Introduction
Statins are competitive inhibitors of
3-hydroxy-3-methylglutaryl-coenzyme A reductase, a liver microsomal
enzyme, which are widely used for the treatment of
hypercholesterolemia (1). In 1999,
Mundy et al (2) first
reported that simvastatin and lovastatin stimulated in vivo
bone formation in rodents and increased nascent bone volume in
cultures from mouse calvariae. Statins have been investigated
during the development of bone anabolic agents (3,4).
Simvastatin is the most widely used statin, which has been the
subject of extensive research.
In recent years, simvastatin has been shown to
promote in vitro osteoblastic differentiation and inhibit
the adipocytic differentiation of pluripotent cell lines or marrow
stromal cells in individuals of all ages (5–10).
However, few studies have been performed to examine the in
vivo effect of simvastatin on ovariectomized (OVX) animal
models of osteoporosis, and the conclusions to date remain
inconsistent (11–19). Previous studies have found that
statins promote bone formation and mineralization and may inhibit
bone resorption (11–15); other studies do not support the
hypothesis that simvastatin is able to increase bone mineral
density (BMD) and reduce the fracture risk (16–19).
Potential reasons for the contradictory results among these
experiments include: Large age range of the selected animal models
(3–6 months); large drug dose range administered to the model
animals (0.3–20 mg/kg/d), which exhibits a lack of clear criteria;
and the bone sites examined varied, and included the tibia, femur
and vertebrae.
Osteoporosis is a metabolic and systemic bone
disease characterized by BMD and microarchitectural deterioration,
which results in increased bone fragility and fracture risk
(20). Fracture, which is the most
severe consequence of osteoporosis, is associated with enormous
costs and substantial morbidity and mortality (21); the risk of lumbar vertebral fractures
in osteoporosis fractures is ~50% (21). Therefore, lumbar vertebrae were
investigated in the present study.
To evaluate the effect of simvastatin on
osteogenesis in the lumbar vertebrae, a postmenopausal osteoporosis
model was created using 6-month-old OVX rats and various doses of
simvastatin. The present findings in model rats may help to
determine whether simvastatin is able to effectively prevent
osteoporosis from bone loss in the axial skeleton in postmenopausal
women. No analogous research has been reported in humans.
Materials and methods
Animals
A total of 60 female 5-month-old Sprague Dawley rats
(body weight, 382±20 g) were purchased (Sino-British SIPPR/BK Lab
Animal, Ltd., Shanghai, China) and housed in pairs at 22.2°C at
40–70% humidity with a 12:12 light/dark cycle and were allowed free
access to water and food pellets consisting of a commercial natural
diet (SIPPR/BK Lab Animal, Ltd.). Following 2 weeks of
acclimatization to the research facility, rats were divided into
six groups (n=10); one group comprised the sham group, and the
remaining five groups were bilaterally OVX. From 2 weeks
post-surgery, four OVX groups were treated daily with 5, 10, 20 or
40 mg/kg simvastatin (MSD Pharmaceutical Co., Ltd., Hangzhou,
China) via oral gavage for 90 days. The remaining OVX group was the
control group. Sham and control groups were administered a vehicle
consisting of physiological saline for 90 days. Simvastatin dosage
was adjusted every 2 weeks according to the weight of the rats.
Rats were subcutaneously injected with 25 mg/kg tetracycline (Bio
Basic Canada, Inc., Markham, ON, Canada) 15 and 5 days prior to
sacrifice. All rats were sacrificed by cervical dislocation
following administration of 0.4 g/kg chloral hydrate (Baomanbio,
Shanghai, China) anesthesia.
Bone densitometry
L4 vertebrae were harvested and prepared by removing
the appendix, including the vertebral lamina. Subsequently, total
BMD was determined ex vivo using dual energy X-ray
absorptiometry (DEXA; Hologic, Inc., Marlborough, MA, USA). The
Hologic Discover A (version 3.3.0.1; Hologic, Inc.) small animal
model scanning software used for small animal bones automatically
selected a small X-ray source collimator and employed a
high-resolution protocol to scan the vertebra from the proximal to
the distal ends. Following scanning, all the vertebrae from the
respective rats were fixed in 70% ethanol at 4°C for subsequent
analysis.
Peripheral quantitative computed
tomography (pQCT) analysis
L4 vertebrae were removed from 70% ethanol and
scanned via pQCT densitometry in increments (slices of 0.8–1 mm)
with 0.09-mm resolution. The median coronal slice was chosen for
detection. All vertebrae were subsequently fixed in 70% ethanol at
4°C.
The following measurements were obtained: Total bone
content (TOT_CNT), total bone mineral density (TOT_DEN), total area
(TOT_A), trabecular bone content (TRAB_CNT), trabecular bone
mineral density (TRAB_DEN), trabecular bone area (TRAB_A), cortical
bone content (CRT_CNT), cortical bone mineral density (CRT_DEN),
cortical bone area (CRT_A), cortical thickness of circumference
(CRT_THK_C), periosteal circumference (PERI_C), endocortical
circumference (ENDO_C), and bone strength and mechanical properties
in the three axial planes X, Y, and Z (SSI X, Y, and Z).
Bone mineral apposition rate
Following pQCT measurement, L4 vertebrae were
dehydrated and the fat was removed prior to embedding in methyl
methacrylate and subsequent sectioning into 50-µm-thick sections.
Sections from each specimen remained unstained for epifluorescence
microscopy. The mineral apposition rate (MAR) was calculated by
dividing the distance between the two labels by the interlabeling
period in days.
