Introduction
Paget's disease of bone (PDB) is a metabolic bone
disease characterized by increased bone resorption and active bone
formation. The prevalence of PDB in Japan is relatively lower
(2.8/1,000,000 individuals) than that of North American and
European countries (1). Mixed lytic
and sclerotic changes are present on the roentgenograms of patients
with PDB, and bone scintigraphy using technetium-99m is
often more sensitive than plain radiographs (2). Because of the lower prevalence of PDB
observed in Japan than in Western countries, diagnosis of PDB
remains difficult for Japanese physicians. In a report by the
committee for PDB within the Japan Osteoporosis Society, 55% of
Japanese patients with PDB underwent diagnostic biopsy of the
affected bones (1). Since
publication of the report, PDB has become more widely recognized
and diagnosis without biopsy has become more common. Another
characteristic sign of PDB is elevation of serum alkaline
phosphatase (ALP) levels. Generally, diagnosis of PDB is confirmed
by radiological findings, high tracer accumulation in bone
scintigraphy, and a high serum ALP level (1,2).
Although PDB is often asymptomatic, it can cause bone pain,
osteoarthritis, pathological fractures, bone deformity, deafness,
and nerve compression syndromes (3).
Patients with symptomatic PDB or PDB that affects the
weight-bearing bones are candidates for medical treatments to
reduce bone turnover. The treatment of choice is a potent
nitrogen-containing bisphosphonate, such as alendronate,
risedronate, pamidronate or zoledronic acid. Treatment monitoring
is divided into two categories, biochemical marker analysis and
imaging analysis. The total serum ALP level is the most commonly
used marker due to its low cost and high reproducibility (2). We propose that bone scintigraphy should
be the imaging technique of choice, as tracer uptake is directly
associated with disease activity and may be observed in advance of
any radiographic evidence (4). For
quantitative bone scintigraphic analysis; however, obtaining
results at different time points is not straightforward and may be
inaccurate due to the lack of suitable image analysis software.
Computer-assisted diagnosis (CAD) systems for bone scintigraphy,
which predominantly target metastatic bone tumors, have recently
been developed (5,6). The representative system, BONENAVI
(Fujifilm RI Pharma, Tokyo, Japan), is based on artificial neural
networks (ANNs) and evaluates the risk of bone metastasis from bone
scintigraphy data. BONENAVI is a bone scintigraphy diagnosis
support software established with a Japanese database. It is used
for the diagnosis and follow-up observation of patients with bone
metastasis (7,8). This system evaluates three parameters:
Bone scan index (BSI), ANN value, and hotspot number (HSn). The ANN
value ranges from 0 to 1 and indicates the probability of
metastasis as follows: ANN=0.00–0.25, bone metastasis is not
suspected in the bone scans; ANN=0.26–0.50, bone metastasis may not
be fully excluded; ANN=0.51–0.75, bone metastasis is suspected; and
ANN=0.76–1.00, bone metastasis is highly suspected (9). BSI represents the total amount of
abnormal tracer accumulation and is shown as a value relative to
the whole body (0–100%). HSn is the number of abnormal lesions and
is determined by the ANN. We hypothesized that the BSI and HSn may
be useful for the quantitative evaluation of bone metabolism in
patients with PDB. Furthermore, whether BONENAVI parameters
correlate with well-known bone metabolic markers was evaluated.
Materials and methods
Patients
The present study included seven Japanese patients
with PDB diagnosed in our department who were treated between 2008
and 2011. The seven patients comprised four female and three male
patients with an average age of 60 years (range, 33–80 years). The
characteristics of the patients are summarized in Table I. Three patients underwent biopsy to
establish the diagnosis of PDB. In accordance with the guidelines
of the Japanese Committee for PDB within the Japan Osteoporosis
Society (10), we have recently
begun to establish the diagnosis of PDB based on imaging studies
and serum bone metabolic markers, and this was performed in
Patients 4–7 (Table I). All patients
underwent bisphosphonate treatment under informed consent. Three
patients exhibited symptoms associated with the affected bone. The
remaining four patients were treated owing to concern regarding
future complications associated with the affected weight-bearing
bones (Patients 1, 5 and 6) or cervical spine (Patient 3). Four
patients received 17.5 mg risedronate (Eisai, Co., Ltd., Tokyo,
Japan) daily for 8 weeks in accordance with previously established
Japanese guidelines (10). The
remaining three patients were administered a reduced dose of weekly
17.5 mg risedronate or monthly minodronate (Ono Pharmaceutical Co.,
Ltd., Osaka, Japan) due to of adverse effects (gastritis in Patient
2) or lack of symptoms (Patients 5 and 6).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Number | Age | Gender | Affected site | Biopsy | Symptom | Bisphosphonate |
|---|
| 1 | 59 | Female | Skull, pelvis,
femur | + |
| E, R |
| 2 | 76 | Female | Humerus |
|
| + | + |
|
| 3 | 48 | Male | Cervical spine,
humerus | + |
| Ra |
| 4 | 80 | Male | Pelvis, humerus |
| + | R |
| 5 | 33 | Female | Skull, femur |
|
| Ra |
| 6 | 64 | Female | Pelvis |
|
|
|
| Ma |
| 7 | 59 | Male | Sacrum |
|
|
| + | R |
Bone metabolic markers
Serum total ALP, bone-specific ALP (BAP), and type I
collagen cross-linked N-telopeptide (sNTx) levels were examined
before and after the initiation of therapy. Urinary markers,
deoxypyridinoline (DPD) and urine NTx (uNTx), were also analyzed.
