Introduction
Hypoparathyroidism is a frequent and serious
complication of thyroid surgery. The reported incidence of
postoperative hypoparathyroidism ranges from 1.7 to 68% (1–3), and
clinically, mild to severe symptoms of hypocalcemia are observed.
Hypocalcemia may lead to significant morbidity and impaired quality
of life. Hypocalcemia post-thyroidectomy has been attributed to the
extent of disease, previous thyroid operations, and other factors,
but more notably, to insufficient understanding of parathyroid
anatomy (4), particularly in
inexperienced surgeons examining parathyroid identification
(5).
Currently, parathyroid recognition depends primarily
on a surgeon's personal experience and their interpretation of the
morphological and topographical criteria (6). Routine frozen section examination of a
parathyroid biopsy is performed to confirm the presence of
parathyroid tissue and to provide pathological tissue confirmation
for potential parathyroid autotransplantation (7). To date, there has been no more
reliable, simpler, minimally invasive approach or technique
proposed to replace frozen section examination and a surgeon's
personal experience.
Parathyroid hormone (PTH) is secreted by the chief
cells of the parathyroid glands to balance blood calcium. Recent
reports have stated that rapid intraoperative parathyroid hormone
(rIO-PTH) levels from tissue fine needle aspiration (FNA) may
predict the presence of parathyroid tissue during
parathyroidectomies for primary or secondary hyperparathyroidism
(8–12) as a highly effective method to
differentiate parathyroid and nonparathyroid tissue during
parathyroidectomy (13).
rIO-PTH assay of suspected parathyroid tissue was
performed using FNA to identify the parathyroids during
thyroidectomy. The sensitivity and specificity of this technique
was investigated to recognize and protect the parathyroids with the
aim of elucidating whether this technique may replace frozen
section analysis in patients undergoing thyroid surgery.
Materials and methods
Patients
A total of 100 consecutive patients undergoing
thyroid surgery in the Department of Thyroid Surgery, China-Japan
Union Hospital of Jilin University (Changchun, China) between
October 2012 and February 2013 were enrolled in the present study.
Patients were randomly divided into two groups, an rIO-PTH group
and a control group, with 50 cases in each group. All patients
underwent the same surgical protocols and level. rIO-PTH was
performed using FNA and histological examination on 194 anatomical
structures from the rIO-PTH group and the relationship between the
rIO-PTH values and histological results was analyzed. FNA and
histological examination was not performed on all of the normal
parathyroids recognized by visualization intraoperatively, in the
present study. Only those anatomical structures that were suspected
as parathyroid tissue were sampled. The present study was approved
by the Ethics Committee of the China-Japan Union Hospital of Jilin
