Introduction
Valacyclovir hydrochloride is an antiviral drug used
in the management of herpes simplex, herpes zoster and herpes B. It
is a prodrug and can be rapidly converted into acyclovir in
vivo (1–3). In addition to neurotoxicity, acute
kidney injury is a well-described side effect of acyclovir
administration, since crystal deposition may lead to the
development of renal failure (4,5). The
side-effects of acyclovir therapy are not well recognized by
clinicians.
Linssen-Schuurmans et al (6) first reported valacyclovir associated
neurotoxicity in 1998, and more cases have been reported cases
since then. Asahi et al (7)
reviewed 20 cases of chronic renal failiue, and both of the other 3
cases, without previous renal failure, had acute renal failure when
valacyclovir neurotoxicity appeared. Three of the 17 patients
(17.6%) had received valacyclovir irregularly, hence their actual
dosage was uncertain; but of the other 14 patients, 8 patients
(57.1%) had clearly received excessive doses. Adair et al
(8) reviewed 30 cases of acyclovir
neurotoxicity. Seven of them had no renal insufficiency before
receiving acyclovir. Acyclovir can produce kidney failure either
through precipitation within the tubular lumen or from acute
interstitial nephritis. With the extensive clinical application of
acyclovir, adverse drug reactions, especially acute renal injury,
have rapidly increased, which were reported by Fleischer et
al (4) and Obada et al
(9).
Acute kidney injury secondary to acyclovir is
characterized by a decrease in renal function that typically
develops within a certain period of time, usually 12–48 h following
drug administration, as indicated by a rapid elevation in the serum
creatinine (5,10). Immediate detection of acute kidney
injury is necessary to prevent the progression and aggravation of
renal diseases (11–14). The current study reviews the clinical
features, diagnosis and management of acyclovir nephrotoxicity,
aiming to add clinical evidence to early diagnosis and treatment of
acyclovir-induced acute renal injury.
Case report
Baseline data
A female patient, aged 35 years, was hospitalized
due to complaints of a hip blister for 6 days, fever enduring for 2
days and kidney dysfunction for 1 day (People's Liberation Army No.
202 Hospital, Shenyang, China) in January 2014. The patient was
admitted to another local hospital and diagnosed with herpes
simplex due to the hip blister with unknown causes accompanied by
slight erythema. The patient was administered with valacyclovir
hydrochloride (dosage unknown) and then presented with lumbar pain.
At 2 days prior to admission, the patient had fever accompanied by
shiver at 37.8°C body temperature. Additionally, the patient
self-reported pain in the lower abdomen, and orally administered
Pudilan anti-inflammatory oral liquid combined with the external
use of iodophor solution. The patient's body temperature was
lowered to 36.9°C, whereas the lumbar discomfort was not evidently
improved; however, dysuria and hypourocrinia were experienced, and
the daytime quantity of urine output was ~400 ml. Subsequently, the
patient was admitted to our hospital for kidney dysfunction
examination.
Comprehensive examinations
Serum creatinine was increased to 592.7 µmol/l (↑)
and uric acid was increased to 624 µmol/l (↑). Physical examination
revealed a body temperature of 36.9°C, pulse of 70 times/min,
respiration rate of 18 times/min and blood pressure of 121/74 mmHg.
No abnormality was noted in the heart, lung and abdomen. No
percussion pain was detected in the bilateral kidney. No tenderness
pain was found in the hypochondrium, upper and medial ureter,
costovertebral angle and costolumbar points. No percussion pain was
noted in the costovertebral angle. Neither bulge nor tenderness was
identified in the suprapubic space. No mass or edema was detected
in bilateral lower extremities. Clusters of erythema and blister
with clear margin were observed in the hip. Laboratory examination
revealed a white blood cell count of 8.6×109/l
(4–10×109/l), neutrophilic leukocyte percentage of 80.4%
(0.5–0.7%), eosinophil percentage of 2.1% (0.005–0.05%), red blood
cell count of 4.2×1012/l (3.5–5×1012/l) and
hemoglobin concentration of 127 g/l (110–150 g/l). Renal function
tests revealed a serum creatinine level of 592.7 µmol/l (41–73
µmol/l), urea nitrogen concentration of 15.37 mmol/l (2.6–7.5
mmol/l), Cystatin C of 2.14 mg/l (0.6–1.4 mg/l), blood uric acid of
624 µmol/l (90–420 µmol/l), total immunoglobulin (Ig)E of 28.9 mg/l
(0.1–0.9 mg/l) and C-reactive protein of 33.60 mg/l (0–3 mg/l).