ALP was analyzed via a colorimetric assay using p-nitrophenyl
phosphate substrate according to the manufacturer's instructions
(Iatro ALP; LSI Medience Co., Tokyo, Japan). BAP/DPD and NTx (serum
and urine) were analyzed by enzyme immunoassay and enzyme-linked
immunosorbent assay, respectively, at a clinical laboratory (SRL
Inc., Kagoshima, Japan). Both DPD and uNTx were adjusted by the
serum creatinine level to account for differences in urine
volume.
Bone scintigraphy and BONENAVI
analysis
Whole-body bone scintigraphy images were acquired 4
h after intravenous injection of 740 MBq technetium-99m-methylene
diphosphonate (Fujifilm RI Pharma) using a dual-head gamma camera
equipped with low-to-mid-energy general-purpose collimators (Symbia
T6; Siemens Medical Solutions, Malvern, PA, USA).
BONENAVI 2.0 (Fujifilm RI Pharma and EXINI Bone;
EXINI Diagnostics, Lund, Sweden) was used to calculate the BSI,
ANN, and HSn. Region-based ANNs in the range of 0.00 to 0.50 were
expressed as blue spots, whereas those ranging from 0.51 to 1.00
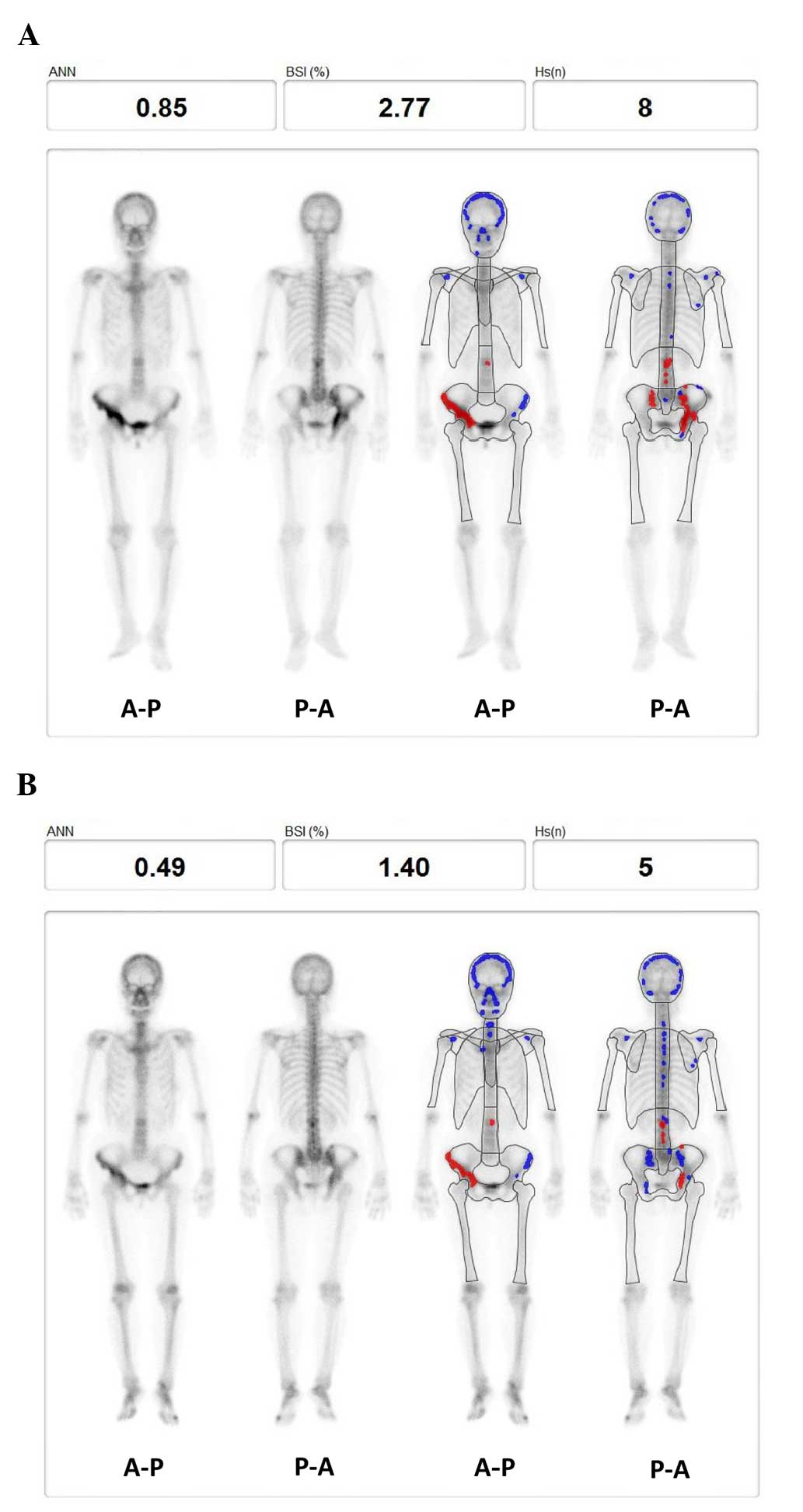
were seen as red hotspots (Fig. 1).