University and written informed consent was obtained from all
patients who participated in the study.
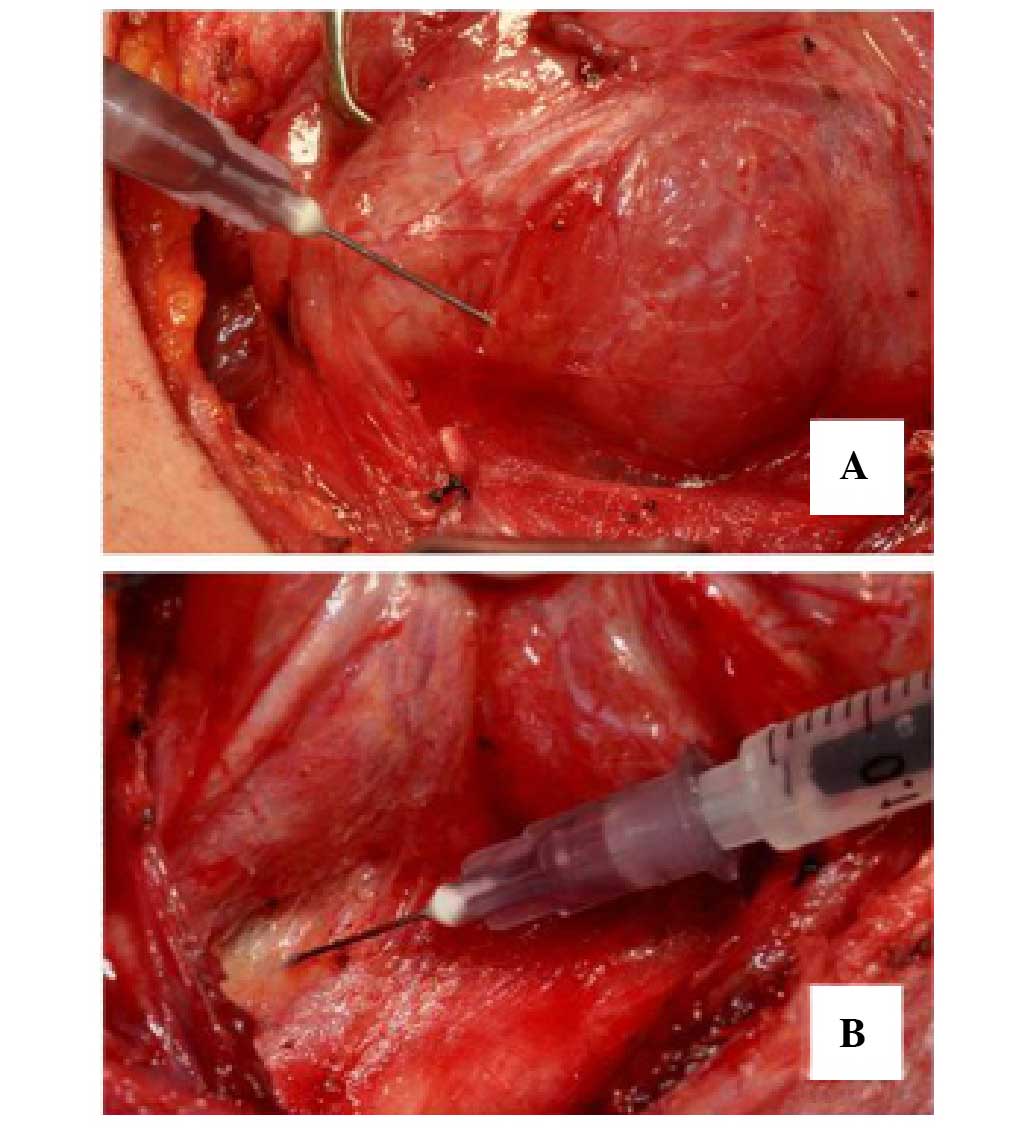
Intraoperative FNA and surgical
management
A 26-gauge needle and a 1-ml syringe containing 0.2
ml saline solution were used to sample the specimens for rIO-PTH
analysis. A total of 3–5 aspirations were performed with each
puncture of the suspect tissue while maintaining appropriate
negative pressure (Fig. 1A and B).
The sample was diluted with 1 ml saline solution and immediately
transferred to the waiting laboratory. rIO-PTH levels were analyzed
using a solid-phase chemiluminescent immunometric assay (Roche
Diagnostics GmbH, Mannheim, Germany). rIO-PTH concentrations were
available within 15 min of receiving the sample.
To analyze the sensitivity and specificity of
rIO-PTH via FNA to identify parathyroids compared with pathological
diagnosis, frozen section examinations of the aspirated tissue were
performed to confirm the histological source. The smallest possible
tissue sample was collected for the frozen section examinations and
results were available within 30 min.
Patients with benign lesions underwent subtotal or
total thyroidectomy. Patients with papillary thyroid carcinoma
lesions confined to a single lobe underwent lobectomy plus
isthmectomy with ipsilateral central cervical lymph node
dissection. If benign nodules were found in the contralateral lobe,
ipsilateral lobectomy plus contralateral subtotal lobectomy with
ipsilateral or bilateral central cervical lymph node dissection was
performed. If papillary thyroid carcinoma lesions were found in
both lobes, total thyroidectomy with bilateral central cervical
lymph node dissection was performed. The above procedures involved
the identification and preservation of the parathyroids.
Statistical analysis
Analyses were performed using the χ2,
Student's t-test, or Mann-Whitney tests, as appropriate. P<0.05
was considered to indicate a statistically significant difference.
Statistical analyses were performed with SPSS version 16.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Clinical characteristics
The mean age of the rIO-PTH group was 42.5 years
(range, 16–56 years); 14 were male and 36 female (ratio, 1:2.6).
Subtotal or total thyroidectomy was performed in 19 patients with
benign lesions, whereas 14/31 patients with papillary thyroid
carcinoma lesions underwent total thyroidectomy, lobectomy plus
contralateral subtotal lobectomy, or isthmectomy (n=17). The age of
control group participants ranged from 20 to 62 years with a mean
of 42.4 years; 13 were male and 37 female (ratio, 1:2.8). A total
of 21 patients with benign lesions underwent subtotal or total
thyroidectomy. Of 29 patients with papillary thyroid carcinoma
lesions, total thyroidectomy was performed in 11 patients, and
lobectomy plus contralateral subtotal lobectomy, or isthmectomy was
performed in 18 patients. No differences in clinical
characteristics were detected between the two groups.