Routine urine examination revealed a urine specific gravity of
1.0100 (↓), occult blood (+++) and urine protein (−); microscopic
examination of red blood cells revealed 1–3/HP, white blood cells
(−), urine IgG of 18.80 mg/l (↑), urine trace albumin of 57.30 mg/l
(↑), urine α1 microglobulin of 21.10 mg/l (↑) and urine transferrin
of 3.280 mg/l (1.9–31 mg/l). Further tests revealed blood potassium
of 4.2 mmol/l (3.5–5.3 mmol/l), sodium of 136 mmol/l (135–145
mmol/l), calcium of 2.25 mmol/l (2.12–2.75 mmol/l), B-type
natriuretic peptide of 1,890 pg/ml (↑), erythrocyte sedimentation
rate of 51 mm/h (↑), fibrinogen of 4.31 g/l ↑, inorganic phosphorus
of 2.01 mmol/l ↑, HBsAg positive, HBcAb positive (↑),
quantification of hepatitis B virus <500 IU/ml, antinuclear
antibody detection and antineutrophil cytoplasmic antibody
detection were negative and ultrasound examination of bilateral
kidneys revealed no renal abnormality. Pulmonary computed
tomography showed lung marking enhancement, multiple lesions in the
bilateral lungs and multiple calcified lesions in the abdomen.
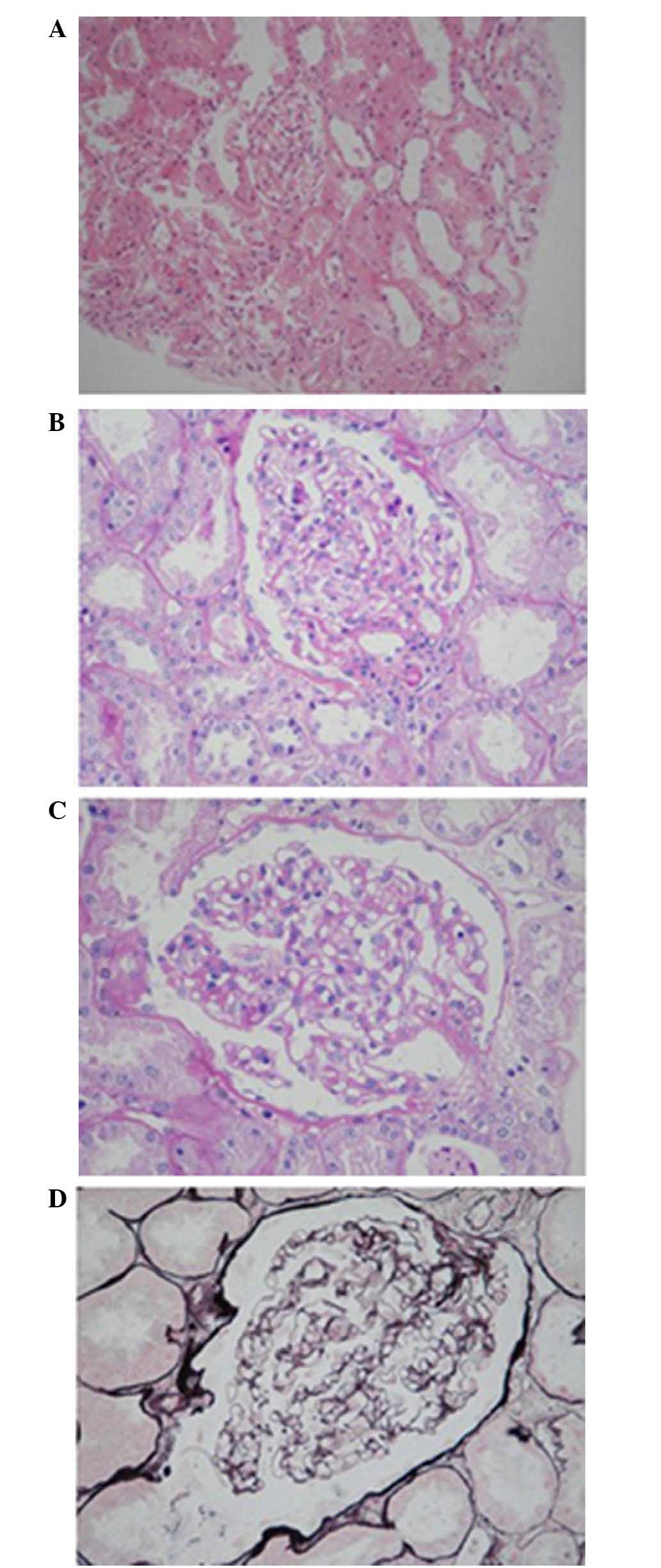
Renal biopsy under light microscopy detected 27 glomeruli and no
signs of glomeruli or segmental sclerosis. Slight hyperplasia of
glomeruli mesangial cell and matrix was detected. No significant
thickening of the basement membrane was noted. Neither epithelial
cell hyperplasia nor crescent formation was documented. Mild
fibrosis was found adjacent to glomeruli sacculus. Renal tubular
epithelial cell granule and vacuolar degeneration were observed.
