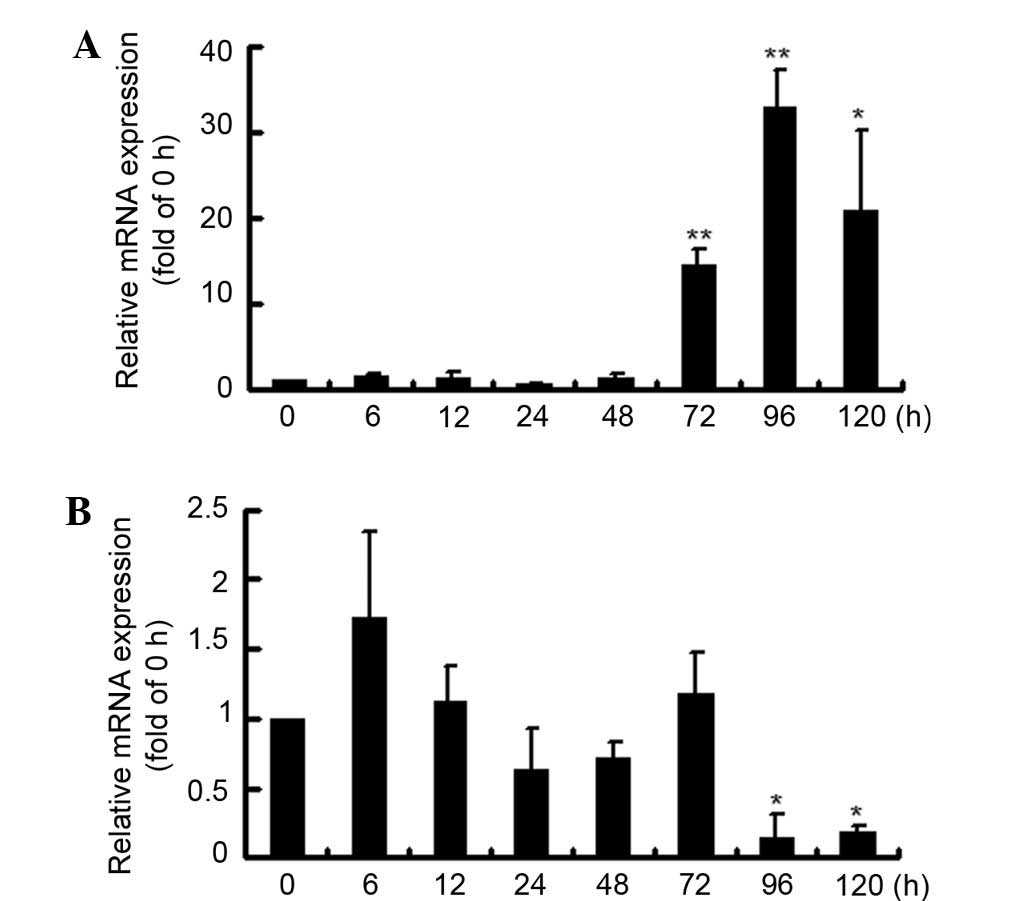
The treatment time was expanded to 120 h, and the
late effects of C-peptide on MMP-9 and MMP-2 expression levels were
investigated. It was found that MMP-9 expression increased markedly
between cells treated for 72 and 96 h, although the early changes
were inconsistent and not significant (Fig. 3A). However, MMP-2 expression was
disordered, but significantly decreased following 96 and 120 h of
treatment (Fig. 3B).