In the rIO-PTH group, 93/194 aspirated anatomical
structures were confirmed as parathyroid tissue, histologically. A
total of 45 of the suspected parathyroid tissue samples were
confirmed to be normal lymph nodes, with only four metastatic lymph
nodes, out of a total of 49. The other non-parathyroid tissues
included 13 thyroid tissue and 39 adipose tissue samples.
rIO-PTH values in parathyroid and
non-parathyroid tissue
A total of 194 tissue samples from the rIO-PTH group
were subdivided into two groups according to the pathological
diagnosis: 93 parathyroid tissues and 101 non-parathyroid tissues.
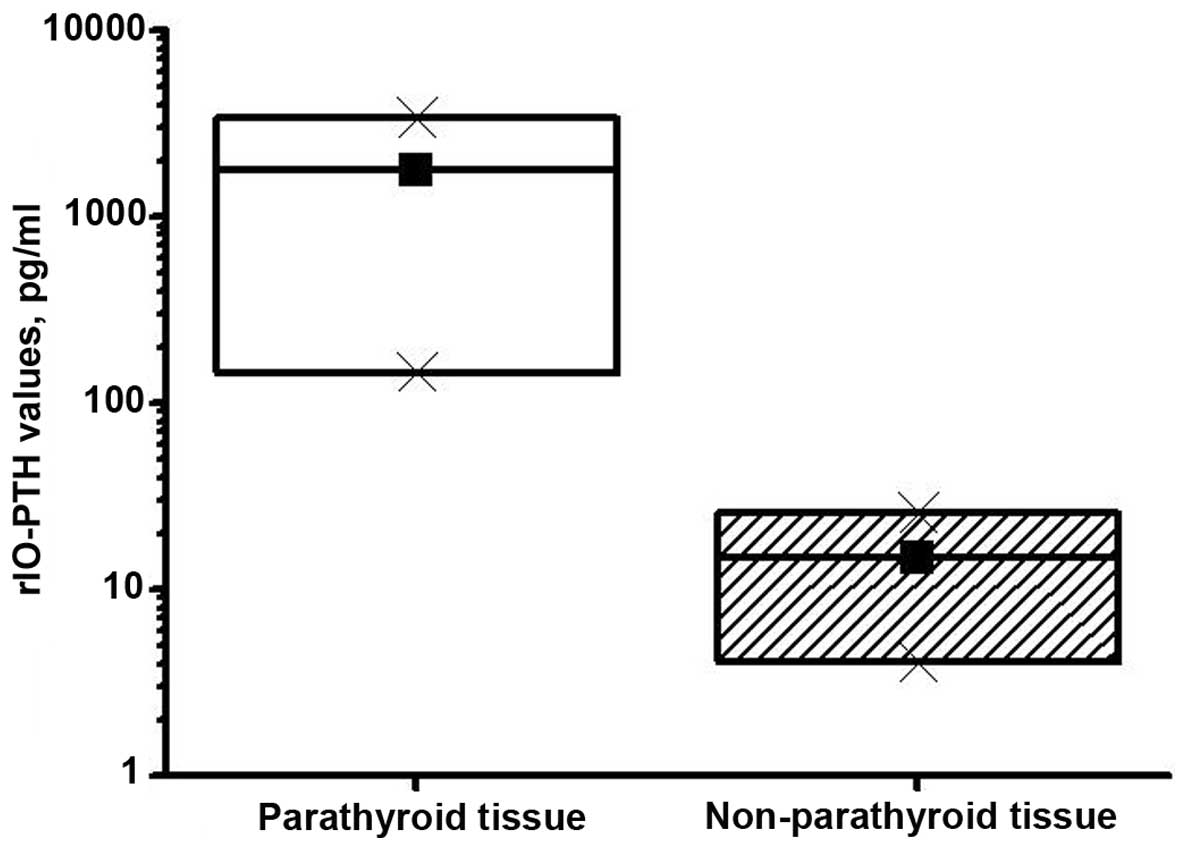
A mean rIO-PTH value of 3,369 pg/ml (range, 145.2–5000 pg/ml) was
obtained from the aspirated confirmed parathyroids. rIO-PTH values
from the non-parathyroid tissues ranged from 4.05 to 39.5 pg/ml,
with a mean of 25.7 pg/ml. Using a box plot distribution, it was
demonstrated that the rIO-PTH values obtained from the parathyroid
tissues did not overlap with the rIO-PTH values obtained from
non-parathyroid tissues (P<0.0001; Fig. 2). The present results showed that the
rIO-PTH median was significantly higher in parathyroid tissue
compared with non-parathyroid tissue.
