Introduction
Nucleus pulposus (NP) has been considered to be
excluded from the development of immunological tolerance as this
part of the intervertebral disc (IVD) normally has no access to
systemic circulation (1). Therefore,
NP has been suggested to trigger an autoimmune response if exposed
to the immune system, which has long been known to be a potent
inducer of pain (2). Historically,
mechanical pressure was considered to be the sole pathophysiologic
mechanism for inducing sciatica, when Mixter and Barr (3) linked the onset of pain to the
mechanical compression of the nerve root. However, biochemical
factors derived from the NP, such as TNF-α and IL-1, have
subsequently been found to be an additional pathophysiologic
mechanism for inducing pain (4). The
current treatment options of NP-mediated pain are not ideal, as
they target symptomatic relief but do not interfere with the
biological mechanisms underlying pain development (5). In clinical practice, reducing the
inflammation can notably ameliorate the clinical outcomes of some
autoimmune diseases (6,7), suggesting that the reduction of
proinflammatory cytokine production may serve as an efficient
therapeutic target for NP-mediated pain. Hence, an ideal
anti-inflammatory should be selected with the ability to interfere
with the biological mechanisms of NP-mediated pain, thus limiting
further degeneration of the IVD.
There is a growing interest in natural bioactive
compounds that may have a pronounced anti-inflammatory effects,
similar to those of corticosteroids (8). Resveratrol
(3,5,4′-trihydroxy-trans-stilbene) is a polyphenolic,
antifungal natural phytoalexin found in various food products, with
particularly high levels in grape skin and red wine. Resveratrol
has been shown to have anti-inflammatory, antioxidant,
antitumorigenic and immunomodulatory properties (9–11).
Recently, resveratrol has been reported to exert direct
cardiovascular protective effects by improving myocardial
perfusion, reducing oxidant stress and inhibiting platelet
aggregation (12–14). However, it is not known if
resveratrol exerts similar protective effects on the NP-mediated
pain. On the basis of these findings, we hypothesized that
resveratrol may exerts similar protective effects on degenerating
IVDs.
Furthermore, a variety of inflammatory mediators
have been implicated in IVD degeneration, including nitric oxide
(NO), interleukin-1 (IL-1), matrix metalloproteinases (MMP),
prostaglandin E2 (PGE2), tumor necrosis factor α (TNF-α) and
various other cytokines (15,16).
There is some direct evidence that the key mediators of these
changes may be TNF-α and IL-1 (17–19),
which are expressed at higher levels in herniated discs compared
with degeneration matched controls (20,21). In
addition, systemic immunomodulatory treatment targeting of TNF-α
activity attenuates these effects in animal models (22–25).
On the basis of the obvious requirement for more
defined, biological treatment options for NP-mediated pain, we
hypothesized that resveratrol may react with proinflammatory
cytokine to relieve the NP-mediated pain. Hence, the purpose of
this study was to investigate the protective effects of resveratrol
on NP cells with regard to proinflammatory cytokines.
Materials and methods
Ethical approval
All experimental procedures were performed in
accordance with protocols approved by the Governmental Animal Care
Committee of the Medical College of Xiamen University (Zhangzhou,
China) and conformed to the National Institute of Health guidelines
on the ethical use of animals.
Model of radiculopathy
Prior surgery, the animals were anesthetized by
intraperitoneal injection of 400 mg/kg chloral hydrate (Beyotime
Institute of Biotechnology, Haimen, China). During surgery, the
rats were placed in a prone position on a warming pad to maintain a
body temperature of 37.0±0.5°C. After surgery, the animals were
returned to individual cages with sufficient water and food and
treated with an intramuscular injection of penicillin (The 175th
Hospital of PLA, Zhangzhou, China) at a dose of 200,000 U/day, for
three days. After 72 h, rats were sacrificed by anesthetic
overdose, with the dose depending on the degree of tolerance to
chloral hydrate.
Adult female Sprague-Dawley rats (n=36; weight,
240–260 g) were randomly assigned into three groups (n=12 per
group): Sham injury (group I), saline-treated (group II) and
resveratrol-treated (group III). Resveratrol was obtained from the
Xi'an Xiaocao Botanical Institute of Biotechnology (Xi'an, China).
The rats were obtained from the Experimental Animal Center of
Xiamen University.
The Autologous Nucleus Pulposus Model of
Radiculopathy was established as described in a previous study
(26). Briefly, after the skin
preparation of the lower back, an incision was made at the L4-S1
level, fascia and multifidus muscle were resected, exposing the L4
and L5 vertebral laminae, and the left L5 nerve root and dorsal
root ganglion (DRG) were exposed by L5-L6 hemilaminectomy on the
left side. Care was taken to avoid trauma to the tissue. After
identifying the annuli fibrosi, autologous NP harvested from the
tail was applied to the DRG in the saline treatment group and the
resveratrol treatment group. In the Sham group, the left L5 nerve
root and DRG were exposed by L5-L6 facetectomy on the left side
without no other procedures were performed.
Resveratrol was administered to the rats in group
III at a dose of 0.1 ml 50 µM solution in saline, while in group II
0.1 ml saline was injected into the underlayer of the epineurium
just distal to the NP prior to closing the incisions. Group I rats
received no treatment throughout the experiment.