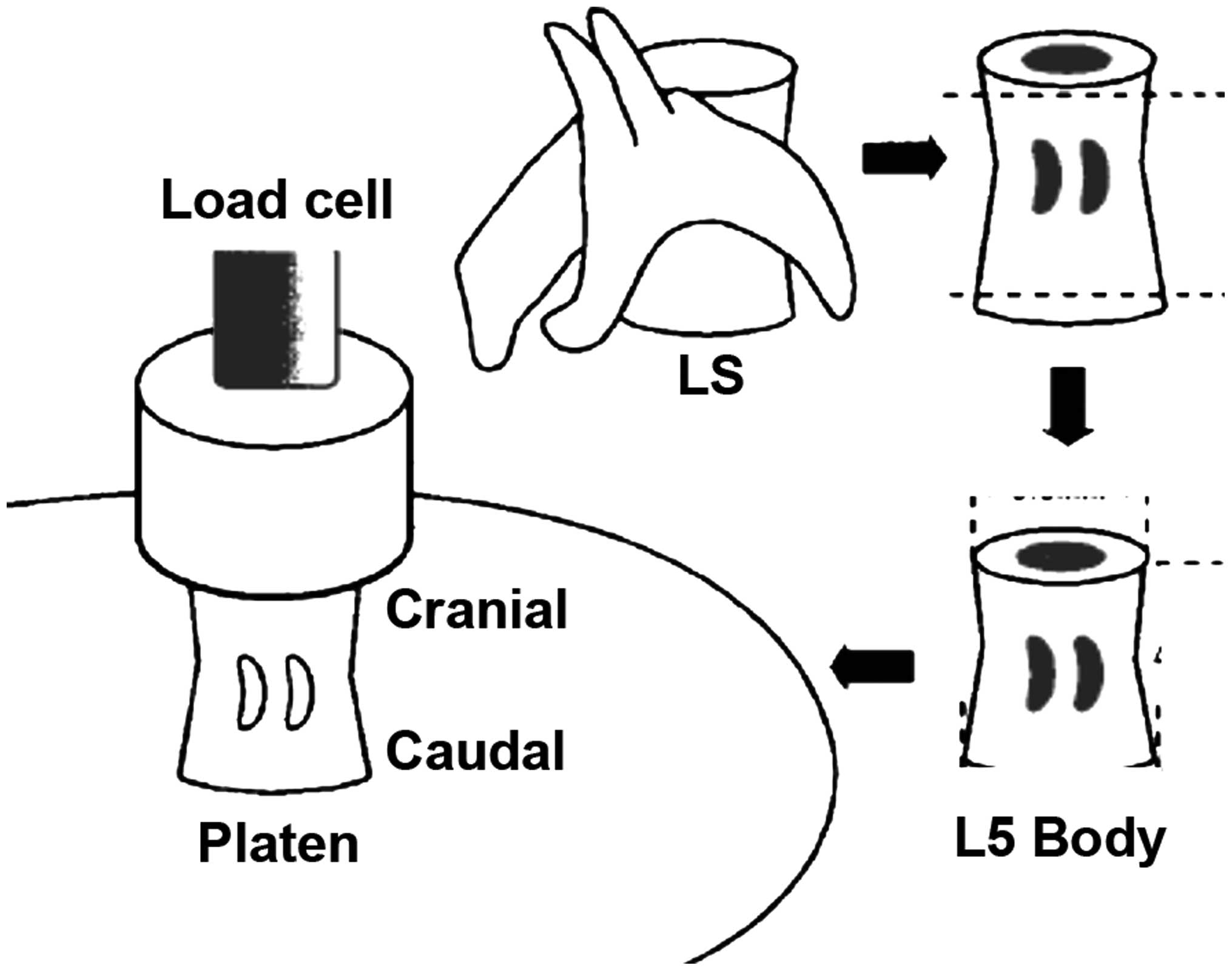
Bone biomechanics
The lumbar spine was mechanically evaluated via a
compression test of the L5 vertebral bodies, which were removed
from the −20°C freezer and thawed in steps. The vertebral arch and
spinous and transverse processes were removed using a low-speed
diamond wheel saw. Vertebral body specimens consisted of the
cancellous bone core surrounded by the cortical rim. Prior
measurement, bones were maintained in gauze with cold normal saline
to avoid drying. Tests were performed using a Lloyd EZ20a system
(Ametek GmbH, Munich, Germany). The ends of vertebrae were trimmed
to provide two parallel surfaces and placed on the center of a
stainless steel plate in the cranial-caudal direction. For each
vertebra, a second stainless steel plate was lowered from above
with a strain rate of 0.5 mm/min along its longitudinal axis until
the vertebra was compressed to failure. The load-strain curve was
recorded, which indicated the mechanical properties of the whole
vertebral body specimen. Ultimate load (F) indicates the
load-bearing capacity. Ultimate stiffness (S) indicates the maximum
slope of the curve. Work to failure (W) indicates the area formed
by the load-strain curve and coordinate axis. Other mechanical
properties were calculated by normalizing the above mechanical data
to the cross-sectional area (CSA) and height (H) of each specimen.