Based on the number of red areas, the software calculates the ANN
to estimate the risk of bone metastasis (11,12). BSI
and HSn were monitored as an objective evaluation of the medical
treatment (Fig. 1).
Statistical analysis
Alterations in the values of the bone metabolic
markers or BONENAVI parameters before and after bisphosphonate
treatment were analyzed by Student's t-test. Correlations between
BONENAVI parameters (ANN, BSI, and HSn) and bone metabolism markers
(ALP, BAP, sNTx, and uNTx) were analyzed by Pearson's correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Ethics statement
All procedures involving human participants were
performed in accordance with the 1964 Helsinki declaration and its
later amendments or comparable ethical standards. Informed consent
was obtained from all individual participants included in the
study.
Results
Bisphosphonate treatment reduces all
three BONENAVI parameters (BSI, ANN and HSn)
A 64-year-old female (patient 5) exhibited a high
pretreatment ANN (0.85) prior to bisphosphonate treatment (Fig. 1A). Post-treatment BONENAVI analysis
showed a reduction in all three parameters, namely BSI, ANN and HSn
(Fig. 1B). Notably, red hotspots
were located around the right pelvis.
Bisphosphonate treatment significantly
reduces ALP, BAP, sNTx and uNTx levels
The average serum ALP level was significantly lower
following bisphosphonate treatment, compared with before (P=0.0052;
Fig. 2A). Serum levels of the other
bone formation marker, BAP, were also significantly lower after
treatment (48.0±19.0) as compared with pre-treatment (93.8±23.0;
P=0.003; Fig. 2B). Among all bone
resorption markers, the reductions of sNTx and uNTx were
statistically significant (P=0.01 and P=0.04, respectively). DPD
was the only marker to not exhibit a significant reduction
following bisphosphonate treatment.
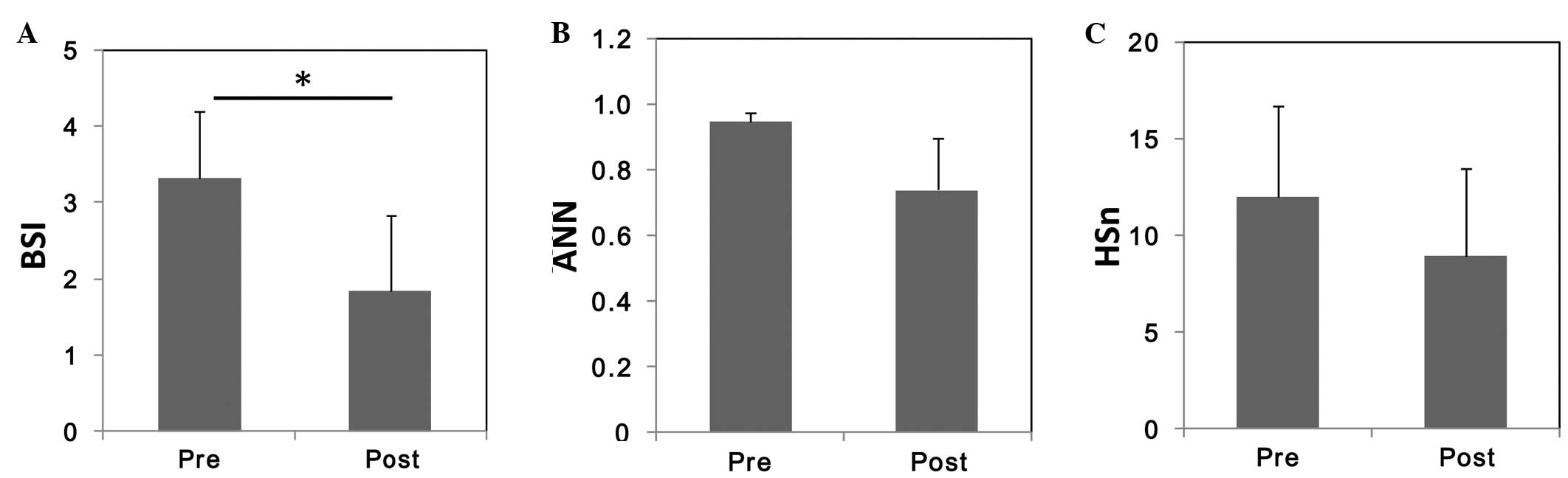
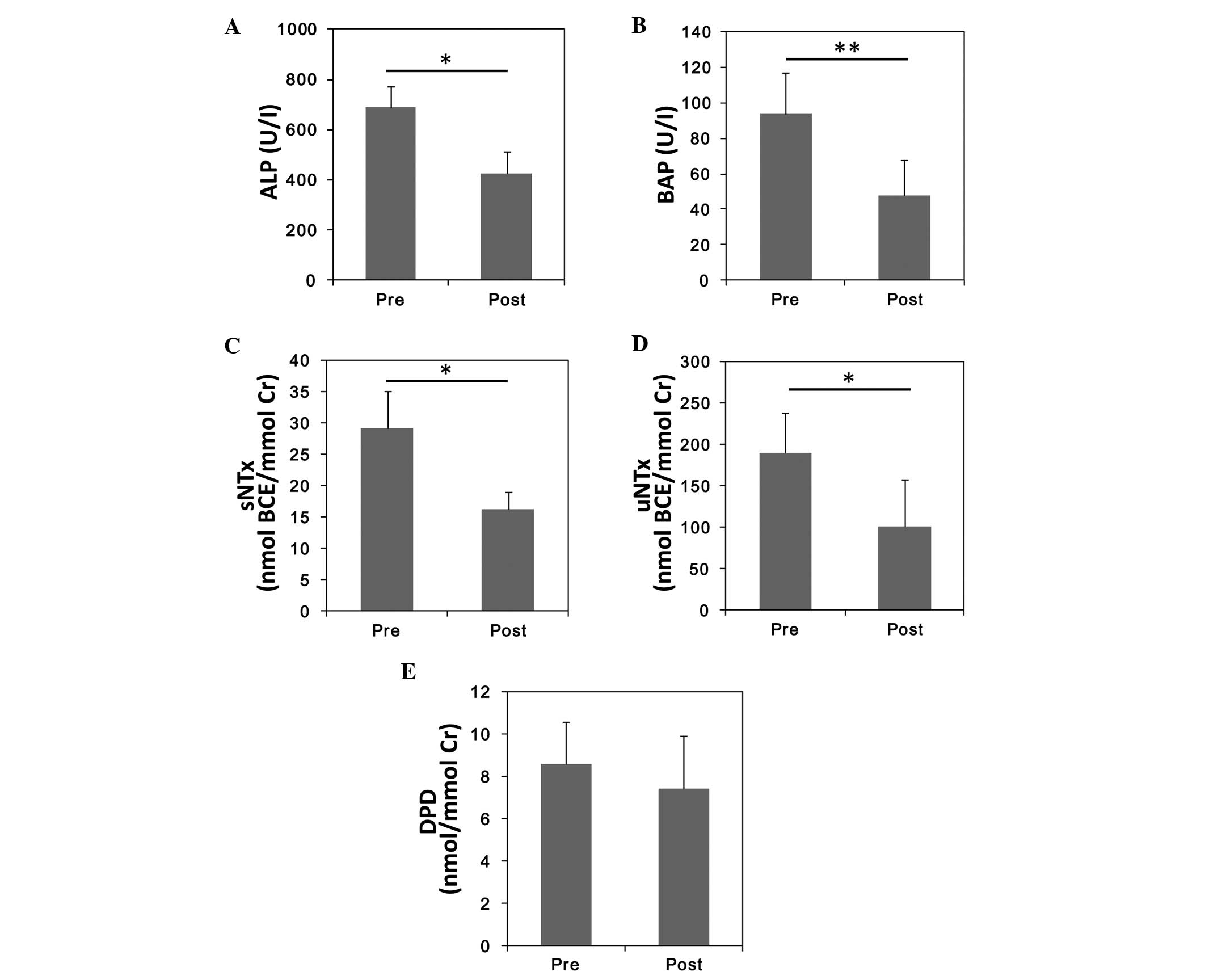 | Figure 2.Alterations in bone metabolic marker
levels before and after treatment with bisphosphonate. Mean values
of (A) ALP, (B) BAP, (C) sNTx, (D) uNTx, and (E) urinary DPD.
*P<0.05; **P<0.005. ALP, serum alkaline phosphatase; BAP,
serum bone-specific ALP; BCE, bone collagen equivalent; sNTx, serum
type I collagen cross-linked N-telopeptide; uNTx, urinary NTx; DPD,
deoxypyridinoline; Cr, creatinine. |
Bisphosphonate treatment significantly
reduces BSI, as detected by BONENAVI
Among the three BONENAVI parameters, only BSI
significantly decreased from a pretreatment value of 3.33±0.90 to a
post-treatment value of 1.84±1.00 (Fig.
3A). Neither the ANN nor the HSn significantly changed with
treatment (Fig. 3B and C,
respectively).