Lumen ectasia was detected in partial renal tubules with absence of
brush border. Renal interstitial edema was noted. Vessel wall
thickening of the arteriole and lumen narrowing were noted.
Immunofluorescent analysis revealed 7 glomeruli, negative outcomes
for IgG, IgM, IgA, C3 and C1q. No marked thickening was noted in
the glomeruli sacculus wall. No evidence of hyperplasia in parietal
layer cells was detected. No significant thickening of the basement
membrane was seen with 250–350 µm in thickness (Fig. 1). Epithelial cell swelling and
vacuolar degeneration were noted. No apparent hyperplasia or dense
deposit was found in mesangial cell paralinin. Renal tubular
epithelial cell vacuolar degeneration, partial lumen ectasia,
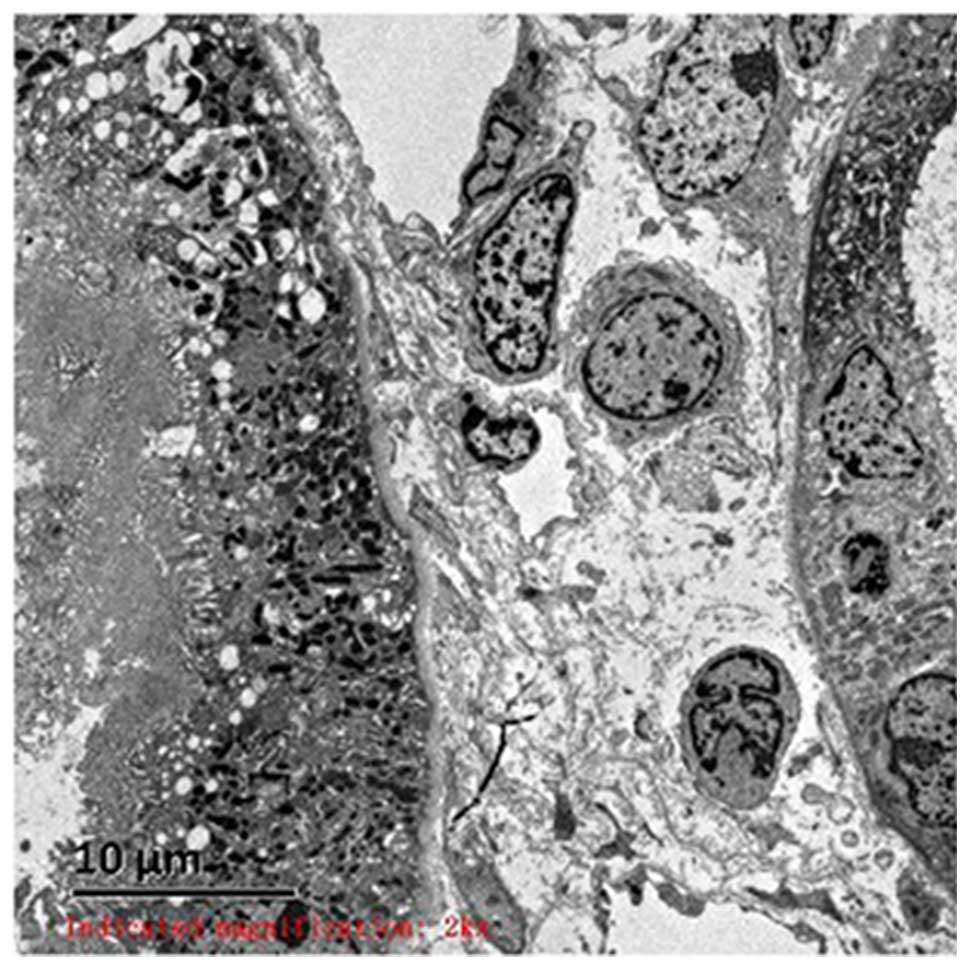
microvilli exfoliation from epithelial cells (Fig. 2A) and renal interstitial edema were
noted (Fig. 2B and C). Evident
vacuolar degeneration of capillary endothelial cells, erythrocyte
aggregation in the partial lumen and opening of the capillary loop
were observed (Fig. 2D). The
findings of light microscope, immunofluorescence and electron
microscopy were consistent with acute renal tubular injury. The
patient was eventually diagnosed with acute renal injury and
drug-induced nephropathy.