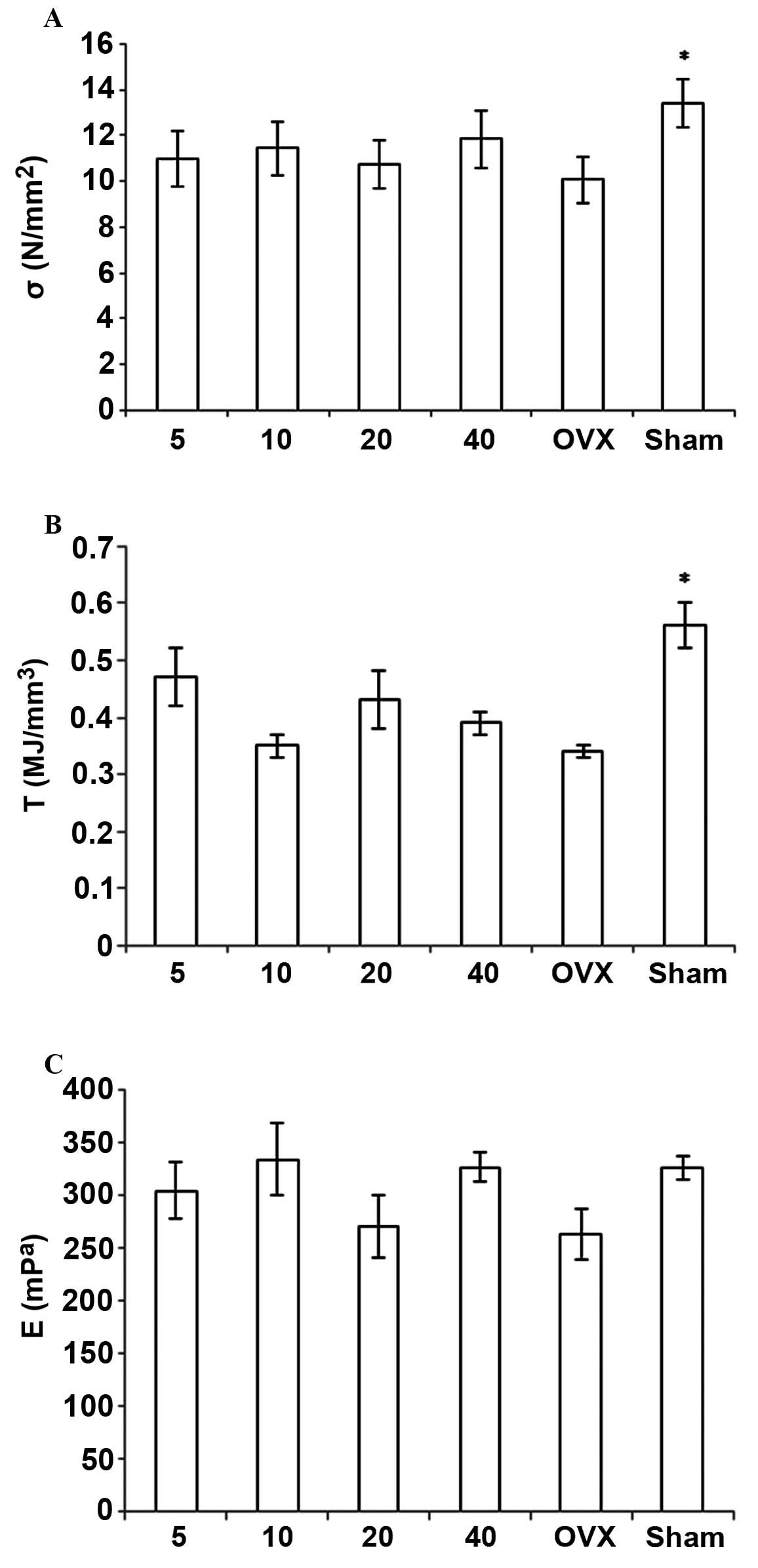
Other properties included the ultimate stress (σ = F / CSA),
Young's modulus [E = S / (CSA / H)], and toughness [T = W / (CSA ×
H)]. The structural property index includes F, S and W; the
material property index includes σ, E and T (Fig. 1).
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed by analysis of variance followed by the least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed with SPSS 11.5 statistical software (SPSS, Inc., Chicago,
IL, USA).
Results
Bone densitometry
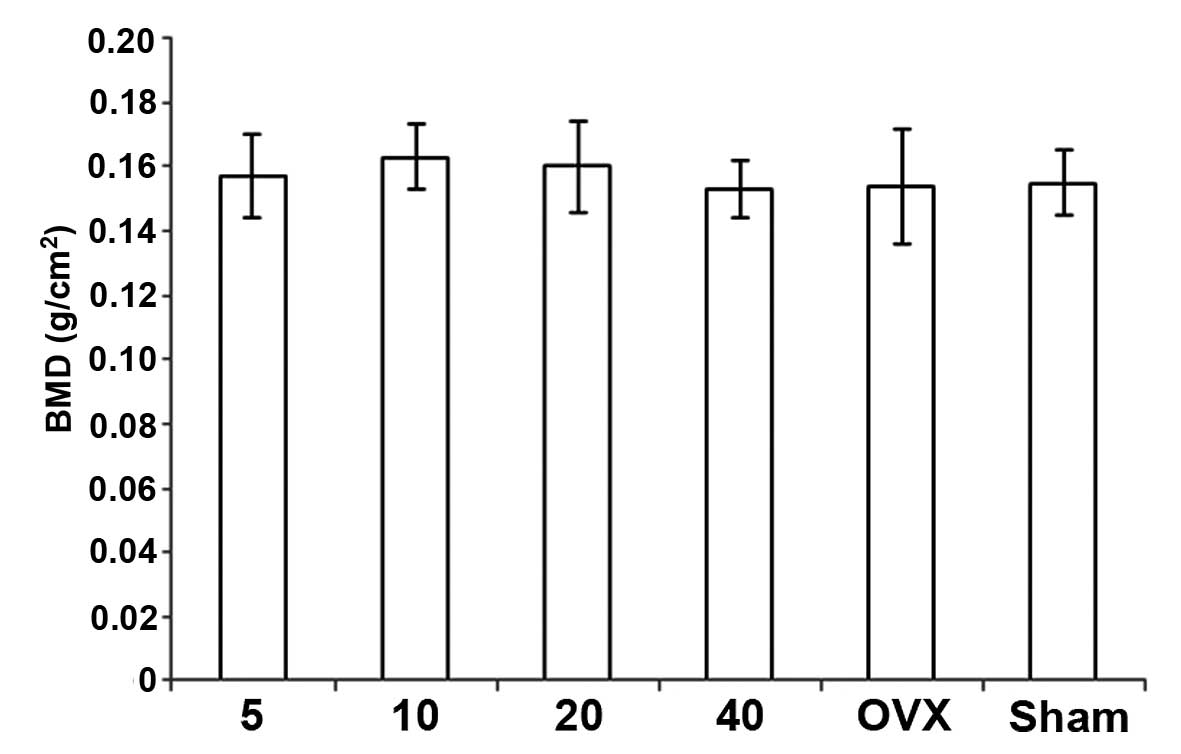
Treatment with simvastatin resulted in a
dose-related decrease in BMD with the highest values observed at 10
mg, but this difference was not significant among the groups. BMD
in the OVX + simvastatin groups was higher than in the OVX +
vehicle group, but the difference was not significant. Notably,
although the BMD in the sham + vehicle group was higher than that
of the OVX + vehicle group, the difference was not significant
(Fig. 2).