Bone formation and reabsorption
markers are significantly correlated
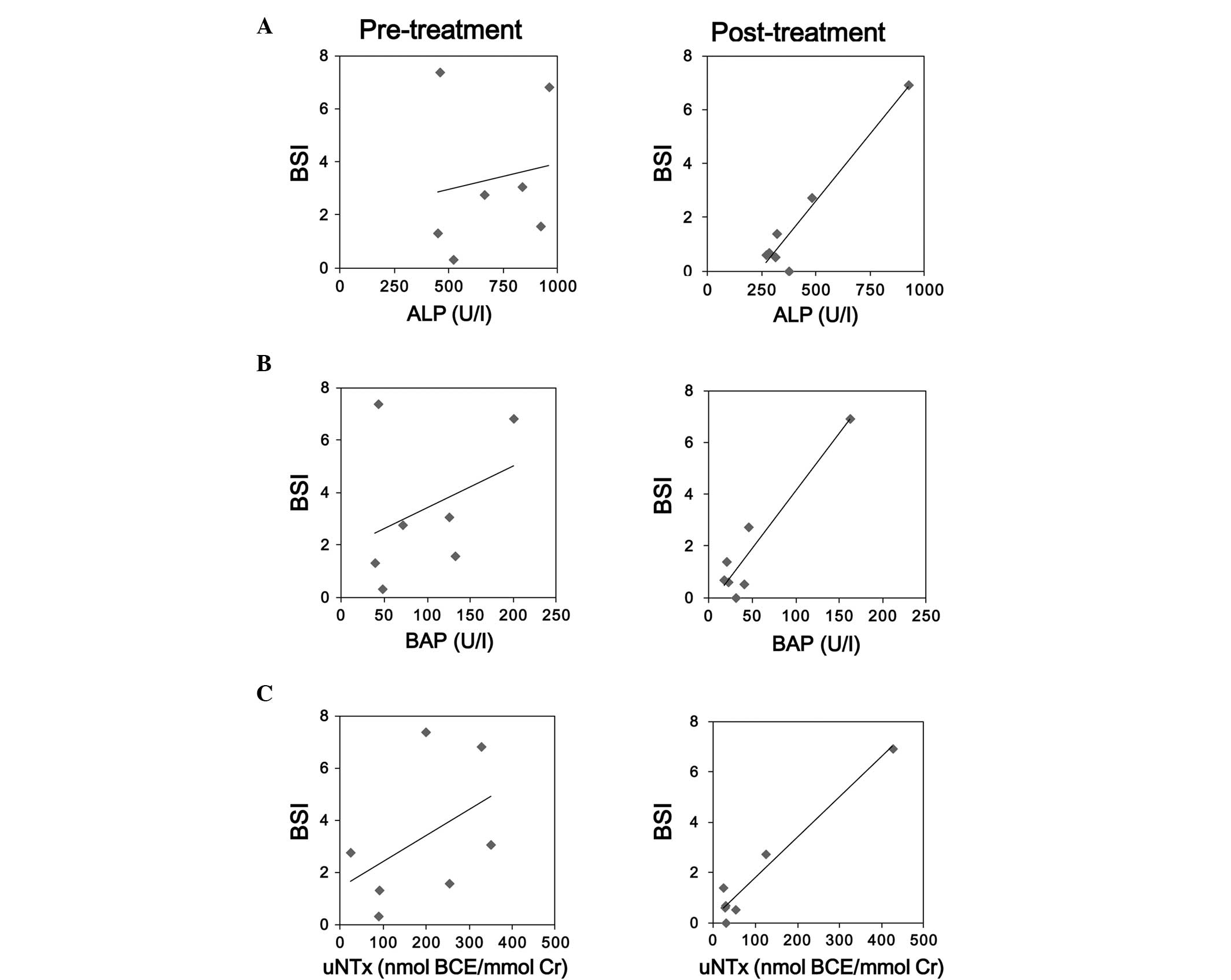
Correlation analysis between the BONENAVI parameters
and bone metabolism markers revealed almost no correlation with the
pretreatment values (Table II). On
the other hand, the post-treatment BSI and HSn showed a significant
correlation with the bone formation and resorption markers (ALP,
BAP, and uNTx) (Fig. 4).
 | Table II.Pearson's correlation index between
BONENAVI system parameters and bone metabolic markers. |
Table II.