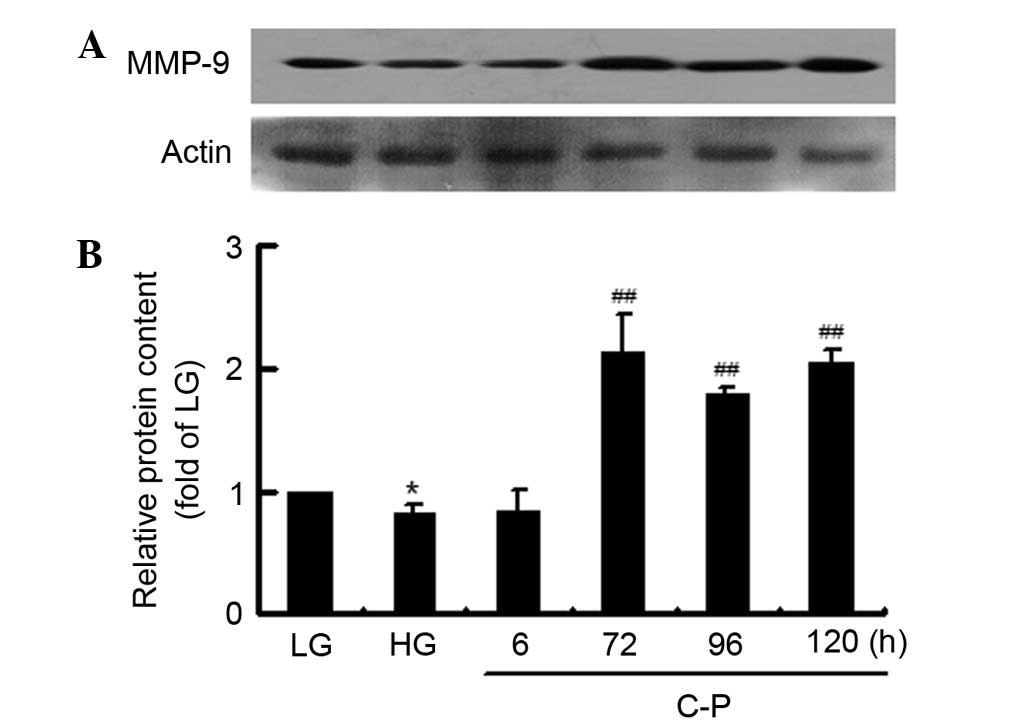
Then, the late induction effect of C-peptide on
MMP-9 expression was further verified by evaluation of its protein
content (Fig. 4). Compared with that
in the LG group, the MMP-9 protein content decreased in the HG
group. The HG-inhibited MMP-9 protein content was significantly
induced by 72, 96 and 120 h of C-peptide treatment. The results
suggest that C-peptide had a late induction effect on MMP-9 in
HG-treated mesangial cells.
Late reversal effect of C-peptide on
HG-suppressed MMP-9 secretion
After treatment, the culture medium was collected
for detection of MMP-9 secretion. The HG-suppressed MMP-9 secretion
from mesangial cells was initially verified. MMP-9 secretion was
found to be suppressed by 25 and 30 mM HG treatment (Fig. 5A). Furthermore, after 30 mM HG
incubation, the MMP-9 secretion time-dependently decreased, with a
significant difference at 24, 48 and 72 h (Fig. 5B).
Thus, the effects of C-peptide on the HG-suppressed
MMP-9 secretion were investigated (Fig.
5C). Compared with the LG group, the MMP-9 secretion was
significantly inhibited by HG incubation. Furthermore,
HG-suppressed MMP-9 secretion was significantly increased at 96 and
120 h C-peptide treatment, although no evident effect was observed
earlier than this. The results demonstrated that C-peptide had a
late reversal effect on the HG-suppressed MMP-9 secretion.
Discussion
Although C-peptide has reversal effects on the
fibrosis of glomerular in DN (18–22), the
underlying mechanism is not clarified. Insufficient MMP-2 and MMP-9
is considered to be a contributor of ECM accumulation (11). Whether C-peptide regulates MMP-2 and
MMP-9 to reverse fibrosis is unclear. In the present study, we
found that C-peptide exhibited a late induction effect on MMP-9 in
HG-stimulated rat mesangial cells, which may represent the
underlying mechanism of C-peptide's reversal effects on DN.
Basal MMP-2 and inducible MMP-9 primarily degrade
collagen and laminin, major components of ECM (28). Although insufficient MMP-2 and MMP-9
may lead to ECM accumulation, the expression levels of MMP-2 and
MMP-9 in DN remain controversial (29). In the present study, marked changes
of MMP-2 expression were not observed for HG stimulation. The MMP-9
expression was markedly inhibited by HG treatment, with the
exception of a sharp increase at 6 h. Furthermore, HG-inhibited
MMP-9 was verified by its secretion detection. The results revealed
that as an inducible enzyme, MMP-9 was more susceptible to be
affected by HG, which predominantly inhibited MMP-9 expression,
indicating that MMP-9 insufficiency may be a contributing factor of
ECM accumulation in DN.
C-peptide has reversal effects on the fibrosis of
glomerular in DN (30). Although a
number of mechanisms have been reported for the protective effects
of C-peptide (23–25), they are not specific to DN and to not
fully explain the anti-fibrosis effects of C-peptide. It has been
reported that C-peptide exerted little effect on MMP-2, which is
basally expressed and was hardly affected by HG (19). On the other hand, whether C-peptide
induces MMP-9 to reverse ECM accumulation is unknown. In the
present study, the short- and long-term effects of C-peptide on
MMP-9 and MMP-2 in HG-treated mesangial cells were
investigated.