Parathyroid glands identified and
postoperative effects in the rIO-PTH vs
control group. A total of 3.76 parathyroid glands
were correctly identified based on visualization and rIO-PTH assay
through FNA in the rIO-PTH group, compared with 2.41 parathyroid
glands in the controls detected by visualization alone.
Mann-Whitney testing confirmed a significant difference between the
two groups (P<0.05; Table I). The
difference between the postoperative serum calcium level and blood
PTH values in the two groups was not statistically significant
(P>0.05; Table I).
 | Table I.Clinical effects of rIO-PTH
treatment. |
Table I.
Clinical effects of rIO-PTH
treatment.
|
| Group (n=50
each) |
|
|---|
|
|
|
|
|---|
| Parameter | rIO-PTH | Control | P-value |
|---|
| Parathyroid glands
(n) | 188 | 122 | <0.05 |
| Serum calcium
(mmol/l) | 2.25 | 2.21 | >0.05 |
| Blood PTH levels
(ng/ml) | 48.3 | 39.5 | >0.05 |
No patient in either group experienced postoperative
permanent hypoparathyroidism or other serious complications of
thyroid surgery. The frequencies of transient hypocalcemia were 0%
(0/50) and 12% (6/50), in the rIO-PTH and control groups,
respectively (P<0.05; Table II).
Patients with transient hypocalcemia complained of only minor
symptoms, with serum calcium levels returning to normal values
<6 months after surgery.
 | Table II.Incidence of postoperative transient
hypocalcaemia in rIO-PTH. |
Table II.
Incidence of postoperative transient
hypocalcaemia in rIO-PTH.
|
|
| Postoperative
transient hypocalcaemia |
|
|
|---|
|
|
|
|
|
|
|---|
| Group | Cases, n | Yes, n (%) | No, n (%) | χ2 | P-value |
|---|
| rIO-PTH | 50 | 0 (0) | 50 (100) | 4.43 | P<0.05 |
| Control | 50 | 6 (12) | 44 (88) |
Discussion
Hypoparathyroidism is one of the most common
complications of thyroidectomy and serious symptoms of hypocalcemia
include paresthesia, numbness, or muscle cramps, which may be
permanent lasting >6 months after surgery (1). Hypocalcemia may also lead to
significant morbidity and impaired quality of life. Postoperative
transient hypoparathyroidism (resolved <6 months after surgery)
can be caused by temporary poor parathyroid blood supply, whereas
permanent hypoparathyroidism may result from ischemic necrosis of
the parathyroids or inadvertent removal. To reduce morbidity
associated with postoperative hypoparathyroidism, surgeons strive
for parathyroid protection, thus effective and improved methods of
parathyroid identification are critical.
Currently, parathyroid identification relies on a
surgeon's personal experience to examine the tissue based on
morphological and topographical criteria (6). Therefore, inexperience of the surgeon
is a significant risk factor for permanent hypoparathyroidism. A
review by Paek et al (5)
reported that permanent hypoparathyroidism occurred in 6.5% of
patients treated by surgeons in the first two years of practice,
decreasing to 1.8% in the second two years. Therefore, surgeons
continually search for accurate, reliable, and simple methods for
distinguishing parathyroid from non-parathyroid tissue. Several
experimental techniques have been used previously to identify
parathyroid tissue, including carbon nanoparticle suspension
negative imaging (14), visible red
fluorescence using aminolevulinic acid (15), visible staining by methylene blue
(16) or antiparathyroid antibody
BB5-G1 conjugated to Cibacron® blue (17), and gamma probe identification
(18). Although these techniques may
greatly benefit patients, they are predominantly experimental or
controversial and the clinical applications remain limited. Frozen
section examination can be used to accurately and definitively
distinguish parathyroid from non-parathyroid tissue; however,
histopathological examination may prolong the operative time and
increase the invasiveness of surgery (19).
