Introduction
Abdominal aortic aneurysms (AAA) is a complex
disorder, caused by the interaction of environmental, genetic, and
biochemical factors. A variety of AAA risk factors can all lead to
damage of the aortic wall, activation of inflammatory response,
local inflammatory cell infiltration, degradation of extracellular
matrix and massive induction of apoptosis in vascular smooth muscle
cells, resulting in severe damage to the wall of the blood vessel,
significant reduction of the main artery elasticity and ability to
withstand pressure, and eventually the gradual swelling of the
aortic wall due to failure to withstand the pressure of blood flow
(1–3). Osteopontin (Opn) is a secreted
glycosylated phosphoprotein, having important function in the
extracellular matrix. Previous studies suggested that Opn can
upregulate the expression of matrix metalloproteinases (MMPs) and
thus accelerate the degradation of extracellular matrix, promoting
aneurysm rupture and tumor metastasis (4,5).
Chemokine-like factor 1 (Cklf1) is a newly
identified cytokine by suppression subtractive hybridization
method, sharing structural and functional similarity with
chemokines. It has been shown that the expression of chemokines
increased significantly in AAA lesions, regulating leukocyte
migration towards the inflamed tissues, and promoting inflammatory
reactions during the AAA progression (6,7). Cklf1
exhibited similar effects on leukocytes.
In this study, we aimed to explore the mechanisms
and functions of Opn and Cklf1 in AAA in rats, based on previous
findings and to provide a theoretical basis for identifying novel
therapeutic targets for AAA.
Materials and methods
Materials
Porcine pancreatic elastase (E1250) was purchased
from Sigma (St. Louis, MO, USA); mouse anti-rat Opn antibody was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA); rabbit anti-rat MMP-2 antibody was purchased from Abcam
(Cambridge, UK); rabbit rCklf1 polyclonal antibody was provided by
the Department of Medicine at Peking University (Beijing, China). A
total of 30 healthy male Sprague-Dawley (SD) rats weighing 150–180
g were given free access to water and standard rat chow. RNA
extraction reagent TRIzol was purchased from Tiangen Biotech
(Beijing) Co., Ltd. (Beijing, China), and M-MLV reverse
transcriptase, TaqDNA polymerase, dNTP, primer Oligo (dT) were
purchased from Takara Bio (Dalian, China). All the synthesized
primers used in this study were obtained from Kang Century
Biotechnology Co., Ltd. (Beijing, China). Other reagents were all
of analytical grade or imported. Rats and standard rat chow were
provided by the Model Animal Center of Nanjing University (Nanjing,
China).
Animal groups
Thirty male SD rats, weighing 150–180 g, were
randomly divided into three groups: A, B, and C3 (10 rats/group).
Group A was the elastase perfusion group (AAA group), perfusion
with 1 ml elastase at 20 U/ml; group B was the normal saline
perfusion group (control group), and group C was the untreated
group (sham group). The surgical procedure included the following
steps: Anesthesia, laparotomy, isolation of abdominal aorta within
0.5–1.0 cm, intubation through upper abdominal aortic bifurcation
mouth, perfusion by high pressure, perfusion liquid varies due to
experimental groups, and the abdomen was repaired at the end of the
surgery.
Pathological stain
Specimens were fixed with 4% paraformaldehyde and
stained with hematoxylin and eosin (H&E) and Victoria blue to
label elastic fibers in blue-green and collagen fibers in
yellow.
Immunohistochemistry
Conventional avidin-biotin complex method was used
to detect the distribution of Opn in different AAA layers with
mouse anti-rat Opn antibody (Abcam, Cambridge, MA, USA; dilution
1:500; cat. no.: ab69498) and goat anti-mouse secondary antibody
(Abcam; dilution 1:500; cat. no.: ab6789).
Western blotting
Tissue samples stored in liquid nitrogen at −80°C
were homogenized followed by protein extraction and denaturation.
Protein concentration was determined using a Coomassie brilliant
blue colorimetric assay and proteins separated by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). After
completion of the electrophoresis and rinsing gel with deionized
water, the separated proteins were transferred to nitrocellulose
membrane and blocked in a 5% non-fat milk powder at 4°C overnight.