Clinical treatment
The patient was injected with glutathione (Chongying
Yaoyou Pharmaceutical Co., Ltd., Chongqing, China) to alleviate
renal tubular injury and sodium bicarbonate to cause alkalized
urine to prevent the turbular formation. Then, the patient was
injected with azithromycin (Northwest Pharmaceutical Co., Ltd.,
Shenyang, China) to treat cutaneous infection. Phenolsulfonic acid
calcium capsule (0.25 g × 48; Ningxia Kangya Pharmaceutical Co.,
Ltd., Yinchuan, China) was supplemented to improve kidney
circulation and accelerate the repairing of renal tubular
epithelial cells. Compound polymyxin and topical use of iodophor
(Zhejiang Ri Sheng Chang Pharmaceutical Co., Ltd., Dongyang, China)
were delivered to manage herpes simplex. Upon discharge, the
quantity of urine was significantly increased to >3,000 ml/day,
serum creatinine levels were 76 µmol/l, microglobulin levels were
5.90 mg/l and urine transferrin levels were 2.210 mg/l. Routine
urine examination revealed occult blood (±), urine protein (−),
white blood cell 1–3/HP and red blood cell 6–8/HP. Follow-up for 3
months revealed the normal renal function.
Discussion
Valacyclovir hydrochloride is an antiviral drug used
in the management of herpes simplex, herpes zoster and herpes B.
Valacyclovir hydrochloride is a prodrug and can be rapidly
converted into acyclovir in vivo (1–3). In
addition to neurotoxicity, acute kidney injury is a well-described
side effect of acyclovir administration, since crystal deposition
may lead to the development of renal failure. In recent years,
valacyclovir has largely replaced acyclovir in the treatment of
herpes virus infections, because it is more effective by oral
administration (7). There are
several cases about the acute kidney injury caused by acyclovir
reported, but the side effects of valacyclovir are not well
recognized by clinicians, thus are rarely reported compared with
acyclovir. Sagawa et al (15)
found an elderly diabetic patient treated with valacyclovir was
diagnosed with acyclovir-induced neurotoxicity and acute kidney
injury later, although the patient with no microalbuminuria and a
serum creatinine level seven days before admission, the age,
metabolic disorder maybe the risk factors of the injury. In the
present report, the patient is a young female without any disease
history, and it has more important clinical significance to
identify the possible side effects of valacyclovir.
Acyclovir, which is relatively insoluble in urine,
is rapidly filtered by the glomeri and secreted by the renal
tubules, which can produce high urine concentrations, particularly
in patients with decreased urine flow rates (16–19).
Renal excretion accounts for 60–90% of acyclovir elimination
(20). The present study reports a
case of a patient developing acute kidney injury secondary to
herpes simplex after receiving acyclovir. Other potential
mechanisms of injury include acute interstitial nephritis and acute
tubular necrosis. However, the most commonly reported mechanism is
obstructive nephropathy (20).