The intraoperative use of serum PTH assays to
determine the satisfactory removal of all hyperfunctional
parathyroid tissue was first reported in 1988 (20) and serum PTH is now recognized as the
gold standard in the diagnosis of hyperfunctional parathyroid
tissue. PTH reflects the function of the parathyroids and rIO-PTH
levels from tissue FNA predicts the presence of parathyroid tissue
during parathyroidectomies for primary or secondary
hyperparathyroidism (8,9,12). To
our knowledge, the present study details the first use of rIO-PTH
assay for FNA of suspected parathyroid tissue to distinguish
parathyroid and non-parathyroid tissues during thyroidectomy. To
analyze the sensitivity and the specificity of this technique, the
aspirated tissue underwent frozen section examinations. The results
demonstrated that the rIO-PTH mean value of 3,369 pg/ml was
significantly higher in parathyroid tissues that were confirmed by
pathology compared with the rIO-PTH mean of 25.7 pg/ml in
non-parathyroid tissue. The rIO-PTH lower limit of 145.2 pg/ml in
parathyroid tissue was markedly higher than the maximal rIO-PTH
value of 39.5 pg/ml obtained from non-parathyroid tissues,
demonstrating that this technique correctly predicted parathyroid
tissue in every case with higher sensitivity and specificity
compared with frozen section examination.
Even in routine cases, frozen section examinations
are often recommended to verify the identity and confirm the
presence of parathyroid tissue, and to provide pathological tissue
confirmation for potential parathyroid autotransplantation
(7,21,22).
However, frozen section examination can be time-consuming, costly,
and require the excision of a significant portion of tissue,
rendering it impractical in various settings (13). Compared with frozen section
examinations, it was demonstrated in the present study that the
rIO-PTH assay through FNA was more reliable, simple, minimally
invasive, and required a reduced surgical duration. rIO-PTH assay
through FNA helped successfully identify parathyroid and
non-parathyroid tissues with the above advantages; thus this
technique may replace frozen section examination.
No serious complications were detected in either
group following the thyroid surgery, including postoperative
hemorrhage and vocal cord paralysis, indicating that this novel
rIO-PTH technology did not increase the surgical risks.
Postoperative transient hypoparathyroidism did not occur in the
rIO-PTH group compared with six cases in the control group, showing
that this technique was an effective method of avoiding transient
hypocalcemia. Notably, a significantly increased number of
parathyroids were detected in the visualization combined with
rIO-PTH assay through FNA method in the rIO-PTH group, compared
visualization alone. The security and practicality of this
technology were effective for protecting parathyroids that are
difficult to identify in subsequent surgery, and for improving
parathyroid identification by inexperienced surgeons. There was no
statistically significant difference in the postoperative serum
calcium level and blood PTH values between the rIO-PTH group and
control group, which indicated that to avoid postoperative
hypocalcemia, surgeons should stress intraoperative in-situ
conservation and autotransplantation of the parathyroids, as well
as improve parathyroid identification.
In conclusion, the present study demonstrated the
value of rIO-PTH assay through FNA as an effective method for
differentiating parathyroid and non-parathyroid tissue. The
technique is highly reliable, quick, simple, and non-invasive and
may replace frozen section examination and a reliance on surgeons'
parathyroid visualization using topographic or morphologic
criteria. The clinical significance of this novel technique and the
reference value index of rIO-PTH require further investigation with
larger numbers of cases across multiple institutions.
Acknowledgements
This research was funded by the National Natural
Science Foundation of China (grant no. 81202552) and the Natural
Science Foundation of Jilin Provincial Science and Technology
Department (grant no. 201101046).
Glossary
Abbreviations
Abbreviations:
|
rIO-PTH
|
rapid intraoperative parathyroid
hormone
|
|
FNA
|
fine needle aspiration
|
|
PTH
|
parathyroid hormone
|
References
|
1
|
Reeve T and Thompson NW: Complications of
thyroid surgery: How to avoid them, how to manage them, and
observations on their possible effect on the whole patient. World J
Surg. 24:971–975. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quiros RM, Pesce CE, Wilhelm SM, Djuricin
G and Prinz RA: Intraoperative parathyroid hormone levels in
thyroid surgery are predictive of postoperative hypoparathyroidism
and need for vitamin D supplementation. Am J Surg. 189:306–309.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosato L, Avenia N, Bernante P, De Palma
M, Gulino G, Nasi PG, Pelizzo MR and Pezzullo L: Complications of
thyroid surgery: Analysis of a multicentric study on 14,934
patients operated on in Italy over 5 years. World J Surg.