Subsequently, the blocking solution was removed and the membrane
was rinsed with TBST 3 times. Primary antibody (dilution of 1:500)
was then incubated with the membrane at room temperature on a
shaker for 2 h followed by rinsing with TBST, and incubation with
horseradish peroxidase-labeled goat anti-mouse secondary antibody
on a shaker at room temperature for 1 h. The diaminobenzidine (DAB)
chromogenic detection system was used for signal visualization and
pro 4.0 gel absorbance measurement/analysis software was used to
perform protein quantification.
Results evaluation
Sections treated with specific staining and
immunohistochemical staining without hematoxylin were analyzed
using a microscope (Olympus, Tokyo, Japan) at a magnification of
×100. Ten visual fields were randomly selected, followed by
analysis using a computer image system. Detection criteria was the
average absorbance value of positive staining multiplied by
relative area of staining (s %).
Statistical analysis
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for data analysis. Results were presented as mean
± standard deviation. The t-test and variance analysis was
performed to assess significant difference. The ANOVA single factor
analysis of variance was used for comparing multiple groups of
data. A correlation analysis was performed using Spearman
correlation analysis.
Results
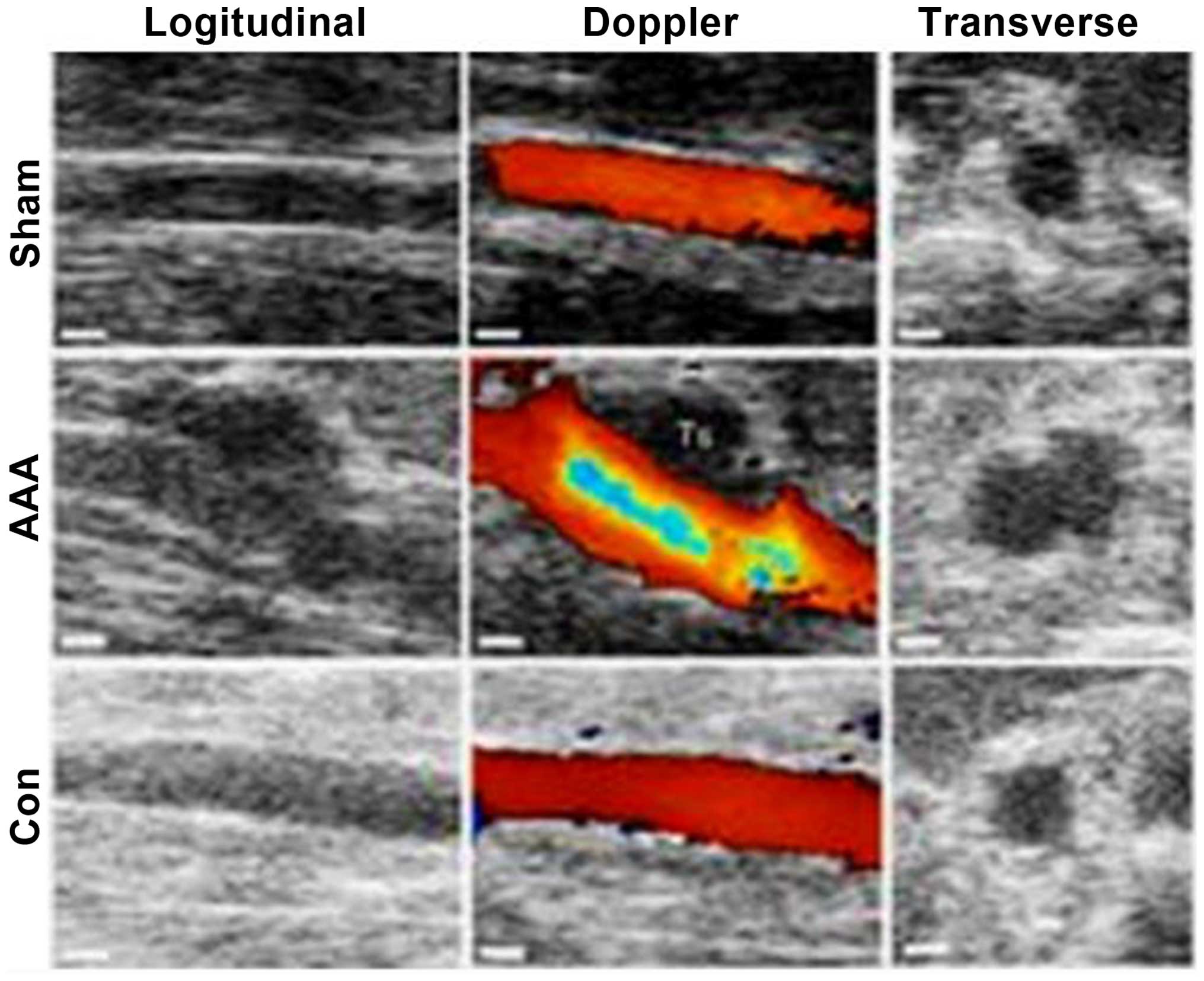
Abdominal aortic diameter change
All the rats survived except one rat from the AAA
group, which died after surgery. On the 14th day after surgery,
abdominal aortic dilatation in rats from the AAA group was
2.38-fold its original aortic diameter, while in rats from the
control group, abdominal aortic dilatation was only 1.12-fold the
original and there was no abdominal aortic dilatation in rats from
the sham group. The t-test analysis revealed that compared to the
control group (group B) and sham group (group C), the abdominal
aortic dilatation ratio in rats from the AAA group (group A) was
significantly higher (P<0.01), while there was no significant
difference in the dilatation ratio between groups B and C
(P>0.05; Table I).