pQCT analysis
Values of CRT_CNT, CRT_A, CRT_DEN, CRT_THK_C,
TOT_DEN, TRAB_DEN and SSI (X, Y, and Z) in the OVX + vehicle group
were lower than those in the sham + vehicle group. Differences in
CRT_DEN and CRT_THK_C were statistically significant.
Values of TOT_CNT, TOT_A, TOT_DEN, CRT_CNT, CRT_A,
CRT_DEN, TRAB_DEN, PERI_C, CRT_THK_C and SSI (X, Y, Z) in the OVX +
simvastatin groups decreased in a dose-related manner and the
maximum value was obtained at 10 mg simvastatin. TRAB_CNT, TRAB_A
and ENDO_C in the OVX + simvastatin groups increased with dosage,
but the differences were not significant among the groups. Although
all these measurements were higher than those in the OVX + vehicle
group, these increases were not statistically significant (Fig. 3).
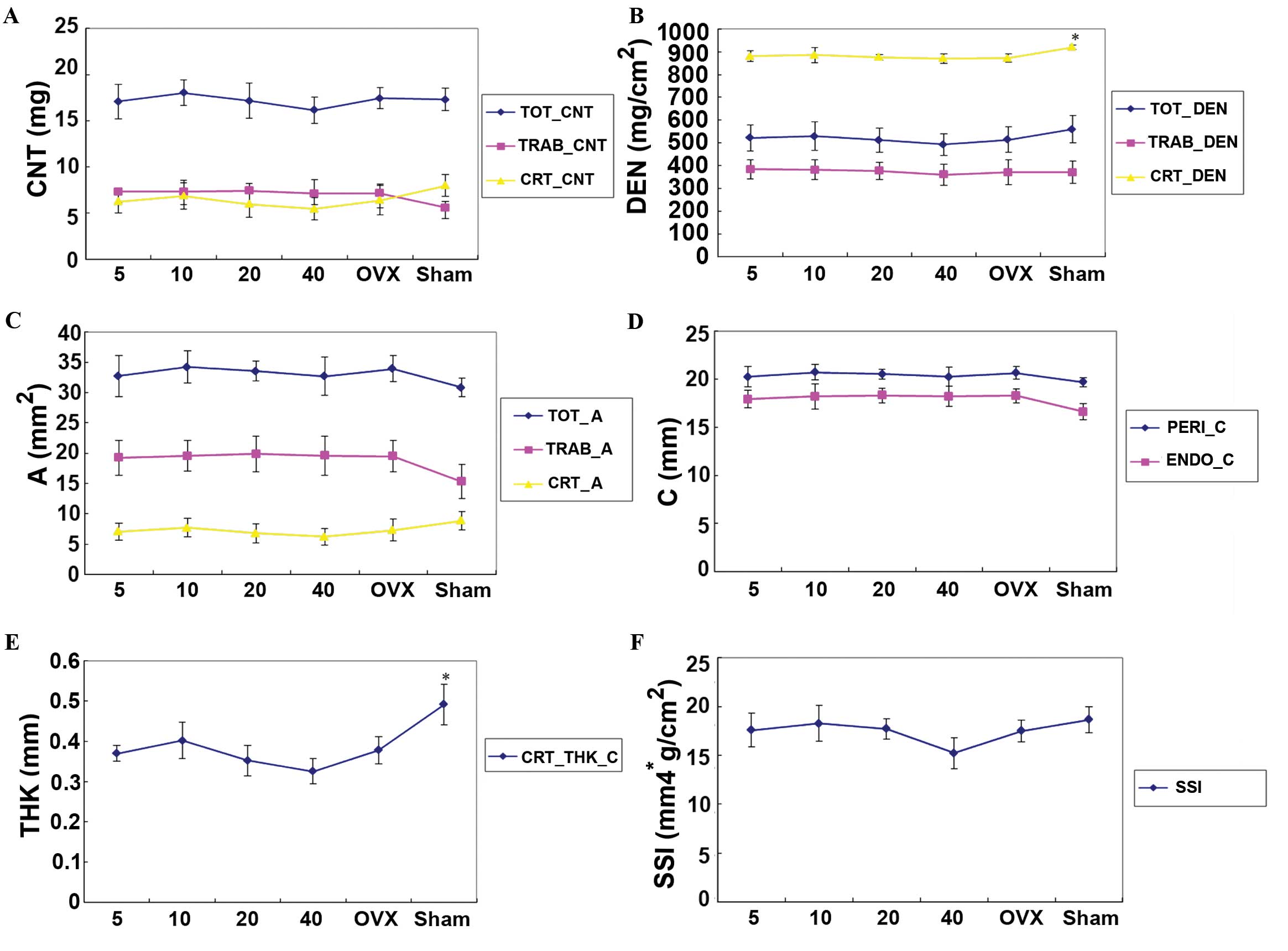 | Figure 3.Analysis of the L4 vertebral body by
pQCT in OVX rats treated with 5, 10, 20 and 40 mg/kg/day simvastin,
and the sham and OVX model groups. *P<0.05 vs. the OVX group.