Behavioral testing
The behavioral testing was evaluated by the paw
withdrawal threshold of the left hind limb. At days 0 (baseline),
3, 7, 14 and 21, all animals underwent behavioral testing by
evaluating the left hind paw withdrawal response to von Frey hair
stimulation of the plantar surface of the footpad. Briefly, von
Frey filaments (27) with a
calibrated mechanical stimuli (Stoelting Co., Wood Dale, IL, USA)
between 1 and 29 g were sequentially applied twice to the paw
surface. The determined withdrawal force was verified with a
negative test of next lower filament as well as by confirming the
initial response after a time lag of 5 min. Lower withdrawal
thresholds was considered to be a sign of mechanical
hypersensitivity, which is correlated to pain behavior in this
animal model.
Hematoxylin and eosin (HE) staining
for detecting pathological changes
For HE staining, 5-µm transverse sections (8 µm) of
tissue at 7 and 14 days post surgery in each group were
deparaffinized and put into fresh xylene for 15 min twice. Sections
were rehydrated in 100% alcohol for 5 min twice, then 95% alcohol
and 70% alcohol once for 3 min. Subsequently, sections were washed
briefly in ddH2O, stained in Harris hematoxylin
(Beyotime Institute of Biotechnology) solution for 5 min. Sections
were then washed in running tap water for 8 min and differentiated
in 1% acid alcohol for 30 sec, blued in 0.2% ammonia water for 30
sec. The sections were washed in running tap water for 5 min and
rinsed in 95% alcohol ~15 times. Sections were stained in
Eosin-Phloxine (Beyotime Institute of Biotechnology) solution for 1
min then dehydrated through 95 and 100% alcohol (5 min each) and
cleared in two changes of xylene (5 min each). Finally, the
sections were mounted with mounting medium (Beyotime Institute of
Biotechnology). Images were captured using a FV 300 confocal
microscope (Olympus Corporation, Tokyo, Japan).
Immunohistochemical staining
For immunohistochemical staining, each specimen was