Ultrasonography is shown in Fig.
1.
 | Table I.Diameter changes in abdominal aorta
(mm). |
Table I.
Diameter changes in abdominal aorta
(mm).
| Groups | No. | Before | After | T-value | P-value |
|---|
| AAA | 9 | 5.23±1.28 |
12.42±6.81a,b | 12.87 | 0.008 |
| Control | 10 | 5.29±1.29 | 5.92±1.21 | 10.98 | 0.048 |
| Sham | 10 | 5.33±2.18 | 5.23±3.23 | 0.69 | 0.32 |
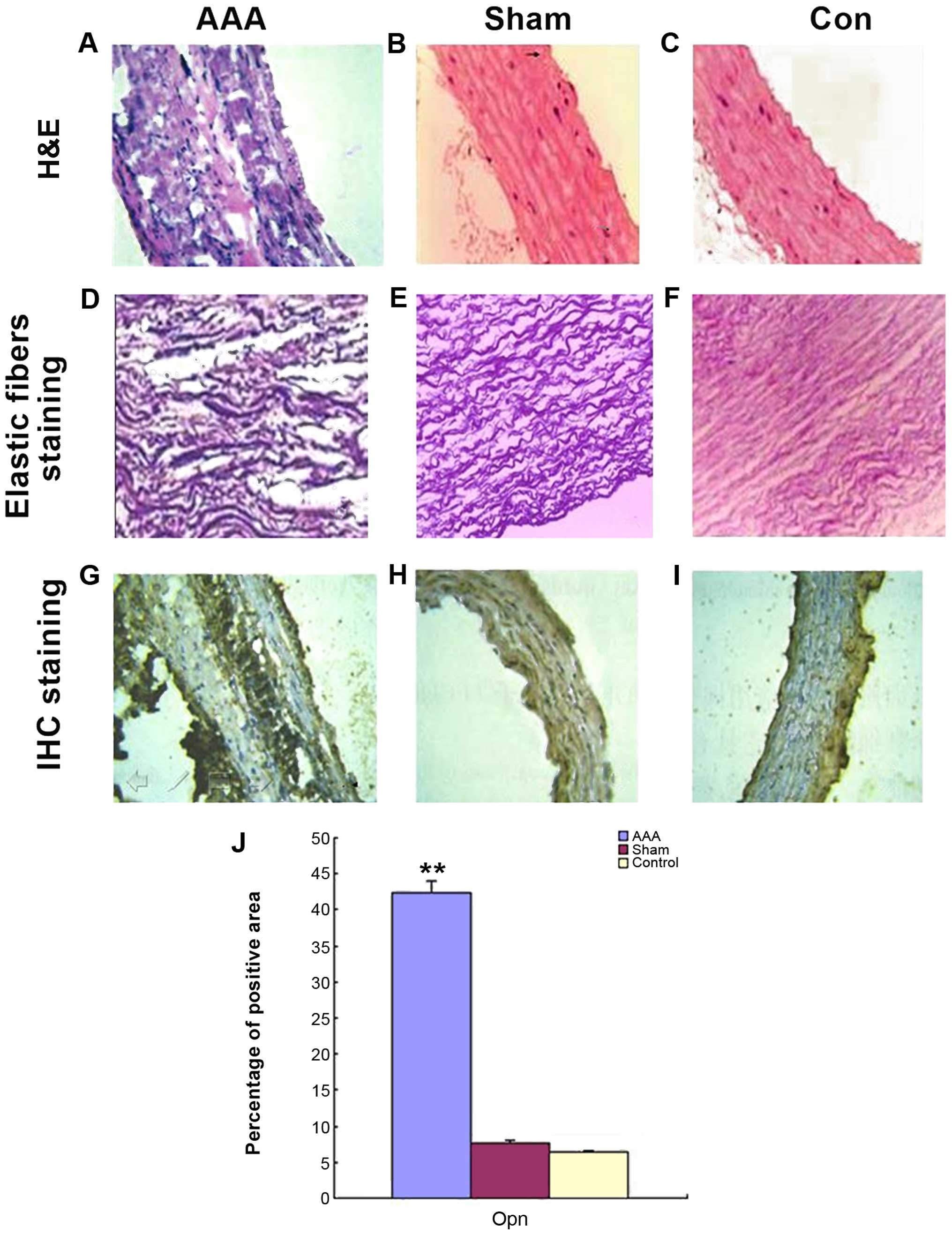
Staining
H&E staining showed that the arterial wall in
rats in the AAA group was thickened, was damaged and had a broken
structure. Debris and notch and the smooth muscle cells were
disorganized with massive inflammatory cell infiltration. On the
other hand, the arterial wall from the control and sham groups
looked much better in terms of thickness, structural integrity,
continuous resistance, smooth muscle cell distribution and the
number of inflammatory cells (Fig.
2A-C). Elastic fibers staining showed that in the AAA group,
elastic fibers in artery medial layer were badly damaged with
irregular morphology, disordered and reduced layers, loss of normal
curvature and continuity, appearance of fracture, fragmentation and
notch, whereas in the control and sham group, elastic fibers
remained relatively intact, resembling regular waves with distinct
layers, fine continuity and curvature (Fig. 2D-F). Opn-specific protein staining
was strongly positive within aneurysms vascular structure in the
AAA group with a high expression located at the medial aneurysm
wall (serious disease area with fracture, fragment and notch)
(Fig. 1C and D). By contrast, in the
control and sham group, Opn expression was weak. Statistical
analysis is shown in Fig. 2E and
Table II.
 | Table II.Expression of Opn, rCklf1 and MMP-2
examined by western blotting. |
Table II.
Expression of Opn, rCklf1 and MMP-2
examined by western blotting.
| Group | Opn,
×105 | rCklf1,