(A-F) Effect of simvastatin on (A) CNT (bone content), (B) DEN
(bone mineral density), (C) A (bone area), (D) C (bone
circumference), (E) THK (bone thickness) and (F) SSI (bone strength
and mechanical properties). pQCT peripheral quantitative computed
tomography; OVX, ovariectomized; CNT, bone content; TOT, total;
TRAB, trabecular; CRT, cortical; DEN, bone mineral density; A,
area; C, circumference; PERI, periosteal; ENDO, endocortical; THK,
thickness; CRT_THK_C, cortical thickness of the circumference; SSI,
bone strength and mechanical properties. |
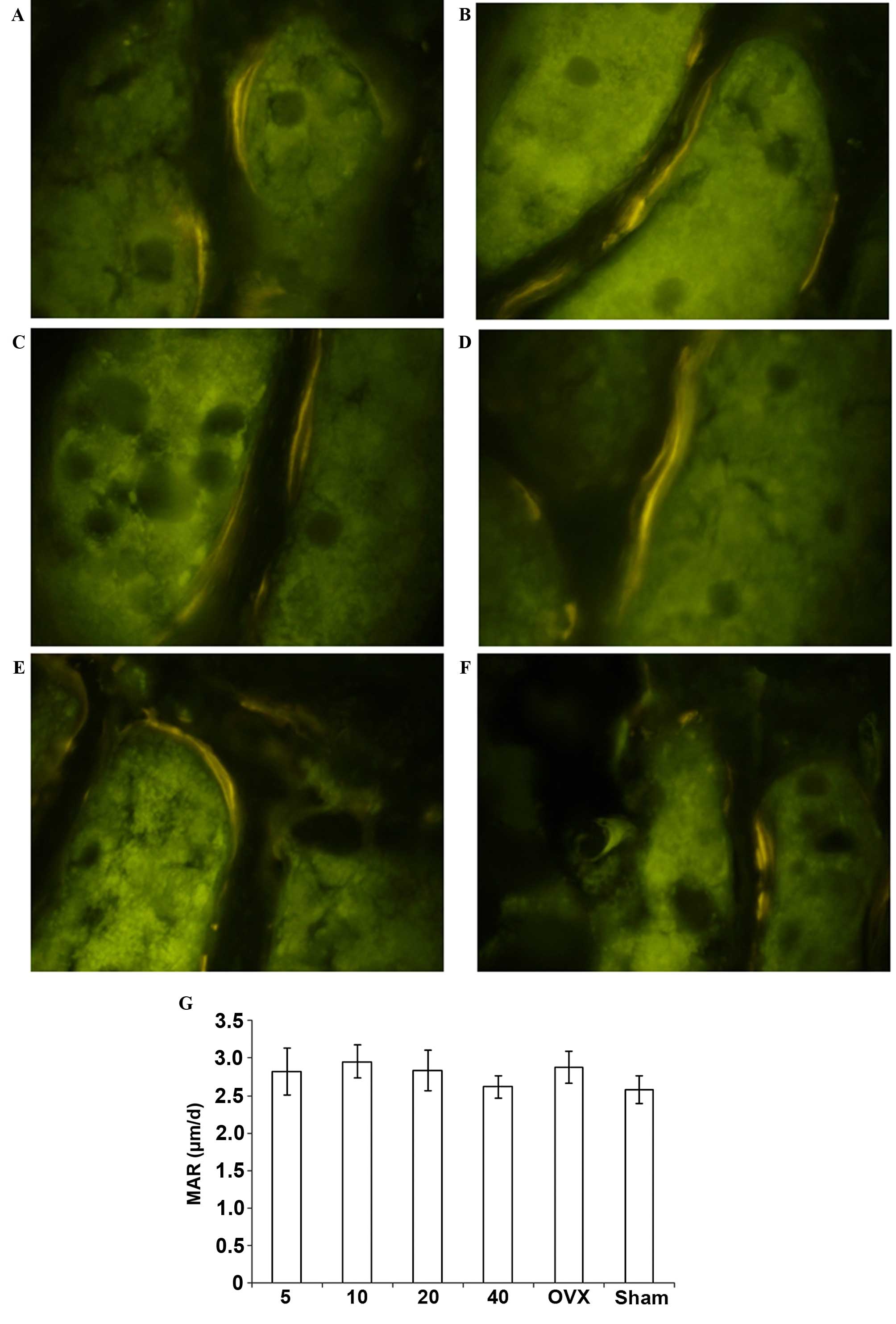
Bone MAR
MAR was unaffected by simvastatin treatment. No
significant changes were demonstrated between the OVX + simvastatin
and OVX + vehicle groups (Fig.
4).