embedded in paraffin and six serial sections (three for TNF-α and
three for IL-1) were cut using a microtome. The immunohistochemical
study of TNF-α and IL-1 was performed using an avidin-biotin
peroxidase complex technique and Histostain SP kit (Maixin-Bio,
Inc., Fuzhou, China) according to the manufacturer's instructions.
Mouse anti-TNF-α (1:1,000; cat. no. 23456-1-AP; Endogen, Inc.,
Rockford, IL, USA) and anti-IL-1 polyclonal antibody (1:100; cat.
no. abM50001-5G3-PU; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) were used for this study. Two pathologists who were
unaware of the experimental data were responsible for counting the
TNF-α- and IL-1-positive cells in 10 high-power fields
(magnification, ×400) in each specimen. Finally, the total number
of IL-1 and TNF-α-positive cells per section were counted. Images
were captured using the FV 300 confocal microscope.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 for Windows (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. The Mann-Whitney U-test
were used for statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Surgery outcome
In all rats, the rectal temperature was maintained
at 37±0.5°C during surgery. The mean body weight of sham operated
rats was 371.25±6.81 g (range, 362–385 g; n=12). The mean body
weights of the control and U0126 groups were 377.67±7.1 g (range,
367–388 g; n=12) and 371.75±10.00 g (range, 354–389; n=12),
respectively. All rats underwent the surgical procedure without
complications. No significant differences in physiological
parameters were detected between the groups.
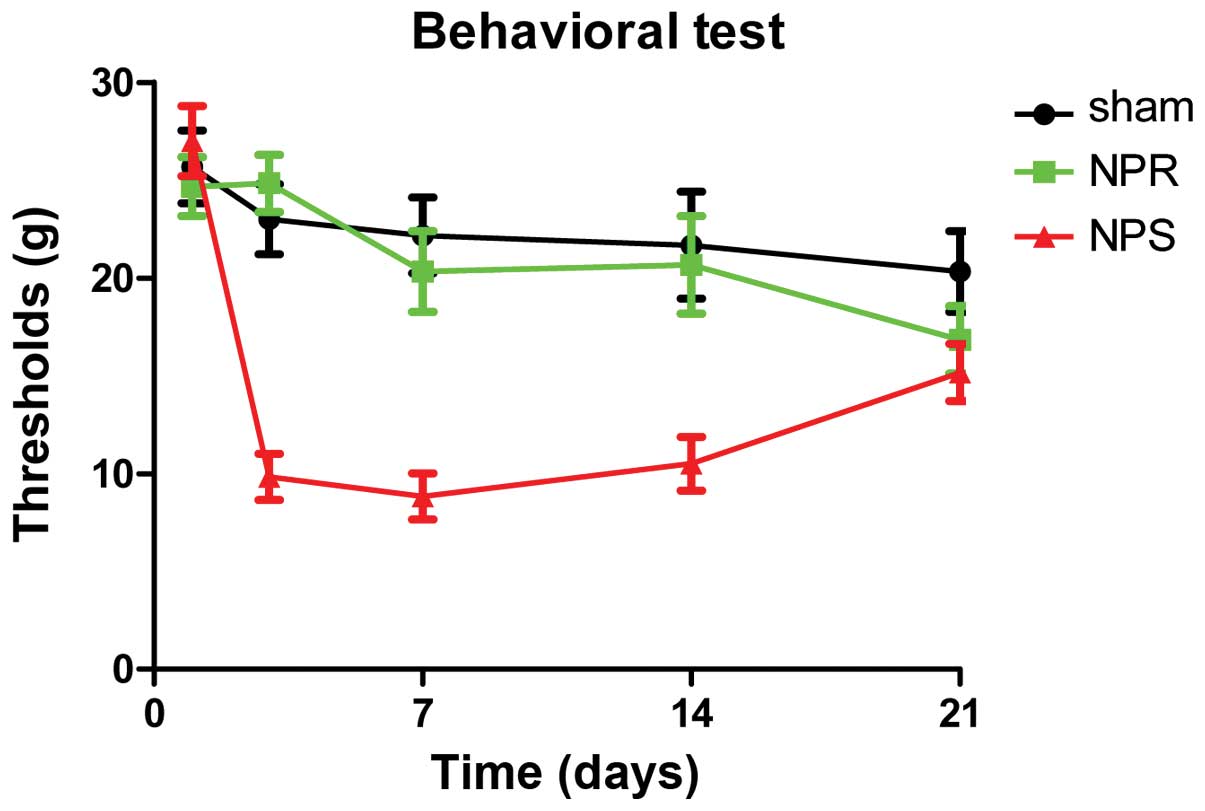
Behavioral test
To evaluate the extent of motor function recovery,
non-noxious mechanical stimulation with von Frey filaments was
employed. Animal behavior in response to the stimulation was
assessed for the three groups at different time points after
surgery. In group I, which (as expected) showed stable mechanical