×105 | MMP-2,
×105 |
|---|
| AAA | 2.78±2.81 | 6.02±6.78 | 9.37±6.72 |
| Sham | 4.57±1.27 | 4.78±4.17 | 8.98±7.28 |
| Control | 2.18±1.94 | 4.37±7.02 | 2.76±3.47 |
Western blotting
Expression of Opn, rCklf1 and MMP-2 was elevated in
the AAA group, while in the control and sham groups, the expression
of all three proteins was low. Analysis revealed that the
expression of Opn, rCklf1 and MMP-2 was higher in the AAA group
than that in the control and sham group and the difference was
statistically significant (P<0.05). However, the difference was
not considered significant between the control and sham group
(P>0.05; Table II).
Additionally, the expression of Opn, rCklf1 and MMP-2 was
positively correlated with each other (Table III).
 | Table III.Spearman correlation analysis. |
Table III.
Spearman correlation analysis.
|
| Western blotting |
|---|
|
|
|
|---|
|
| Opn | rCklf1 | MMP-2 |
|---|
|
|
|
|
|
|---|
| Group | P-value | r-value | P-value | r-value | P-value | r-value |
|---|
| Opn | – | – | <0.01 | 0.885 | <0.05 | 1.298 |
| rCklf1 | <0.01 | 0.876 | – | – | <0.01 | 1.045 |
| MMP-2 | <0.01 | 0.576 | <0.01 | 0.028 | – | – |
Discussion
Aortic aneurysm primarily affects male populations
over the age of 55 with a mortality rate as high as 50–80% if a
rupture occurs, making it clinically significant to investigate the
mechanism controlling mthe initiation and progression of AAA as
well as to improve clinical outcomes in patients (8). At present, scholars at home and abroad
have created AAA rat models through a range of methods including
genetic defect induction, calcium chloride-mediated injury and
elastase perfusion (9). Among these
methods, the elastase perfusion model is superior in mimicking the
native pathological conditions in AAA patients (10). In the present study, the AAA rat
model was successfully generated using a micro-surgery method.