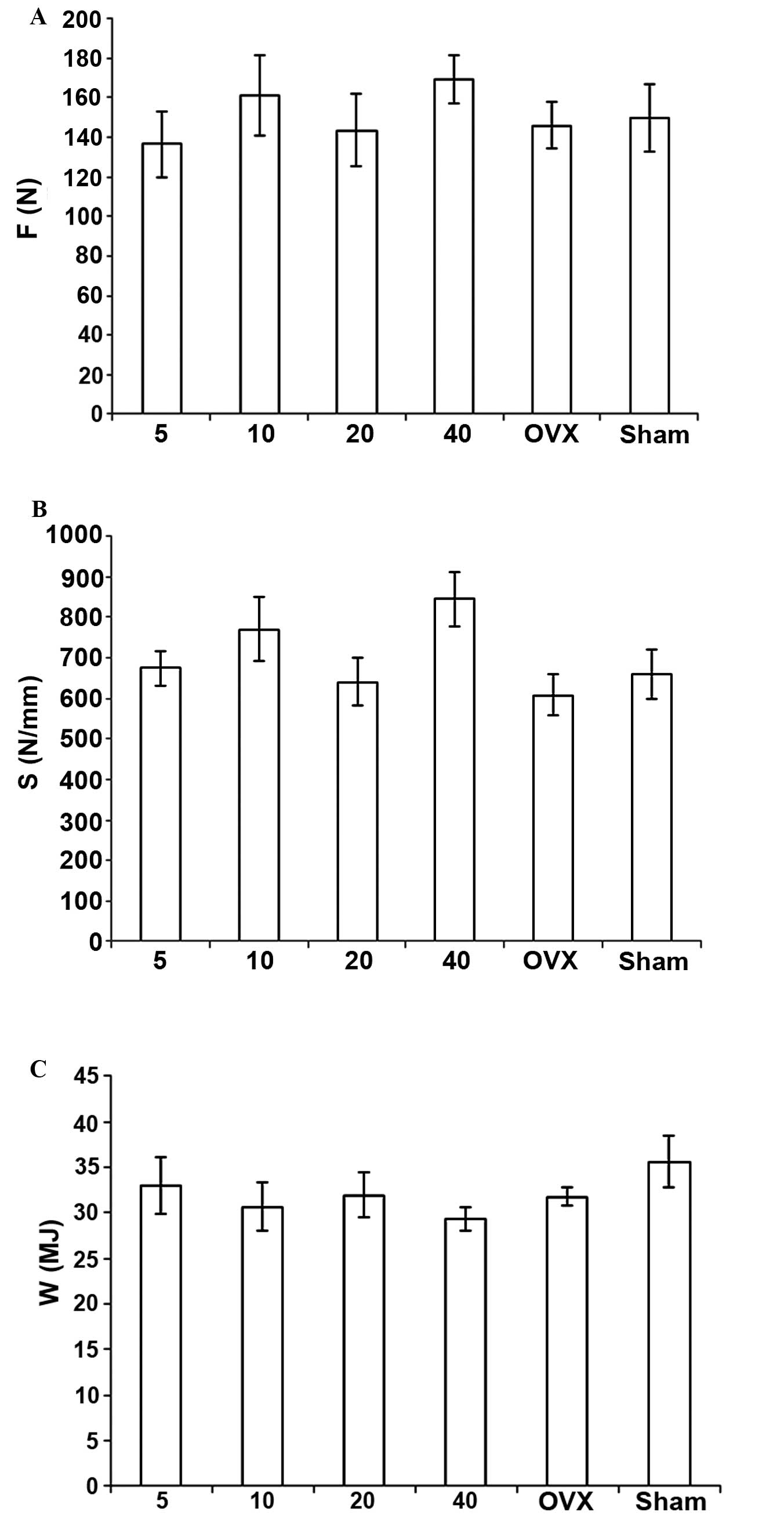
Biomechanical measurements
F, σ, s, E, W and T were increased in the OVX +
simvastatin groups, as compared with those values in the OVX +
vehicle group; however, no significant differences were detected
among the groups. All of these measurements in the OVX + vehicle
group were lower than those in the sham + vehicle group, and the
differences in σ and T were statistically significant (Figs. 5 and 6).
Discussion
Bone loss in OVX rats is similar to bone loss in
postmenopausal women; therefore, the United States Food and Drug
Administration and the World Health Organization recommend OVX rats
as an animal model of postmenopausal osteoporosis (22). In previous studies, 3-month-old
female rats have been used as an OVX model due to their sexual
maturity (11–13,15–17);
however, the skeletons of 3-month-old rats are not mature and
continue to rapidly grow. The effect of anabolic drugs on
osteogenesis may be unclear due to the effects of development on
bone formation. Therefore, it is easy to obtain false positive or
false negative errors, which influence the precise evaluation of
drug efficacy in the treatment of osteogenesis and may be one of
the primary reasons for inconsistent findings in previous studies
(11–13,15–17). In
contrast, 6-month-old rats have reached sexual maturity and peak
bone mass (23). This is the optimal
age for OVX rat modeling because the influence of bone development
on osteogenesis can be avoided. Therefore, in the present study
6-month-old OVX rats were selected as a postmenopausal model
(24).
Differences in drug tolerance between humans and
animals are substantial; therefore, clinical doses of
pharmacological agents must be converted into doses that can be
safely administered to experimental animals to explore the novel
functions of these agents. Currently, the maximum safe dose for
oral simvastatin is 80 mg/day, and regular dosages include 10, 20,
and 40 mg/day, which were provided in the simvastatin instructions
issued by the U.S. FDA in 2007. Dosages were converted into rat
gavage dosages according to the equivalent conversion of drugs
between humans and animals (25,26) and
the report issued by Illingworth et al (27), which investigated the clinical
toxicity and equivalent analysis of simvastatin. Finally,
simvastatin dosages administered to rats were divided into 5, 10,
20 and 40 mg/kg/day portions and administered by gavage. Therefore,
the simvastatin dosages used in the present study were safe,
reliable and clinically comparable to those in humans.
DEXA is primarily used to measure total BMD in
animal experiments. It serves as a preliminary measurement for bone
quantitative analysis and can quickly yield a macro impression of
the bone quality of specimens. The present results indicated that
the total (T)-BMD of lumbar vertebrae in the OVX + simvastatin
groups had a tendency to gradually increase as the simvastatin
dosage was reduced and was higher than that in the OVX + vehicle
group. Significant differences were not observed.
DEXA is a two-dimensional detection method. T-BMD
from DEXA is predominantly determined by cancellous BMC. However,
the loss rate of BMC in cancellous bone exhibits marked variation
after ovariectomy depending on the skeletal site measured. It has
been reported that significant cancellous bone loss in the lumbar
spine typically occurs by 180 days post-ovariectomy (24,28). The
post-ovariectomy period in the present study was 90 days. This may
explain why the initial BMD analysis by the DEXA did not produce a
positive result. pQCT is a relatively novel and more precise 3D
bone measurement method than DEXA. It is advantageous as it
accurately distinguishes between cortical bone and trabecular bone
and separately calculate cancellous bone histomorphometry, cortical
bone structure and the relevant BMC and BMD; simultaneously, it can
also be used to deduce bone strength and other biomechanical
indices (29–32). For peripheral bones and small animal
skeletons, pQCT is more sensitive than DEXA and more beneficial in
evaluating the effect of therapeutic interventions on different
skeletal sites (33). Therefore, it
is necessary to apply pQCT to scan the lumbar vertebrae by layers,
which allows for separate assessment of the effect of simvastatin
on the cortex and cancellous bones and helps to further confirm the
experimental results.