Pearson's correlation index between
BONENAVI system parameters and bone metabolic markers.
|
| Pre-treatment | Post-treatment |
|---|
|
|
|
|
|---|
|
| ANN | BSI | HSn | ANN | BSI | HSn |
|---|
| ALP | 0.24 | 0.16 | 0.53 | 0.25 | 0.96a | 0.96a |
| BAP | 0.29 | 0.35 | 0.75 | 0.33 | 0.94a | 0.96a |
| sNTx | 0.63 | 0.28 | 0.74 | 0.30 | 0.73 | 0.75 |
| uNTx | 0.59 | 0.46 | 0.88a | 0.45 | 0.98a | 0.96a |
Representative case
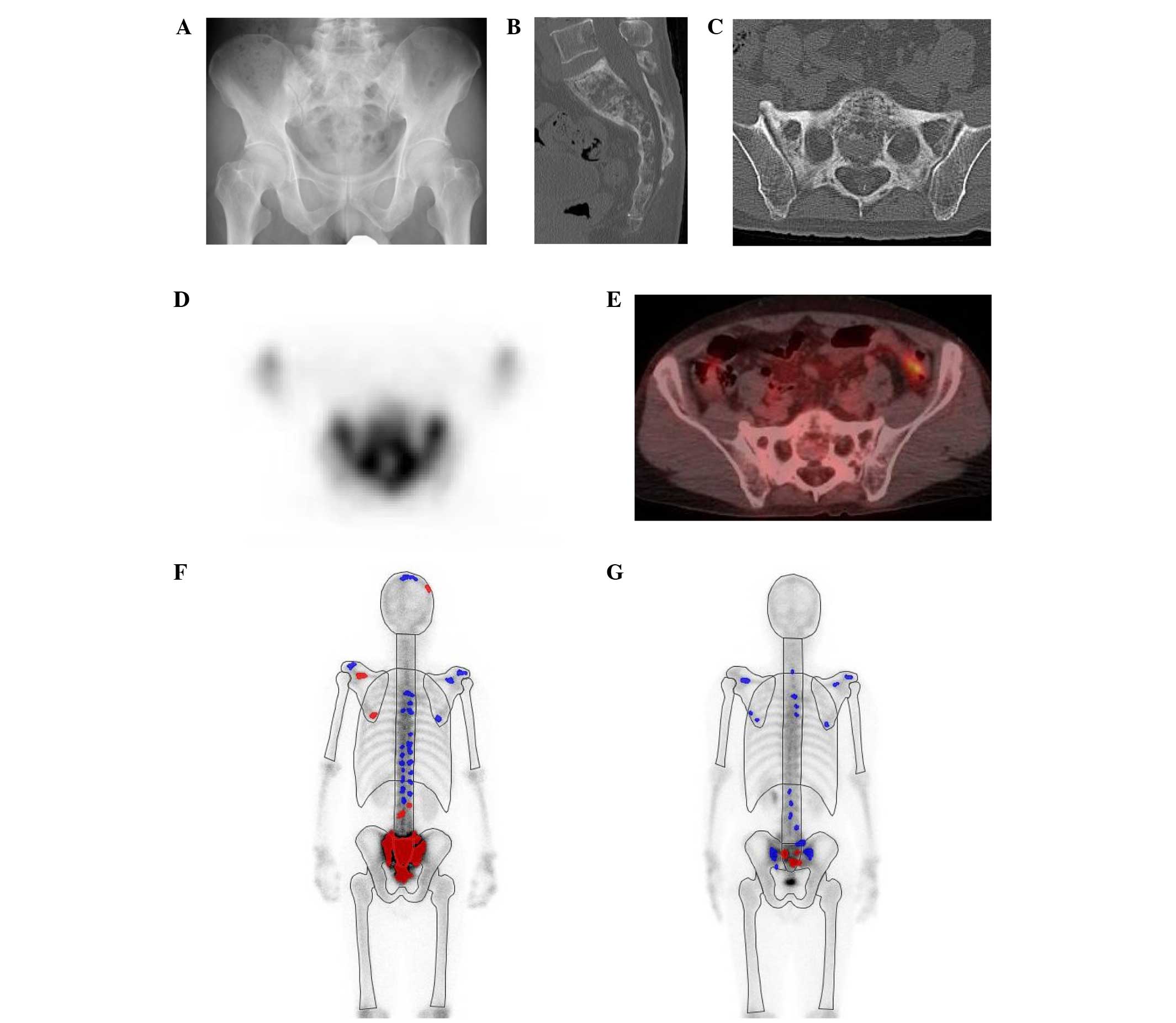
A representative case is shown in Fig. 5. This 59-year-old man had been
referred to our institution due to abnormal tracer accumulation in
the sacrum during a bone scan, accompanies by elevated ALP levels
(Fig. 5A-D). Positron-emission
tomography with fluorodeoxyglucose was performed, which revealed no
accumulation of fluorodeoxyglucose in the sacral area (Fig. 5E). The patient was diagnosed with PDB
and administered risedronate treatment for 8 weeks. As compared
with prior to treatment (Fig. 5F),
post-treatment BONENAVI imaging revealed a markedly reduced
tracer-positive area on the bone scan (Fig. 5G). The ALP level also significantly
decreased from a pretreatment value of 498 U/l to a post-treatment
value of 269 U/l.