Victoria blue staining revealed the disappearance of elastic
fibers, degeneration of vascular muscle cells and a significant
increase in collagen fibers, in agreement with human AAA
pathological conditions. Previous findings have shown that
inflammation plays an important role in the pathogenesis of AAA and
the regulation of monocyte-macrophage cells, lymphocyte activation
and migration by chemokine is one of the key steps to develop AAA
(11,12). Additionally, the expression of
chemokines was significantly increased in AAA patients and
macrophages as well as lymphocytes in blood infiltrating the
arterial wall when induced by chemokines, causing the release of
excess MMP and further degradation of structural proteins (elastin,
collagen) in the aorta wall and damage of elastic fibers and
collagen fibers (13). Cklf, a newly
identified chemokine, has exhibited chemotactic activities on
neutrophils, lymphocytes and monocyte in vitro and in
vivo. In addition, Cklf is involved in the regulation of
endometrial hyperplasia, atherosclerosis and other inflammatory
processes (14,15).
In the present study, the rat AAA models have been
successfully created by elastase perfusion surgery. In AAA rats,
the aortic diameter expansion rate after surgery was significantly
higher (P<0.01), elastin in medial layer was significantly
reduced and inflammatory cell infiltration was increased
significantly compared to the control rats. In addition,
immunohistochemical staining showed that the expression of Opn,
Cklf1 and MMP-2 in the AAA group was significantly increased
(P<0.05). Furthermore, western blot analysis revealed that the
expression of Opn, Cklf1 and MMP-2 in the AAA group was
significantly higher than that in the sham and control groups
(P<0.01) and Opn as well as rCklf1 was positively correlated
with MMP-2; rs (Opn)=1.298, rs (MMP-2)=1.045 (P<0.05). The
results of the present study suggest that Opn, and rCklf1
upregulate the expression of extracellular matrix MMP and elevated
MMP destroys the balance between anabolic and catabolic activity in
extracellular matrix outside the arterial wall. Thus, the
degradation of extracellular matrix is accelerated and the
pathogenesis of AAA is promoted. Previous studies suggested that
the occurrence of aortic aneurysms involved the decomposition and
denaturation of arterial elastin, collagen and other factors,
resulting in damage in arterial medial layer and the support plate
(16). Under the impact of high
blood flow, the release of MMP and other proteinases from
macrophages and aortic smooth muscle cells were further
accelerated. Additionally, widely infiltrated lymphocytes and
macrophages in the aortic wall produced a variety of cytokines
(such as interleukins, tumor necrosis factor and immunoreactive
fibronectin) (17) as well as
immunoglobulins, which in turn lead to activation of the protease
cascade. Opn levels increased at the inflammatory lesion in chronic
inflammatory and autoimmune diseases caused by injury, and this
upregulation was more evident in particular in activated T
lymphocytes, mononuclear macrophages and adjacent parts (18).
In summary, findings of the present study have shown
that, in the rat model of AAA, Opn and Cklf1 synergistically
upregulated the expression of MMP-2 in tumor tissue, which in turn,
accelerated the degradation of extracellular matrix, eventually
resulting in the development of aortic aneurysms.
References
|
1
|
Chen HZ, Wang F, Gao P, Pei JF, Liu Y, Xu
TT, Tang X, Fu WY, Lu J, Yan YF, et al: Age-associated sirtuin 1
reduction in vascular smooth muscle links vascular senescence and
inflammation to abdominal aortic aneurysm. Circ Res. 119:1076–1088.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaheen B, Downs EA, Serbulea V, Almenara
CC, Spinosa M, Su G, Zhao Y, Srikakulapu P, Butts C, McNamara CA,
et al: B-Cell Depletion Promotes Aortic Infiltration of
Immunosuppressive Cells and Is Protective of Experimental Aortic
Aneurysm. Arterioscler Thromb Vasc Biol. 36:2191–2202. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shingaki M, Kato M, Motoki M, Kubo Y,
Isaji T and Okubo N: Endovascular repair for abdominal aortic
aneurysm followed by type B dissection. Asian Cardiovasc Thorac
Ann. Sep 15–2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan Y, Wang Y, Li L, Xia J, Peng S and He
Y: Chemokine-like factor 1-derived C-terminal peptides induce the
proliferation of dermal microvascular endothelial cells in
psoriasis. PLoS One. 10:e01250732015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moores AE, Cahill MS and Villines TC:
Myocardial infarction and aortic root mycotic aneurysm complicating
aortic valve endocarditis: Utility of cardiac CT. Case Rep Med.