Firstly, the early effects of C-peptide on MMP-9 and
MMP-2 expression levels at 24 h were detected. The MMP-2 expression
showed no significant changes, consistent with previous results
(22). Physiological concentrations
of C-peptide inhibited MMP-9 expression at 24 h treatment, except
for a sharp increase at 6 h, revealing the early dual effects of
C-peptide on MMP-9 expression. Next, the treatment time was
expanded to 120 h to investigate the late effects of C-peptide on
MMP-9 and MMP-2 expression levels. Notably, it was found that MMP-9
expression was markedly induced, while MMP-2 expression was
inhibited. In addition, the changes of MMP-9 protein content
confirmed the late induction effect of C-peptide on MMP-9
expression.
Furthermore, ELISA results showed that C-peptide had
a significant late reversal effect on the HG-inhibited MMP-9
secretion, although the early effect was unchanged. The decreased
MMP-9 secretion in response to HG stimulation and unchanged MMP-9
secretion for short-period C-peptide treatment indicated that the
sharp increases in MMP-9 mRNA expression in response to HG and
C-peptide at 6 h may be due to the inducibility of MMP-9 mRNA.
In conclusion, the results demonstrated that
C-peptide exhibited a late induction effect on MMP-9 in
HG-stimulated rat mesangial cells, which may be associated with the
underlying mechanism of C-peptide's reversal effects on DN.
Acknowledgements
This study was supported by Grants from the Major
State Basic Research Development Program of China (973 Program;
grant no. 2012CB518601), the National Natural Science Foundation of
China (grant no. 81070658), the Hebei Natural Science Foundation
(grant no. H2012206005).
References
|
1
|
Kato M and Natarajan R: Diabetic
nephropathy-emerging epigenetic mechanisms. Nat Rev Nephrol.
10:517–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Xiao HQ, Wang Y, Yang ZS, Dai LJ
and Xu YC: Differential expression and therapeutic efficacy of
microRNA-346 in diabetic nephropathy mice. Exp Ther Med.
10:106–112. 2015.PubMed/NCBI
|
|
3
|
Zhang L, Zhang J, Liu X, Liu S and Tian J:
Tribbles 3 regulates the fibrosis cytokine TGF-β1 through
ERK1/2-MAPK signaling pathway in diabetic nephropathy. J Immunol
Res. 2014:2403962014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller CG, Pozzi A, Zent R and
Schwarzbauer JE: Effects of high glucose on integrin activity and
fibronectin matrix assembly by mesangial cells. Mol Biol Cell.
25:2342–2350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galliera E, Tacchini L and Romanelli MM
Corsi: Matrix metalloproteinases as biomarkers of disease: Updates
and new insights. Clin Chem Lab Med. 53:349–355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tauro M, McGuire J and Lynch CC: New
approaches to selectively target cancer-associated matrix
metalloproteinase activity. Cancer Metastasis Rev. 33:1043–1057.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piperi C and Papavassiliou AG: Molecular
mechanisms regulating matrix metalloproteinases. Curr Top Med Chem.
12:1095–1112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukami K, Yamagishi S, Coughlan MT,
Harcourt BE, Kantharidis P, Thallas-Bonke V, Okuda S, Cooper ME and
Forbes JM: Ramipril inhibits AGE-RAGE-induced matrix
metalloproteinase-2 activation in experimental diabetic
nephropathy. Diabetol Metab Syndr. 6:862014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong Y, Zhang X, Cai X, Wang K, Chen Y
and Deng Y: Puerarin attenuated early diabetic kidney injury
through down-regulation of matrix metalloproteinase 9 in
streptozotocin-induced diabetic rats. PLoS One. 9:e856902014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu H, Cao X, Zhang H, Sun G, Fan G, Chen L
and Wang S: Imbalance between MMP-2, 9 and TIMP-1 promote the
invasion and metastasis of renal cell carcinoma via SKP2 signaling
pathways. Tumour Biol. 35:9807–9813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun H, Ge N, Shao M, Cheng X, Li Y, Li S
and Shen J: Lumbrokinase attenuates diabetic nephropathy through
regulating extracellular matrix degradation in
Streptozotocin-induced diabetic rats. Diabetes Res Clin Pract.