28:271–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bliss RD, Gauger PG and Delbridge LW:
Surgeon's approach to the thyroid gland: Surgical anatomy and the
importance of technique. World J Surg. 24:891–897. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paek SH, Lee YM, Min SY, Kim SW, Chung KW
and Youn YK: Risk factors of hypoparathyroidism following total
thyroidectomy for thyroid cancer. World J Surg. 37:94–101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pelizzo MR, Losi A, Boschin IM, Toniato A,
Pennelli G, Sorgato N, Faggian D and Plebani M: Rapid
intraoperative parathyroid hormone assay in fine needle aspiration
for differential diagnosis in thyroid and parathyroid surgery. Clin
Chem Lab Med. 48:1313–1317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baumann DS and Wells SA Jr: Parathyroid
autotransplantation. Surgery. 113:130–133. 1993.PubMed/NCBI
|
|
8
|
Lo CY, Chan WF, Leung P and Luk JM:
Applicability of tissue aspirate for quick parathyroid hormone
assay to confirm parathyroid tissue identity during
parathyroidectomy for primary hyperparathyroidism. Arch Surg.
140:146–149; discussion 150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiblut NK, Cussac JF, Soudan B, Farrell
SG, Armstrong JA, Arnalsteen L, Biechlin A, Delattre AA and Proye
CA: Fine needle aspiration and intraparathyroid intact parathyroid
hormone measurement for reoperative parathyroid surgery. World J
Surg. 28:1143–1147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan RK, Ibrahim SI, Pil P, Tanasijevic M
and Moore FD: Validation of a method to replace frozen section
during parathyroid exploration by using the rapid parathyroid
hormone assay on parathyroid aspirates. Arch Surg. 140:371–373.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conrad DN, Olson JE, Hartwig HM, Mack E
and Chen H: A prospective evaluation of novel methods to
intraoperatively distinguish parathyroid tissue utilizing a
parathyroid hormone assay. J Surg Res. 133:38–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boggs JE, Irvin GL III, Carneiro DM and
Molinari AS: The evolution of parathyroidectomy failures. Surgery.
126:998–1002; discussion 1002–1003. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horányi J, Duffek L, Szlávik R, Takács I,
Tóth M and Romics L Jr: Intraoperative determination of PTH
concentrations in fine needle tissue aspirates to identify
parathyroid tissue during parathyroidectomy. World J Surg.
34:538–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang K, Luo D, Huang M, Long M, Peng X
and Li H: Protection of parathyroid function using carbon
nanoparticles during thyroid surgery. Otolaryngol Head Neck Surg.
149:845–850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu WW, Li CQ, Guo ZM, Li H, Zhang Q and
Yang AK: Fluorescence identification of parathyroid glands by
aminolevulinic acid hydrochloride in rats. Photomed Laser Surg.
29:635–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel HP, Chadwick DR, Harrison BJ and
Balasubramanian SP: Systematic review of intravenous methylene blue
in parathyroid surgery. Br J Surg. 99:1345–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
King RC, Mills SL and Medina JE: Enhanced
visualization of parathyroid tissue by infusion of a visible dye
conjugated to an antiparathyroid antibody. Head Neck. 21:111–115.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grubbs EG, Mittendorf EA, Perrier ND and
Lee JE: Gamma probe identification of normal parathyroid glands
during central neck surgery can facilitate parathyroid
preservation. Am J Surg. 196:931–935; discussion 935–936. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farrag T, Weinberger P, Seybt M and Terris
DJ: Point-of-care rapid intraoperative parathyroid hormone assay of
needle aspirates from parathyroid tissue: A substitute for frozen
sections. Am J Otolaryngol. 32:574–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nussbaum SR, Thompson AR, Hutcheson KA,
Gaz RD and Wang CA: Intraoperative measurement of parathyroid
hormone in the surgical management of hyperparathyroidism. Surgery.
104:1121–1127. 1988.PubMed/NCBI
|
|
21
|
Shidham VB, Asma Z, Rao RN, Chavan A,
Machhi J, Almagro U and Komorowski RA: Intraoperative cytology
increases the diagnostic accuracy of frozen sections for the
confirmation of various tissues in the parathyroid region. Am J
Clin Pathol. 118:895–902. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Westra WH, Pritchett DD and Udelsman R:
Intraoperative confirmation of parathyroid tissue during
parathyroid exploration: A retrospective evaluation of the frozen
section. Am J Surg Pathol. 22:538–544. 1998. View Article : Google Scholar : PubMed/NCBI
|