2016:37563022016.PubMed/NCBI
|
|
6
|
Ijaz T, Tilton RG and Brasier AR: Cytokine
amplification and macrophage effector functions in aortic
inflammation and abdominal aortic aneurysm formation. J Thorac Dis.
8:E746–E754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomez I, Ozen G, Deschildre C, Amgoud Y,
Boubaya L, Gorenne I, Benyahia C, Roger T, Lesèche G, Galardon E,
et al: Reverse regulatory pathway (H2S/PGE2/MMP) in human aortic
aneurysm and saphenous vein varicosity. PLoS One. 11:e01584212016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan SM, Wang J, Huang HR and Jing H:
Osteopontin expression and its possible functions in the aortic
disorders and coronary artery disease. Rev Bras Cir Cardiovasc.
26:173–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tu M, Li Y, Zeng C, Deng Z, Gao S, Xiao W,
Luo W, Jiang W, Li L and Lei G: MicroRNA-127-5p regulates
osteopontin expression and osteopontin-mediated proliferation of
human chondrocytes. Sci Rep. 6:250322016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shang T, Ran F, Qiao Q, Liu Z and Liu CJ:
Tanshinone IIA attenuates elastase-induced AAA in rats via
inhibition of MyD88-dependent TLR-4 signaling. Vasa. 43:39–46.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tarín C, Fernández-Laso V, Sastre C,
Madrigal-Matute J, Gómez M, Zaragoza C, Egido J, Burkly LC,
Martín-Ventura JL and Blanco-Colio LM: Tumor necrosis factor-like
weak inducer of apoptosis or Fn14 deficiency reduce elastase
perfusion-induced aortic abdominal aneurysm in mice. J Am Heart
Assoc. 3:e0007232014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang GY, Chen X, Sun YC, Ma CL and Qian G:
Chemokine-like factor 1 (CLFK1) is over-expressed in patients with
atopic dermatitis. Int J Biol Sci. 9:759–765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson RW and Baxter BT: MMP inhibition
in abdominal aortic aneurysms. Rationale for a prospective
randomized clinical trial. Ann N Y Acad Sci. 878:159–178. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang ZZ, Zhang Y, Yuan YH and Chen NH:
Developmental expression of chemokine-like factor 1, a novel member
of chemokines family, in postnatal rat cerebral cortex. Neurosci
Lett. 519:51–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng Y, Guo C, Zhang Y, Qi H, Sun Q, Xu
E, Zhang Y, Ma D and Wang Y: Alleviation of murine allergic
rhinitis by C19, a C-terminal peptide of chemokine-like factor 1
(CKLF1). Int Immunopharmacol. 11:2188–2193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carmo M, Colombo L, Bruno A, Corsi FR,
Roncoroni L, Cuttin MS, Radice F, Mussini E and Settembrini PG:
Alteration of elastin, collagen and their cross-links in abdominal
aortic aneurysms. Eur J Vasc Endovasc Surg. 23:543–549. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martín-Alonso M, García-Redondo AB, Guo D,
Camafeita E, Martínez F, Alfranca A, Méndez-Barbero N, Pollán Á,
Sánchez-Camacho C, Denhardt DT, et al: Deficiency of MMP17/MT4-MMP
proteolytic activity predisposes to aortic aneurysm in mice. Circ
Res. 117:e13–e26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Chang Q, Qian X, Tian C and Sun X:
Angiotensin II induces an increase in MMP-2 expression in
idiopathic ascending aortic aneurysm via AT1 receptor and JNK
pathway. Acta Biochim Biophys Sin (Shanghai). 47:539–547. 2015.
View Article : Google Scholar : PubMed/NCBI
|