The use of acyclovir can damage the kidney via a
number of mechanisms. Firstly, the severity of renal injury induced
by acyclovir is associated with the dose used (10). It directly causes cell membrane
injury, alters the membrane permeability and ion transportation. In
addition, it destroys cytoplasm mitochondria, inhibits enzymatic
activity and protein synthesis (21), promotes calcium internal flow and
leads to cytoskeleton structural damage and epithelial cell
necrosis. Furthermore, it is able to produce oxygen free radicals.
It can also affect epithelial cell DNA, induces crosslinking or
inhibits DNA replication of related enzymes and suppresses renal
tubular epithelial cell metabolism. Secondly, acyclovir is
primarily discharged from the urine and is almost insoluble in the
urine, forms crystals and occludes the renal tubule (22). Thirdly, it is mediated by a variety
of immune factors (22). Fourthly,
it may cause thrombotic microangiopathy (23). Fifthly, the physiological nature of
the kidney is highly susceptible to drug-induced nephropathy
(21). The abundant renal capillary
network and rich blood allow for high concentrations of drugs
passing through a large contacted area. The counter-current
multiplication mechanism also contributes to elevated drug
concentration in the medulla renis. Furthermore, the variation in
urine pH is likely to cause crystals and drug sediment in the renal
tubule. The enzymes contained in the kidney would be deactivated by
the medication. In the current report, the patient presented with
valacyclovir hydrochloride-induced nephropathy, which has been
rarely reported in clinical practice.
The female patient was orally administered with
valacyclovir hydrochloride tablets to treat herpes simplex.
Subsequently, the patient experienced a significant reduction in
quantity of urine produced, and an apparent elevation in serum
creatinine levels. However, the patient presented with no clinical
manifestations of thrombotic microangiopathy. The patient was
pathologically diagnosed with acute renal tubular injury, which
resulted from the use of acyclovir and renal obstruction.
Clinical manifestations of the patient in the
present study suggest that, following the application of antiviral
agents, hydration, alkalization of urine, pro-discharge of
medication and use of antagonistic agents should be adopted as
preventive measures of adverse events following the use of
antiviral agents. High-risk populations should be avoided with
regards to using such medication. The proportion of drug-induced
interstitial nephritis and nephropathy is relatively high in the
cases of acute renal failure, which deserves widespread attention
and emphasis. The significance of monitoring renal function in
hospitalized patients on acyclovir is strongly supported by the
present study. In addition, there is strong evidence that acyclovir
can cause neurotoxicity in kidney injury, which could further
complicate the patient's clinical status (24–26).
Early detection, intervention and treatment contribute to the
favorable prognosis of acyclovir-induced acute renal injury.
References
|
1
|
Vachvanichsanong P, Patamasucon P, Malagon
M and Moore ES: Acute renal failure in a child associated with
acyclovir. Pediatr Nephrol. 9:346–347. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baker DA, Blythe JG and Miller JM:
Once-daily valacyclovir hydrochloride for suppression of recurrent
genital herpes. Obstet Gynecol. 94:103–106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomori K, Isozumi K, Motohashi S,
Komatsumoto S and Fukuuchi Y: A young patient of acute encephalitis
complicated with acyclovir encephalopathy without renal
dysfunction. Rinsho Shinkeigaku. 43:470–476. 2003.(In Japanese).
PubMed/NCBI
|
|
4
|
Fleischer R and Johnson M: Acyclovir
nephrotoxicity: A case report highlighting the importance of
prevention, detection, and treatment of acyclovir-induced
nephropathy. Case Rep Med. 2010:6027832010.PubMed/NCBI
|
|
5
|
Delluc A, Mocquard Y, Latour P and Goas
JY: Encephalopathy and acute renal failure during acyclovir
treatment. Rev Neurol (Paris). 160:704–706. 2004.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Linssen-Schuurmans CD, van Kan EJ, Feith
GW and Uges DR: Neurotoxicity caused by valacyclovir in a patient
on hemodialysis. Ther Drug Monit. 20:385–386. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asahi T, Tsutsui M, Wakasugi M, Tange D,
Takahashi C, Tokui K, Okazawa S and Okudera H: Valacyclovir
neurotoxicity: clinical experience and review of the literature.
Eur J Neurol. 16:457–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adair JC, Gold M and Bond RE: Acyclovir
neurotoxicity: Clinical experience and review of the literature.