The results of the present study indicated that the
cortical PERI-C and ENDO-C values in the OVX + vehicle group were
increased compared with the sham + vehicle group; however, the
CRT-THK-C and CRT_DEN values in the OVX + vehicle group were
decreased compared with the sham + vehicle group. These decreases
were statistically significant. One reason for this may be that
bone absorption in the endosteum prevailed over osteogenesis in the
periosteum post-ovariectomy. The characteristics demonstrated were
similar to the ‘pencil line’ or ‘picture-framing’ sign of the human
vertebrae in postmenopausal osteoporosis, which indicates cortical
thinning (34). This finding
demonstrated that the CRT-THK-C and CRT_DEN values of lumbar
vertebrae may be effective markers in the OVX rat model of
osteoporosis when ovariectomy is performed <180 days prior and
may be used as reliable indices for evaluating drug effects. The
results of the present study showed that CRT-THK-C and CRT_DEN
values in the OVX + simvastatin groups were increased compared with
the OVX + vehicle group; however, these differences were not
significant. MAR is a dynamic measurement that complements the
statistical measurements of pQCT. However, no changes were produced
by simvastatin treatment.
Although simvastatin did not improve BMD of the
vertebral body in OVX rats according to the results of DEXA, pQCT
and MAR, skeleton biomechanical properties are the ultimate indices
of the assessment of bone quality that reflect the changes in bone
structure (35). BMD may only
represent 60–80% of bone mechanical strength, and the accuracy of
BMD is <70% for the evaluation of the effects of drugs on
osteoporosis. However, if the BMD value is combined with bone
biomechanics, the accuracy of the assessment of the effects of
drugs on osteoporosis may be as high as 90% (36,37).
Therefore, it is necessary to use biomechanical analysis as the
final measurement in the evaluation of the effect of drugs on
osteoporosis.
Bone biomechanical characteristics can be divided
into two parts: Structural mechanical properties and material
mechanical properties (38).
Structural mechanical properties include F, S and W, which reflect
the changes in bone architecture and are predominantly affected by
the geometric shape and size of the bone (36,39).
Material mechanical properties include σ, E, and T, which represent
bone mechanical characteristics that are not relevant to the
geometrical shape of the bone, are calculated by the appropriate
formula, and include bone area, bone height, bone diameter,
structure mechanical index, and calibration factors (cross
sectional moment of inertia) (36,37).
These results showed that all of the biomechanical measurements in
the OVX + simvastatin groups were increased compared with the OVX +
vehicle group; however, statistically significant differences were
not observed. Therefore, the biomechanical result was also
consistent with the DEXA, pQCT and MAR results.
The findings of the present study indicate that
simvastatin did not promote osteogenesis of the lumbar vertebrae in
OVX rats, suggesting it has no effect on the prevention and
treatment of postmenopausal osteoporosis in the vertebrae.
References
|
1
|
Bauer DC: HMG CoA reductase inhibitors and
the skeleton: A comprehensive review. Osteoporos Int. 14:273–282.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mundy G, Garrett R, Harris S, Chan J, Chen
D, Rossini G, Boyce B, Zhao M and Gutierrez G: Stimulation of bone
formation in vitro and in rodents by statins. Science.
286:1946–1949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meier CR, Schlienger RG, Kraenzlin ME,
Schlegel B and Jick H: HMG-CoA reductase inhibitors and the risk of
fractures. JAMA. 283:3205–3210. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang PS, Solomon DH, Mogun H and Avorn J:
HMG-CoA reductase inhibitors and the risk of hip fractures in
elderly patients. JAMA. 283:3211–3216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu M, Wang K, Tang T, Dai K and Zhu Z:
The effect of simvastatin on the differentiation of marrow stromal
cells from aging rats. Pharmazie. 64:43–48. 2009.PubMed/NCBI
|
|
6
|
Song C, Guo Z, Ma Q, Chen Z, Liu Z, Jia H
and Dang G: Simvastatin induces osteoblastic differentiation and
inhibits adipocytic differentiation in mouse bone marrow stromal
cells. Biochem Biophys Res Commun. 308:458–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugiyama M, Kodama T, Konishi K, Abe K,
Asami S and Oikawa S: Compactin and simvastatin, but not
pravastatin, induce bone morphogenetic protein-2 in human
osteosarcoma cells. Biochem Biophys Res Commun. 271:688–692. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maeda T, Matsunuma A, Kawane T and
Horiuchi N: Simvastatin promotes osteoblast differentiation and
mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun.
280:874–877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maeda T, Kawane T and Horiuchi N: Statins
augment vascular endothelial growth factor expression in
osteoblastic cells via inhibition of protein prenylation.
Endocrinology. 144:681–692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maeda T, Matsunuma A, Kurahashi I,
Yanagawa T, Yoshida H and Horiuchi N: Induction of osteoblast
differentiation indices by statins in MC3T3-E1 cells. J Cell
Biochem. 92:458–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Song QS, Wang JY, Leng HJ, Chen ZQ,
Liu ZJ, Dang GT and Song CL: Simvastatin induces estrogen
receptor-alpha expression in bone, restores bone loss, and
decreases ERα expression and uterine wet weight in ovariectomized
rats. J Bone Miner Metab. 29:396–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maritz FJ, Conradie MM, Hulley PA, Gopal R
and Hough S: Effect of Statins on bone mineral density and bone
histomorphometry in rodents. Arterioscler Thromb Vasc Biol.