withdrawals that were overall close to the preoperative baseline
over the entire course of the experiment, indicating that the
surgical intervention alone did not cause any change in pain
behavior. Groups II and III were compared with group I for all time
points. Results indicate that in group II, the mechanical
withdrawal thresholds were significantly decreased for each time
point compared with the baseline values of the sham group up to day
14, indicating that pain was evoked by application of NP tissue to
the DRG. While in group III that was treated with resveratrol,
animal behavior was very similar to group I. Thresholds in group
III were significantly higher compared with group II on days 3, 7
and 14 (P<0.01). However, at day 21, there was no significant
difference anymore between the thresholds of groups II and III
(Fig. 1).
Visual study
Following surgery, there was no obvious tissue
oedema in group I, indicating that the surgical intervention alone
did not cause any change in the DRG and IVD. While in group II and
III, obvious tissue oedema was observed in at 7 days post surgery
and was visibly attenuated at 14 days post surgery.
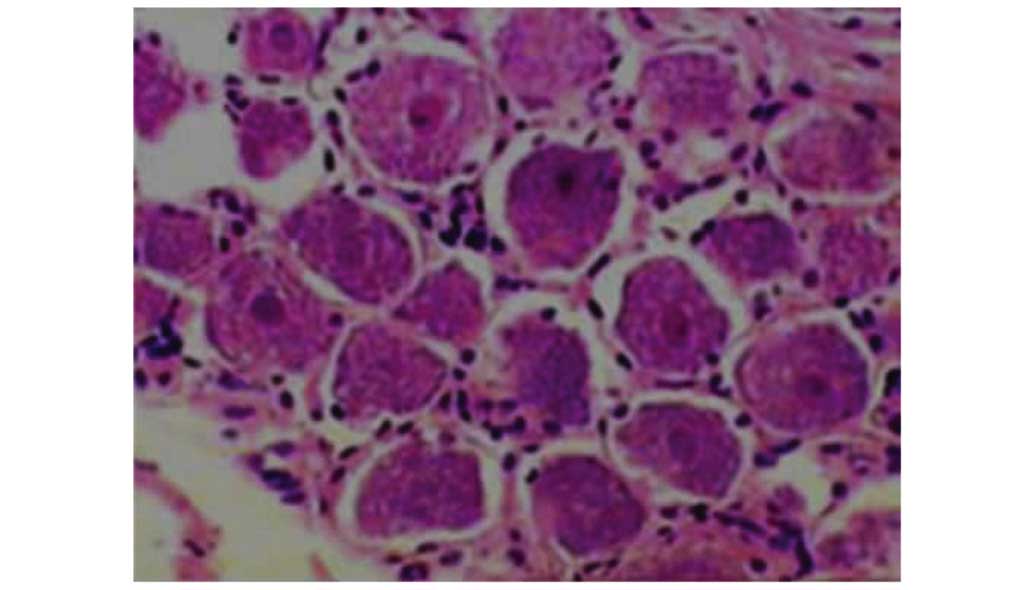
H&E staining
In group I, neurons had a normal appearance, as
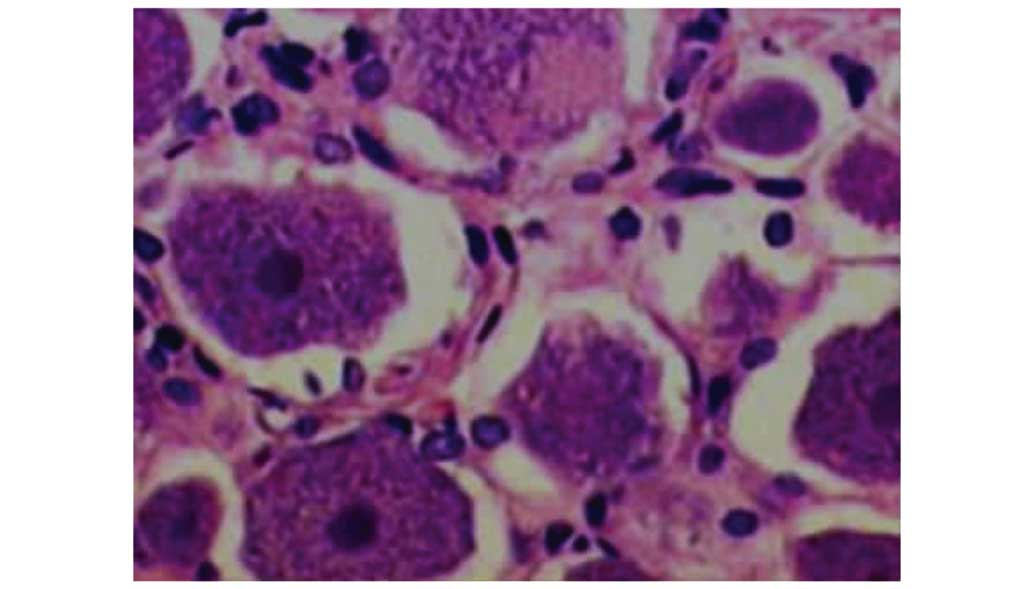
expected (Fig. 2). In group II at 7
and 14 days post surgery, morphological detection indicated
increased inflammatory response in the neurons: Cellular edema of
DRG cells, irregular structure of cytoplasm, nissl body population
decreased and focal hyperaemia (Fig.
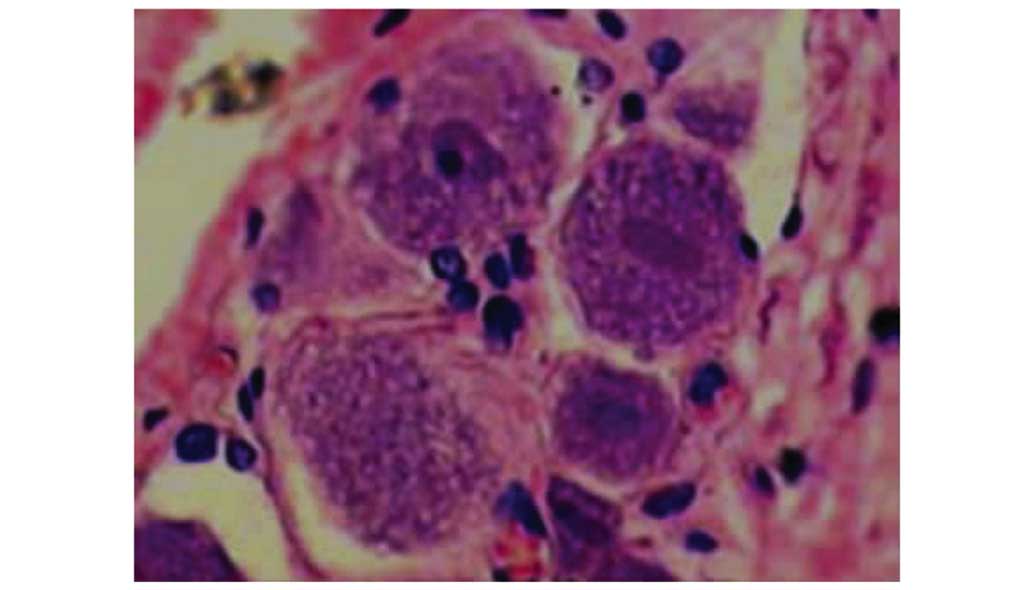
3). In group III, cell structure was improved, with decreased
edema and focal hyperaemia compared with group II (Fig. 4). Mild inflammatory response was
detected in the focal zone, but not as obvious or serious as in the
group II. In summary, the DRG pathological changes that occurred
after surgery were significantly attenuated by resveratrol at 7 and
14 days post surgery.