South Med J. 87:1227–1231. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Obada EN, Level G, Mathieu P, Parent X,
Gilson B and Bindi P: Acute renal failure following a treatment
with acyclovir. Nephrol Ther. 6:125–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rao S, Abzug MJ, Carosone-Link P, Peterson
T, Child J, Siparksy G, Soranno D, Cadnapaphornchai MA and Simões
EA: Intravenous acyclovir and renal dysfunction in children: A
matched case control study. J Pediatr. 166:1462–1468.e1-e4. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rantanen JM, Markvardsen LH, Jakobsen J
and Birn H: Acute kidney injury secondary to the first intravenous
administration of acyclovir. Ugeskr Laeger. 176:pii2014.(In
Danish).
|
|
12
|
Sodhi PK and Ratan SK: A case of chronic
renal dysfunction following treatment with oral acyclovir. Scand J
Infect Dis. 35:770–772. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yavuz BB, Cankurtaran M, Halil M, Dagli N
and Kirkpantur A: Renal dysfunction after oral acyclovir treatment
in a geriatric woman: A case report. Scand J Infect Dis.
37:611–613. 2005.PubMed/NCBI
|
|
14
|
Becker BN, Fall P, Hall C, Milam D,
Leonard J, Glick A and Schulman G: Rapidly progressive acute renal
failure due to acyclovir: Case report and review of the literature.
Am J Kidney Dis. 22:611–615. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sagawa N, Tsurutani Y, Nomura K, Okuyama
T, Kondo M, Sata A, Miyao M and Mizuno Y: Acyclovir-induced
neurotoxicity and acute kidney injury in an elderly diabetic
patient treated with valacyclovir: report of a case. Nihon Ronen
Igakkai Zasshi. 51:581–585. 2014.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lam NN, Weir MA, Yao Z, Blake PG, Beyea
MM, Gomes T, Gandhi S, Mamdani M, Wald R, Parikh CR, et al: Risk of
acute kidney injury from oral acyclovir: A population-based study.
Am J Kidney Dis. 61:723–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seedat A and Winnett G: Acyclovir-induced
acute renal failure and the importance of an expanding waist line.
BMJ Case Rep. 2012:pii2012.
|
|
18
|
Obada EN, Level G, Mathieu P, Parent X,
Gilson B and Bindi P: Acute renal failure following a treatment
with acyclovir. Nephrol Ther. 6:125–127. 2010.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawyer MH, Webb DE, Balow JE and Straus
SE: Acyclovir-induced renal failure. Clinical course and histology.
Am J Med. 84:1067–1071. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Firat H, Brun P, Loirat C and
Jacqz-Aigrain E: Kidney failure induced by administration of
acyclovir. Apropos of 2 cases. Arch Fr Pediatr. 49:641–643.
1992.(In French). PubMed/NCBI
|
|
21
|
Vashishtha AK and Kuchta RD: Effects of
Acyclovir, Foscarnet, and Ribonucleotides on Herpes Simplex Virus-1
DNA Polymerase: Mechanistic Insights and a Novel Mechanism for
Preventing Stable Incorporation of Ribonucleotides into DNA.
Biochemistry. 55:1168–1177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perazella MA: Crystal-induced acute renal
failure. Am J Med. 106:459–465. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mulay SR, Evan A and Anders HJ: Molecular
mechanisms of crystal-related kidney inflammation and injury.
Implications for cholesterol embolism, crystalline nephropathies
and kidney stone disease. Nephrol Dial Transplant. 29:507–514.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Deyne S, De la Gastine B, Gras G,
Dargère S, Verdon R and Coquerel A: Acute renal failure with
acyclovir in a 42-year-old patient without previous renal
dysfunction. Rev Med Interne. 27:892–894. 2006.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Genc G, Ozkaya O, Acikgöz Y, Yapici O, Bek
K, Gülnar Sensoy S and Ozyürek E: Acute renal failure with
acyclovir treatment in a child with leukemia. Drug Chem Toxicol.
33:217–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riba Solé M, Farré Riba R, Badell Serra I
and Mangues Bafalluy MA: Acyclovir-induced acute renal failure in a
paediatric oncology patient. Farm Hosp. 35:281–282. 2011.(In
Spanish). PubMed/NCBI
|