100:85–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thrailkill KM, Bunn R Clay and Fowlkes JL:
Matrix metalloproteinases: Their potential role in the pathogenesis
of diabetic nephropathy. Endocrine. 35:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lewandowski KC, Banach E, Bieńkiewicz M
and Lewiński A: Matrix metalloproteinases in type 2 diabetes and
non-diabetic controls: Effects of short-term and chronic
hyperglycaemia. Arch Med Sci. 7:294–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li SY, Huang PH, Yang AH, Tarng DC, Yang
WC, Lin CC, Chen JW, Schmid-Schönbein G and Lin SJ: Matrix
metalloproteinase-9 deficiency attenuates diabetic nephropathy by
modulation of podocyte functions and dedifferentiation. Kidney Int.
86:358–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Potier M, Elliot SJ, Tack I, Lenz O,
Striker GE, Striker LJ and Karl M: Expression and regulation of
estrogen receptors in mesangial cells: Influence on matrix
metalloproteinase-9. J Am Soc Nephrol. 12:241–251. 2001.PubMed/NCBI
|
|
16
|
Bhatt MP, Lim YC, Hwang J, Na S, Kim YM
and Ha KS: C-peptide prevents hyperglycemia-induced endothelial
apoptosis through inhibition of reactive oxygen species-mediated
transglutaminase 2 activation. Diabetes. 62:243–253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Rasheed NM, Willars GB and Brunskill
NJ: C-peptide signals via Galpha i to protect against
TNF-alpha-mediated apoptosis of opossum kidney proximal tubular
cells. J Am Soc Nephrol. 17:986–995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hills CE, Brunskill NJ and Squires PE:
C-peptide as a therapeutic tool in diabetic nephropathy. Am J
Nephrol. 31:389–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wahren J, Kallas A and Sima AA: The
clinical potential of C-peptide replacement in type 1 diabetes.
Diabetes. 61:761–772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samnegård B, Jacobson SH, Jaremko G,
Johansson BL and Sjöquist M: Effects of C-peptide on glomerular and
renal size and renal function in diabetic rats. Kidney Int.
60:1258–1265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang DY, Richter K, Breidenbach A and
Vallon V: Human C-peptide acutely lowers glomerular hyperfiltration
and proteinuria in diabetic rats: A dose-response study. Naunyn
Schmiedebergs Arch Pharmacol. 365:67–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun W, Gao X, Zhao X, Cui D and Xia Q:
Beneficial effects of C-peptide on renal morphology in diabetic
rats. Acta Biochim Biophys Sin (Shanghai). 42:893–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ishii T, Fukano K, Shimada K, Kamikawa A,
Okamatsu-Ogura Y, Terao A, Yoshida T, Saito M and Kimura K:
Proinsulin C-peptide activates α-enolase: Implications for
C-peptide-cell membrane interaction. J Biochem. 152:53–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luppi P, Geng X, Cifarelli V, Drain P and
Trucco M: C-peptide is internalized in human endothelial smooth
muscle cells via early endosomes. Diabetologia. 52:2218–2228. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindahl E, Nyman U, Zaman F, Palmberg C,
Cascante A, Shafqat J, Takigawa M, Sävendahl L, Jörnvall H and
Joseph B: Proinsulin C-peptide regulates ribosomal RNA expression.
J Biol Chem. 285:3462–3469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Zhao M, Li B and Qi J: Dynamic
localization and functional implications of C-peptide might for
suppression of iNOS in high glucose-stimulated rat mesangial cells.
Mol Cell Endocrinol. 381:255–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Liu D, Liu Y, Li E, Wang H, Liu K
and Qi J: Protein nitration promotes inducible nitric oxide
synthase transcription mediated by NF-κB in high glucose-stimulated
human lens epithelial cells. Mol Cell Endocrinol. 370:78–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lehners A, Lange S, Niemann G, Rosendahl
A, Meyer-Schwesinger C, Oh J, Stahl R, Ehmke H, Benndorf R, Klinke
A, et al: Myeloperoxidase deficiency ameliorates progression of
chronic kidney disease in mice. Am J Physiol Renal Physiol.
307:F407–F417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuno Y, Iyoda M, Shibata T, Hirai Y and
Akizawa T: Sildenafil, a phosphodiesterase type 5 inhibitor,
attenuates diabetic nephropathy in non-insulin-dependent otsuka
long-evans tokushima fatty rats. Br J Pharmacol. 162:1389–1400.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samnegård B, Jacobson SH, Jaremko G,
Johansson BL, Ekberg K, Isaksson B, Eriksson L, Wahren J and
Sjöquist M: C-peptide prevents glomerular hypertrophy and mesangial
matrix expansion in diabetic rats. Nephrol Dial Transplant.
20:532–538. 2005. View Article : Google Scholar : PubMed/NCBI
|