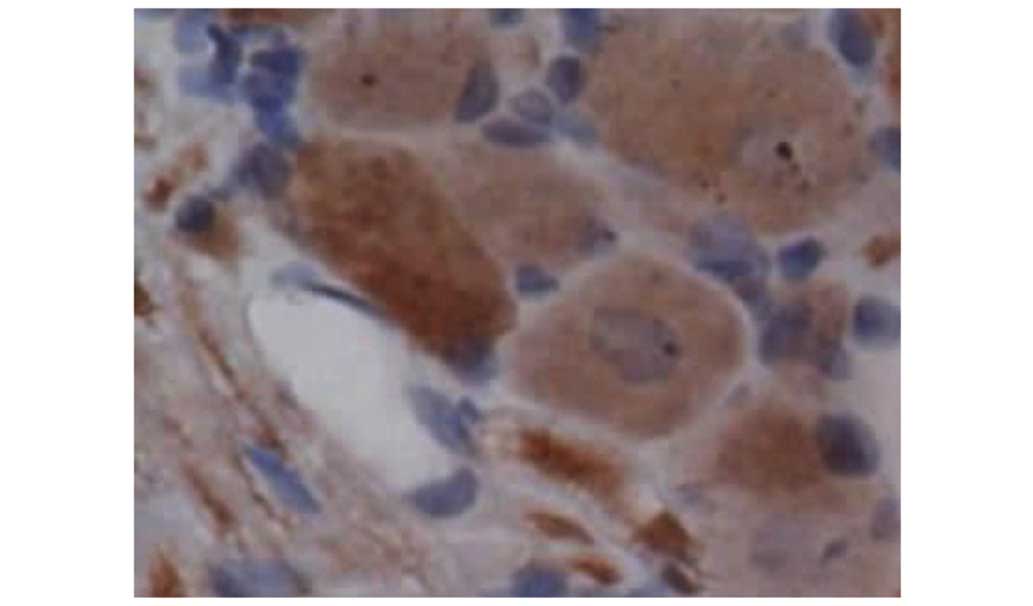
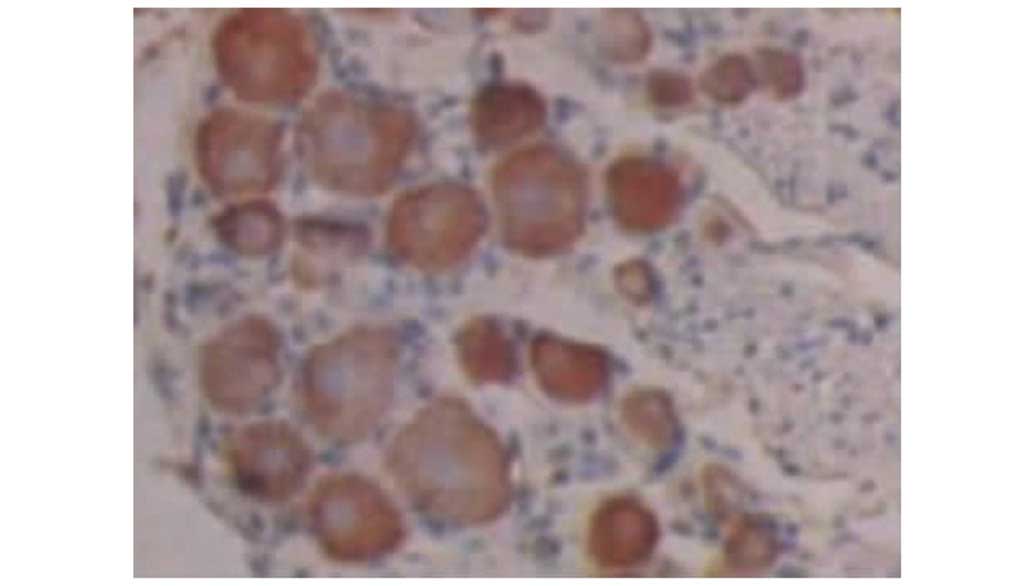
Effects of resveratrol on the protein
expression levels of TNF-α and IL-1
Increased constitutive expression of TNF-α and IL-1
was observed in groups II and III (Tables I and II). However, TNF-α and IL-1
immunohistochemical analysis of tissue sections showed significant
differences between groups II and III. Compared with group II,
resveratrol markedly inhibited the expression of TNF-α and IL-1 was
observed at 7 and 14 days post surgery in group III. This result
indicates that the anti-inflammatory effect of the highest
concentration of resveratrol could be confirmed on the protein
level for TNF-α and IL-1 (Figs.
5–9).
 | Table I.TNF-α-positive cells per section. |
Table I.
TNF-α-positive cells per section.
|
| Post-surgery
(days) |
|---|
|
|
|
|---|
| Group | 7 | 14 |
|---|
| I |
3.00±1.41 |
2.67±1.03 |
| II |
24.00±2.61a |
20.83±2.32a |
| III |
22.83±2.48a,b |
9.17±2.04a,b |
 | Table II.IL-1-positive cells per section. |
Table II.
IL-1-positive cells per section.
|
| Post-surgery
(days) |
|---|
|
|
|
|---|
| Group | 7 | 14 |
|---|
| I |
2.5±1.05 |
1.83±1.17 |
| II |
23.67±1.75a |
20.50±2.07a |
| III |
23.67±4.18a |
9.67±3.08a,b |
Discussion
Lower back pain remains a major public health
concern worldwide. Lower back pain is among the most common reasons
for visiting a doctor and is the most common cause of disability in
patients <45 years old (28).
Amongst a variety of etiologies, degenerative disc disease (leading
to so-called discogenic back pain) that correlated with increased
levels of proinflammatory cytokines has been postulated to be a
crucial cause of lower back pain (21,29,30).