21:1636–1641. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oxlund H and Andreassen TT: Simvastatin
treatment partially prevents ovariectomy-induced bone loss while
increasing cortical bone formation. Bone. 34:609–618. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai L, Xu M, Wu H, Xue L, Yuan D, Wang Y,
Shen Z, Zhao H and Hu M: The functional mechanism of simvastatin in
experimental osteoporosis. J Bone Miner Metab. 34:23–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho ML, Chen YH, Liao HJ, Chen CH, Hung SH,
Lee MJ, Fu YC, Wang YH, Wang GJ and Chang JK: Simvastatin increases
osteoblasts and osteogenic proteins in ovariectomized rats. Eur J
Clin Invest. 39:296–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
von Stechow D, Fish S, Yahalom D, Bab I,
Chorev M, Müller R and Alexander JM: Does simvastatin stimulate
bone formation in vivo? BMC Musculoskelet Disord. 4:82003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pytlik M, Janiec W, Misiarz-Myrta M and
Gubała I: Effects of simavastatin on the development of osteopenia
caused by ovariectomy in rats. Pol J Pharmacol. 55:63–71.
2003.PubMed/NCBI
|
|
18
|
Yao W, Farmer R, Cooper R, Chmielewski PA,
Tian XY, Setterberg RB, Jee WS and Lundy MW: Simvastatin did not
prevent nor restore ovariectomy-induced bone loss in adult rats. J
Musculoskelet Neuronal Interact. 6:277–283. 2006.PubMed/NCBI
|
|
19
|
Rizzo M and Rini GB: Statins, fracture
risk, and bone remodeling: What is true? Am J Med Sci. 332:55–60.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reginster JY and Burlet N: Osteoporosis: A
still increasing prevalence. Bone. 38(2): Suppl 1. S4–S9. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Canalis E, Giustina A and Bilezikian JP:
Mechanisms of anabolic therapies for osteoporosis. N Engl J Med.
357:905–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA guidelines and animal models for osteoporosis. Bone. 17(4):
Suppl. S125–S133. 1995. View Article : Google Scholar
|
|
23
|
Kalu DN, Liu CC, Hardin RR and Hollis BW:
The aged rat madel of ovarian homone deficiency bone loss.
Endocrinology. 124:7–16. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Y, Zhao J, Genant HK, Dequeker J and
Geusens P: Long-term changes in bone mineral and biomechanical
properties of vertebrae and femur in aging, dietary calcium
restricted, and/or estrogen-deprived/-replaced rats. J Bone Miner
Res. 12:820–831. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Food and Drug Administration (FDA), .
Draft Guidance for Industry and ReviewersEstimating the Safe
Starting Dose in Clinical Trials for Therapeutics in Adult Healthy
Volunteers. FDA; Rockville, MD: 2002
|
|
26
|
Food and Drug Administration (FDA), .
Guidance for IndustryFood-Effect Bioavailability and Fed
Bioequivalence Studies. FDA; Rockville, MD: 2002
|
|
27
|
Illingworth DR and Tobert JA: A review of
clinical trials comparing HMG-CoA reductase inhibitors. Clin Ther.
16:365–385. 1994.
|
|
28
|
Jee WS and Yao W: Overview: Animal models
of osteopenia and osteoporosis. J Musculoskel Neuron Interact.
1:193–207. 2001.
|
|
29
|
Gasser JA: Assessing bone quantity by
pQCT. Bone. 17(4): Suppl. S145–S154. 1995. View Article : Google Scholar
|
|
30
|
Ma YF, Ferretti JL, Capozza RF, Cointry G,
Alippi R, Zanchetta J and Jee WS: Effects of on/off anabolic hPTH
and remodeling inhibitors on metaphyseal bone of immobilized rat
femurs. Tomographical (pQCT) description and correlation with
histomorphometric changes in tibial cancellous bone. Bone. 17(4):
Suppl. S321–S327. 1995. View Article : Google Scholar
|
|
31
|
Ferretti JL, Capozza RF and Zanchetta JR:
Mechanical validation of a tomographic (pQCT) index for noninvasive
estimation of rat femur bending strength. Bone. 18:97–102. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eriksson SA, Isberg BO and Lindgren JU:
Prediction of vertebral strength by dual photon absorptiometry and
quantitative computed tomography. Calcif Tissue Int. 44:243–250.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujita T, Fujii Y and Goto B: Measurement
of forearm bone in children by peripheral computed tomography.
Calcif Tissue Int. 64:34–39. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quek ST and Peh WC: Radiology of
osteoporosis. Semin Musculoskelet Radiol. 6:197–206. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Turner CH: Biomechanics of bone:
Determinants of skeletal fragility and bone quality. Osteoporos In.
13:97–104. 2002. View Article : Google Scholar
|
|
36
|
Ferretti JL, Capozza RF, Mondelo N and
Zanchetta JR: Interrelationships between densitometric, geometric,
and mechanical properties of rat femora: Inferences concerning
mechanical regulation of bone modeling. J Bone Miner Res.
8:1389–1396. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bauss F, Lalla S, Endele R and Hothorn LA:
Effects of treatment with ibandronate on bone mass, architecture,
biomechanical properties, and bone concentration of ibandronate in
ovariectomized aged rats. J Rheumatol. 29:2200–2208.
2002.PubMed/NCBI
|
|
38
|
Bouxsein ML and Seeman E: Quantifying the
material and structural determinants of bone strength. Best Pract
Res Clin Rheumatol. 23:741–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nordin M and Frankel VH: Basic
Biomechanics of the Musculoskeletal System. 2nd. Lea & Febiger;
Philadelphia, PA: pp. 35–36. 1989
|