Discussion
The concept of the BSI for quantitative evaluation
of bone scans in patients with cancer was first established by a
group from the Memorial Sloan-Kettering Cancer Center (13). They reported that the BSI may be
useful for stratifying patients with prostate cancer entering
treatment protocols. However, the BSI was calculated from the
weight and the fractional involvement of each bone, expressed as
percentages of the entire skeleton; this is largely dependent upon
each observer's visual evaluation (13). Following establishment of the
CAD-based BONENAVI system for automatic calculation of the BSI, its
usefulness in the diagnosis and monitoring of bone metastasis has
been reported by several authors (11,14,15).
BONENAVI is now routinely used during bone scans in various
facilities, including our institution. Therefore, data from
patients with bone metastasis will be accumulated to determine how
to adapt BONENAVI analysis in the management of bone metastases in
the future. To our knowledge, no studies have evaluated the use of
BONENAVI in patients with PDB. Although the number of patients in
the present study was limited by the extremely low incidence of PDB
in Japan, the present findings demonstrated that the BSI reflects
the response to bisphosphonate treatment in patients with PDB. The
advantage of using BONENAVI over the measurement of bone metabolic
markers is the ability to spatially evaluate bone metabolism. It
would be helpful to evaluate the correlation between changes in
bone metabolism and symptoms associated with the affected bone
after bisphosphonate treatment in patients with polyostotic PDB.
However, it is not possible to separately evaluate each affected
lesion using only bone metabolic markers, particularly in patients
with polyostotic PDB. For instance, Patient 4 had mild pain in the
right upper extremity. Pretreatment analysis revealed hotspots
predominantly in the right humerus and left pelvis (Table I). Although the hotspots in the
pelvic area remained unchanged after treatment, accumulation in the
left humerus was reduced (blue), resulting in relief of the
patient's pain. Therefore, we consider that the bisphosphonate
treatment in this patient was effective for the symptomatic lesion
in the humerus, but not substantially for the pelvic lesion.
Biochemical assessment of PDB has been studied
previously (16). Since
bisphosphonates are antiresorptive, three markers in this study
were monitored in the present study (sNTx, uNTx, and DPD). The
present finding that uNTx better demonstrated the effect of
bisphosphonate treatment than DPD is consistent with previous
reports (16,17). It has previously been demonstrated
that short-term reduction of uNTx may predict the final
post-treatment result evaluated by ALP (18). As markers of bone formation, ALP,
BAP, and procollagen type 1 N-terminal propeptide perform similarly
in the diagnosis and assessment of polyostotic PDB (17). In particular, in patients with
monostotic PDB with limited disease activity, BAP has been shown to
be increased despite a normal ALP level (19). In the present patient series, both
BAP and ALP were elevated in all patients and were demonstrated to
be significantly reduced after treatment, as compared with before.
For the reasons outlined in the present study, in addition to
previous research and its cost-effectiveness, we conclude that ALP
has the most established role in the evaluation and monitoring of
PDB (16,17).
It should be emphasized that the present study is
the first to have shown a good correlation between quantitative
bone scan results and biomarkers in patients with PDB. A previous
study evaluated bone scan results using a manually and visually
quantified ‘scintigraphic index’ (16). However, to become a useful tool for
physicians, such analysis should not only provide reproducible
results, but its performance should also feasible. BONENAVI is a
reliable analysis of bone metabolic diseases, including PDB;
however, how it is correlated with bone metabolic markers remains
unknown. In this context, the correlation index between BONENAVI
parameters and biochemical markers was analyzed. Unexpectedly, the
pretreatment BONENAVI values were not significantly correlated with
any bone metabolic markers, except HSn and uNTx (Table II). Iwase et al (20) recently studied the correlation
between the BSI and bone metabolic markers in patients with bone
metastatic breast cancer. They showed that BSI was significantly
correlated with the ALP level at the time of initial treatment. The
present study demonstrated that the post-treatment values of ALP,
BAP, and uNTx were significantly correlated with the BSI and HSn
(Table II). Therefore, although the
BSI is not correlated with pretreatment biochemical markers, it is
an imaging biomarker that is as reliable as bone metabolic markers
to evaluate the efficacy of bisphosphonate treatment.
The main limitation of the present study was the
small sample size, which was largely due to the low prevalence rate
of PDB in Japan (1). Based on this
pilot study, further validation studies from Western countries are
required to elucidate whether this CAD-based system is useful in
the management of PDB.
In conclusion, the present study is the first to
demonstrate that automated quantitative and BONENAVI qualitative
evaluation software for bone scans may be used to monitor treatment
response in patients with PDB. Although the majority of the bone
metabolic markers decreased in response to bisphosphonate
treatment, only BSI significantly decreased among the three
BONENAVI parameters evaluated. A significant correlation between
the post-treatment BSI and bone metabolic markers was also
demonstrated. The use of BONENAVI software in combination with bone
metabolic markers may allow bone scintigraphy to quantitatively and
spatially evaluate treatment responses to bisphosphonates,
particularly in patients with polyostotic PDB.