Current treatments for lower back pain remain contested, indicating
that current treatment options are not ideal, as current
therapeutic strategies do affect the underlying biological
mechanisms of pain development (5).
For those reasons, injectable anti-inflammatory substances that
specifically target the metabolism of IVD cells may serve as novel
and useful minimally-invasive treatment options (31,32).
TNF-α has been reported to produce radicular pain
caused by lumbar disc herniation in adult patients, as mentioned
earlier (22–24). Recently, cytokines such as IL-1,
IL-6, and TNF-α have been associated with lower back and radicular
pain (7,33). The cytokines IL-1 and TNF-α are
overexpressed in degenerated IVD, which has led to them being
implicated in the matrix degradation that characterizes disk
degeneration (34–36). Furthermore, it has been reported that
a single intravenous infusion of the TNF-α inhibitor infliximab was
effective in treating sciatic pain caused by lumbar disc herniation
in clinical practice (20). Cohen
et al (37) reported a
preclinical safety study of transforaminal epidural etanercept
(another TNF-α inhibitor) for the treatment of sciatica caused by
disc herniation in 24 patients. The results indicated that the
effectiveness was dependent on the dose of etanercept. Among these,
proinflammatory cytokines such as TNF-α and IL-1 have been the
focus of a number of studies investigating the pathogenesis of
intervertebral disc degeneration, herniation and sciatic pain
(22–24).
There is growing evidence over the past decade that
resveratrol may impact multiple biological systems, particularly
the immune system (9–12). Recently, resveratrol has gained
considerable attention due to its anticancer (38), cardiovascular protective (39) and anti-inflammatory effects (40). A previous study has shown that
resveratrol can effectively reduce mRNA levels of major
proinflammatory cytokines (IL-6 and IL-8), TLR2, and matrix
degrading enzymes (MMP1, MMP3 and MMP13) (41), which have previously been shown to be
involved in disc degeneration and pain induction (42). Furthermore, recent studies
demonstrate that resveratrol can effectively prevent and treat
experimental inflammatory diseases by suppressing the production of
inflammatory cytokines in various types of cells (43–45).
Notably, in a study performed by Li et al (46), resveratrol has additionally been
shown to increase proteoglycan synthesis and to reduce TNF-α- and
IL-1-induced proteoglycan loss in bovine IVD cells, therefore
providing further evidence that resveratrol may be an innovative
treatment for NP-mediated back and leg pain (16,41,46).
The present study investigated the effect of
resveratrol on autologous NP model of radiculopathy as an in
vivo model. This animal model of radiculopathy involved placing
autologous tail NP tissue onto an exposed lumbar DRG in treated
animals. The advantage of this model is that using tail NP ensures
that the lumbar disc is neither injured by annular puncture nor
weakened by NP evacuation. This aids evaluation of inflammatory and
immune changes at the DRG specific to the presence of autologous
NP, particularly when annular incision alone can cause
radiculopathy in animal models (47–49).
As shown in the present study, resveratrol
suppresses the expression of TNF-α and the TNF-α-induced production
of IL-1. The present results also suggest that resveratrol may have
potential as a treatment for NP-mediated back and leg pain.
Resveratrol treatment was able to prevent the pain-related behavior
to a certain degree for 14 days in rats. This may be due to
resveratrol reducing or inhibiting the expression cytokines that
are released from the NP tissue in vivo, similar to the
mechanism observed in previous in vitro cell culture studies
(41,46).
In conclusion, this study has underlined the
potential of resveratrol for attenuating NP-mediated pain. Since
resveratrol is clinically widely used, its beneficial effects in
preventing and treating IVD can be transplanted to the bedside.
Resveratrol may provide a new therapeutic approach in treatment of
IVD.
References
|
1
|
Geiss A, Larsson K, Rydevik B, Takahashi I
and Olmarker K: Autoimmune properties of nucleus pulposus: An
experimental study in pigs. Spine (Phila Pa 1976). 32:168–173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki M, Inoue G, Gemba T, Watanabe T,
Ito T, Koshi T, Yamauchi K, Yamashita M, Orita S, Eguchi Y, et al:
Nuclear factor-kappaB decoy suppresses nerve injury and improves
mechanical allodynia and thermal hyperalgesia in a rat lumbar disc
herniation model. Eur Spine J. 18:1001–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mixter WJ and Barr J: Rupture of the
intervertebral disc with involvement of the spinal canal. N Engl J
Med. 211:210–215. 1934. View Article : Google Scholar
|
|
4
|
Olmarker K and Larsson K: Tumor necrosis
factor alpha and nucleus-pulposus-induced nerve root injury. Spine
(Phila Pa 1976). 23:2538–2544. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharifi S, Bulstra SK, Grijpma DW and
Kuijer R: Treatment of the degenerated intervertebral disc;
closure, repair and regeneration of the annulus fibrosus. J Tissue
Eng Regen Med. 9:1120–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ancuţa C, Ancuţa E, Miu S, Iordache C,
Belibou C and Chirieac R: Adalimumab therapy in patients with
active rheumatoid arthritis. Rev Med Chir Soc Med Nat Iasi.
113:710–715. 2009.PubMed/NCBI
|
|
7
|
Yount S, Sorensen MV, Cella D, Sengupta N,
Grober J and Chartash EK: Adalimumab plus methotrexate or standard
therapy is more effective than methotrexate or standard therapies
alone in the treatment of fatigue in patients with active,
inadequately treated rheumatoid arthritis. Clin Exp Rheumatol.
25:838–846. 2007.PubMed/NCBI
|
|
8
|
Fontana G, See E and Pandit A: Current
trends in biologics delivery to restore intervertebral disc
anabolism. Adv Drug Deliv Rev. 84:146–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harikumar KB and Aggarwal BB: Resveratrol:
A multitargeted agent for age-associated chronic diseases. Cell
Cycle. 7:1020–1035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soleas GJ, Diamandis EP and Goldberg DM:
The world of resveratrol. Adv Exp Med Biol. 492:159–182. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burns J, Yokota T, Ashihara H, Lean ME and
Crozier A: Plant foods and herbal sources of resveratrol. J Agric
Food Chem. 50:3337–3340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmitt CA, Heiss EH and Dirsch VM: Effect
of resveratrol on endothelial cell function: Molecular mechanisms.
Biofactors. 36:342–349. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Robich MP, Osipov RM, Nezafat R, Feng J,
Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ and Sellke
FW: Resveratrol improves myocardial perfusion in a swine model of
hypercholesterolemia and chronic myocardial ischemia. Circulation.
122:(Suppl 11). S142–S149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Crescente M, Jessen G, Momi S, Höltje HD,
Gresele P, Cerletti C and de Gaetano G: Interactions of gallic
acid, resveratrol, quercetin and aspirin at the platelet
cyclooxygenase-1 level. Functional and modelling studies. Thromb
Haemost. 102:336–346. 2009.PubMed/NCBI
|
|
15
|
Cassinelli EH, Hall RA and Kang JD:
Biochemistry of intervertebral disc degeneration and the potential
for gene therapy applications. Spine J. 1:205–214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang JD, Stefanovic-Racic M, McIntyre LA,
Georgescu HI and Evans CH: Toward a biochemical understanding of
human intervertebral disc degeneration and herniation.
Contributions of nitric oxide, interleukins, prostaglandin E2, and
matrix metalloproteinases. Spine (Phila Pa 1976). 22:1065–1073.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murata Y, Onda A, Rydevik B, Takahashi K
and Olmarker K: Distribution and appearance of tumor necrosis
factor-alpha in the dorsal root ganglion exposed to experimental
disc herniation in rats. Spine (Phila Pa 1976). 29:2235–2241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi N, Kikuchi S, Shubayev VI,
Campana WM and Myers RR: TNF-alpha and phosphorylation of ERK in
DRG and spinal cord: Insights into mechanisms of sciatica. Spine
(Phila Pa 1976). 31:523–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aoki Y, Rydevik B, Kikuchi S and Olmarker
K: Local application of disc-related cytokines on spinal nerve
roots. Spine (Phila Pa 1976). 27:1614–1617. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weiler C, Nerlich AG, Bachmeier BE and
Boos N: Expression and distribution of tumor necrosis factor alpha
in human lumbar intervertebral discs: A study in surgical specimen
and autopsy controls. Spine (Phila Pa 1976). 30:44–53; discussion
54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le Maitre CL, Hoyland JA and Freemont AJ:
Catabolic cytokine expression in degenerate and herniated human
intervertebral discs: IL-1beta and TNFalpha expression profile.
Arthritis Res Ther. 9:R772007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murata Y, Olmarker K, Takahashi I,
Takahashi K and Rydevik B: Effects of selective tumor necrosis
factor-alpha inhibition to pain-behavioral changes caused by
nucleus pulposus-induced damage to the spinal nerve in rats.
Neurosci Lett. 382:148–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murata Y, Onda A, Rydevik B, Takahashi K
and Olmarker K: Selective inhibition of tumor necrosis factor-alpha
prevents nucleus pulposus-induced histologic changes in the dorsal
root ganglion. Spine (Phila Pa 1976). 29:2477–2484. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olmarker K and Rydevik B: Selective
inhibition of tumor necrosis factor-alpha prevents nucleus
pulposus-induced thrombus formation, intraneural edema, and
reduction of nerve conduction velocity: Possible implications for
future pharmacologic treatment strategies of sciatica. Spine (Phila
Pa 1976). 26:863–869. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onda A, Yabuki S and Kikuchi S: Effects of
neutralizing antibodies to tumor necrosis factor-alpha on nucleus
pulposus-induced abnormal nociresponses in rat dorsal horn neurons.