References
|
1
|
Hashimoto J, Ohno I, Nakatsuka K,
Yoshimura N, Takata S, Zamma M, Yabe H, Abe S, Terada M, Yoh K, et
al: Prevalence and clinical features of Paget's disease of bone in
Japan. J Bone Miner Metab. 24:186–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Langston AL and Ralston SH: Management of
Paget's disease of bone. Rheumatology (Oxford). 43:955–959. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ralston SH, Langston AL and Reid IR:
Pathogenesis and management of Paget's disease of bone. Lancet.
372:155–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scutellari PN, Giorgi A, De Sario V and
Campanati P: Correlation of multimodality imaging in Paget's
disease of bone. Radiol Med. 110:603–615. 2005.(In English,
Italian). PubMed/NCBI
|
|
5
|
Sadik M, Jakobsson D, Olofsson F, Ohlsson
M, Suurkula M and Edenbrandt L: A new computer-based
decision-support system for the interpretation of bone scans. Nucl
Med Commun. 27:417–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sadik M, Hamadeh I, Nordblom P, Suurkula
M, Höglund P, Ohlsson M and Edenbrandt L: Computer-assisted
interpretation of planar whole-body bone scans. J Nucl Med.
49:1958–1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wakabayashi H, Nakajima K, Mizokami A,
Namiki M, Inaki A, Taki J and Kinuya S: Bone scintigraphy as a new
imaging biomarker: The relationship between bone scan index and
bone metabolic markers in prostate cancer patients with bone
metastases. Ann Nucl Med. 27:802–807. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizumi M, Wagatsuma K, Miyaji N, Murata
T, Miwa K, Takiguchi T, Makino T and Koyama M: Evaluation of a
computer-assisted diagnosis system, BONENAVI version 2, for bone
scintigraphy in cancer patients in a routine clinical setting. Ann
Nucl Med. 29:138–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horikoshi H, Kikuchi A, Onoguchi M,
Sjöstrand K and Edenbrandt L: Computer-aided diagnosis system for
bone scintigrams from Japanese patients: Importance of training
database. Ann Nucl Med. 26:622–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takata S, Hashimoto J, Nakatsuka K,
Yoshimura N, Yoh K, Ohno I, Yabe H, Abe S, Fukunaga M, Terada M, et
al: Guidelines for diagnosis and management of Paget's disease of
bone in Japan. J Bone Miner Metab. 24:359–367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koizumi M, Wagatsuma K, Miyaji N, Murata
T, Miwa K, Takiguchi T, Makino T and Koyama M: Evaluation of a
computer-assisted diagnosis system, BONENAVI version 2, for bone
scintigraphy in cancer patients in a routine clinical setting. Ann
Nucl Med. 29:138–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kikushima S, Hanawa N and Kotake F:
Diagnostic performance of bone scintigraphy analyzed by three
artificial neural network systems. Ann Nucl Med. 29:125–131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Imbriaco M, Larson SM, Yeung HW, Mawlawi
OR, Erdi Y, Venkatraman ES and Scher HI: A new parameter for
measuring metastatic bone involvement by prostate cancer: The bone
scan index. Clin Cancer Res. 4:1765–1772. 1998.PubMed/NCBI
|
|
14
|
Tokuda O, Harada Y, Ohishi Y, Matsunaga N
and Edenbrandt L: Investigation of computer-aided diagnosis system
for bone scans: A retrospective analysis in 406 patients. Ann Nucl
Med. 28:329–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahashi Y, Yoshimura M, Suzuki K,
Hashimoto T, Hirose H, Uchida K, Inoue S, Koizumi K and Tokuuye K:
Assessment of bone scans in advanced prostate carcinoma using fully
automated and semi-automated bone scan index methods. Ann Nucl Med.
26:586–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reid IR, Davidson JS, Wattie D, Wu F,
Lucas J, Gamble GD, Rutland MD and Cundy T: Comparative responses
of bone turnover markers to bisphosphonate therapy in Paget's
disease of bone. Bone. 35:224–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shankar S and Hosking DJ: Biochemical
assessment of Paget's disease of bone. J Bone Miner Res. 21:(Suppl
2). P22–P27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papapoulos SE and Frölich M: Prediction of
the outcome of treatment of Paget's disease of bone with
bisphosphonates from short-term changes in the rate of bone
resorption. J Clin Endocrinol Metab. 81:3993–3997. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alvarez L, Guañabens N, Peris P, Monegal
A, Bedini JL, Deulofeu R, de Osaba MJ Martinez, Muñoz-Gomez J,
Rivera-Fillat F and Ballesta AM: Discriminative value of
biochemical markers of bone turnover in assessing the activity of
Paget's disease. J Bone Miner Res. 10:458–465. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwase T, Yamamoto N, Ichihara H, Togawa T,
Nagashima T and Miyazaki M: The relationship between
skeletal-related events and bone scan index for the treatment of
bone metastasis with breast cancer patients. Medicine (Baltimore).
93:e2692014. View Article : Google Scholar : PubMed/NCBI
|