Spine (Phila Pa 1976). 28:967–972. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shamji MF, Allen KD, So S, Jing L, Adams
SB Jr, Schuh R, Huebner J, Kraus VB, Friedman AH, Setton LA and
Richardson WJ: Gait abnormalities and inflammatory cytokines in an
autologous nucleus pulposus model of radiculopathy. Spine (Phila Pa
1976). 34:648–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woodwell D: National ambulatory medical
care survey: 1996 summary. Adv Data. 17:1–25. 1997.
|
|
29
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayashi S, Taira A, Inoue G, Koshi T, Ito
T, Yamashita M, Yamauchi K, Suzuki M, Takahashi K and Ohtori S:
TNF-alpha in nucleus pulposus induces sensory nerve growth: A study
of the mechanism of discogenic low back pain using
TNF-alpha-deficient mice. Spine (Phila Pa 1976). 33:1542–1546.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An HS, Thonar EJ and Masuda K: Biological
repair of intervertebral disc. Spine (Phila Pa 1976). 28:(Suppl
15). S86–S92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
An H, Boden SD, Kang J, Sandhu HS, Abdu W
and Weinstein J: Summary statement: Emerging techniques for
treatment of degenerative lumbar disc disease. Spine (Phila Pa
1976). 28:(Suppl 15). S24–S25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burke JG, Watson RW, McCormack D, Dowling
FE, Walsh MG and Fitzpatrick JM: Intervertebral discs which cause
low back pain secrete high levels of proinflamatory mediators. J
Bone Joint Surg Br. 84:196–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoyland JA, Le Maitre CL and Freemont AJ:
Investigation of the role of IL-1 and TNF in matrix degradation in
the intervertebral disc. Rheumatology (Oxford). 47:809–814. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Korhonen T, Karppinen J, Malmivaara A,
Autio R, Niinimäki J, Paimela L, Kyllönen E, Lindgren KA, Tervonen
O, Seitsalo S and Hurri H: Efficacy of infliximab for disc
herniation-induced sciatica: One-year follow-up. Spine (Phila Pa
1976). 29:2115–2119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cohen SP, Bogduk N, Dragovich A,
Buckenmaier CC III, Griffith S, Kurihara C, Raymond J, Richter PJ,
Williams N and Yaksh TL: Randomized, double-blind,
placebo-controlled, dose-response, and preclinical safety study of
transforaminal epidural etanercept for the treatment of sciatica.
Anesthesiology. 110:1116–1126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aluyen JK, Ton QN, Tran T, Yang AE,
Gottlieb HB and Bellanger RA: Resveratrol: Potential as anticancer
agent. J Diet Suppl. 9:45–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu JM, Hsieh TC and Wang Z:
Cardioprotection by resveratrol: A review of effects/targets in
cultured cells and animal tissues. Am J Cardiovasc Dis. 1:38–47.
2011.PubMed/NCBI
|
|
40
|
Recio MC, Andujar I and Rios JL:
Anti-inflammatory agents from plants: Progress and potential. Curr
Med Chem. 19:2088–2103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wuertz K, Quero L, Sekiguchi M, Klawitter
M, Nerlich A, Konno S, Kikuchi S and Boos N: The red wine
polyphenol resveratrol shows promising potential for the treatment
of nucleus pulposus-mediated pain in vitro and in vivo. Spine
(Phila Pa 1976). 36:E1373–E1384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Li K, Han X, Mao C, Zhang K, Zhao T
and Zhao J: The imbalance between TIMP3 and matrix-degrading
enzymes plays an important role in intervertebral disc
degeneration. Biochem Biophys Res Commun. 469:507–514. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roy S, Sannigrahi S, Majumdar S, Ghosh B
and Sarkar B: Resveratrol regulates antioxidant status, inhibits
cytokine expression and restricts apoptosis in carbon tetrachloride
induced rat hepatic injury. Oxid Med Cell Longev. 2011:7036762011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Culpitt SV, Rogers DF, Fenwick PS, Shah P,
De Matos C, Russell RE, Barnes PJ and Donnelly LE: Inhibition by
red wine extract, resveratrol, of cytokine release by alveolar
macrophages in COPD. Thorax. 58:942–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang OH, Jang HJ, Chae HS, Oh YC, Choi JG,
Lee YS, Kim JH, Kim YC, Sohn DH, Park H and Kwon DY:
Anti-inflammatory mechanisms of resveratrol in activated HMC-1
cells: Pivotal roles of NF-kappaB and MAPK. Pharmacol Res.
59:330–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, Phillips FM, An HS, Ellman M, Thonar
EJ, Wu W, Park D and Im HJ: The action of resveratrol, a
phytoestrogen found in grapes, on the intervertebral disc. Spine
(Phila Pa 1976). 33:2586–2595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kayama S, Konno S, Olmarker K, Yabuki S
and Kikuchi S: Incision of the annulus fibrosus induces nerve root
morphologic, vascular, and functional changes. An experimental
study. Spine (Phila Pa 1976). 21:2539–2543. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ulrich JA, Liebenberg EC, Thuillier DU and
Lotz JC: ISSLS prize winner: Repeated disc injury causes persistent
inflammation. Spine (Phila Pa 1976). 32:2812–2819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shamji MF, Allen KD, So S, Jing L, Adams
SB Jr, Schuh R, Huebner J, Kraus VB, Friedman AH, Setton LA and
Richardson WJ: Gait abnormalities and inflammatory cytokines in an
autologous nucleus pulposus model of radiculopathy. Spine (Phila Pa
1976). 34:648–654. 2009. View Article : Google Scholar : PubMed/NCBI
|