Introduction
D-dimer is a fibrin degradation product that is
observed in the blood following clot degeneration. Currently, tests
determining the concentration of D-dimer in the blood are widely
employed in various clinical practices. Determination of D-dimer
levels in combination with clinical probability assessment can be
used to safely rule-out the diagnosis of pulmonary embolism
(1–3). In addition, D-dimer levels are used for
predicting the risk of recurrent venous thromboembolism and
determining the duration of anticoagulation therapy in these
patients (4–6).
The levels of D-dimer in the blood are significantly
increased in patients with liver cirrhosis, and are gradually
elevated further as the degree of liver dysfunction increases in
severity (7,8). It has been also suggested that D-dimer
levels are influenced by the presence and treatment of ascites
(9). Furthermore, the levels of this
protein are significantly higher in cirrhotic patients with ascites
compared with those without ascites, and are significantly
decreased subsequently to ascite resolution (9). More recently, a meta-analysis performed
by our group indicated that D-dimer levels are significantly
associated with the presence of portal vein thrombosis in liver
cirrhosis and may predict the development of portal vein thrombosis
following splenectomy (10).
Therefore, D-dimer may be a prognostic factor negatively associated
with outcomes of liver cirrhosis.
In the present study, the correlation of D-dimer
levels with the Child-Pugh and Model for End-Stage Liver Disease
(MELD) scores was retrospectively analyzed, and determined the
predictive ability of D-dimer levels for the in-hospital mortality
in liver cirrhosis.
Subjects and methods
Study design
Patients consecutively admitted to the General
Hospital of Shenyang Military Area (Shenyang, China) between
January 2011 and June 2014 were retrospectively reviewed. The
inclusion criteria were as follows: i) Patients were diagnosed with
liver cirrhosis (based on clinical presentations, routine
laboratory tests and imaging, and biopsy if necessary); and ii)
D-dimer level was tested. The exclusion criteria were as follows:
i) Patients were diagnosed with malignant tumors; ii) patients were
diagnosed with venous or arterial thrombosis; iii) Child-Pugh and
MELD scores on admissions could not be calculated; and iv) the
survival during their hospitalizations could not be evaluated.
Repeated admissions were not excluded for patients meeting the
aforementioned criteria. Certain of these patients have also been
included in our previous studies (11–14). The
present study was approved by the Ethics Committee of the General
Hospital of Shenyang Military Area. Due to the retrospective nature
of this study, the patients' written informed consents were
waived.
Data collection
The following data were collected from the
electronic medical records of the patients: Age, gender, etiology
of liver diseases, presence of ascites, hepatic encephalopathy, red
blood cell (RBC) count, hemoglobin (Hb) level, white blood cell
(WBC) count, platelet level, total bilirubin (TBIL), albumin (ALB),
alanine transaminase (ALT), aspartate aminotransferase (AST),
alkaline phosphatate (ALP), γ-glutamine transferase (GGT),
creatinine (Cr), prothrombin time (PT), activated partial
thromboplastin time (APTT), international normalized ratio (INR),
D-dimer levels, Child-Pugh (15) and
MELD scores (16), and in-hospital
mortality. In cases where the D-dimer level was measured two or
more times, the maximum D-dimer value was selected for use in the
current analysis.
Statistical analysis
Continuous data are presented as the mean ± standard
deviation, and were compared using the independent sample t test or
one-way analysis of variance. Categorical data are expressed as
frequencies (i.e., rates of patients) and were compared using the
χ2 test. The correlation of D-dimer levels with the
Child-Pugh or MELD scores was evaluated by Pearson's χ2
test. Boxplots were also constructed to demonstrate the difference
in the D-dimer levels among the various Child-Pugh classes (namely
A-C) and MELD (namely scores >15 or <15). Receiver operating
curve (ROC) analysis was employed to evaluate the specificity and
sensitivity of D-dimer for predicting the in-hospital mortality.
The optimal cut-off value was defined as the value at which the
specificity plus sensitivity was maximal. In addition, the area
under the ROC (AUROC) and the 95% confidence intervals (CIs) were
also calculated. A two-sided P-value of <0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using the MedCalc statistical software,
version 15.10 (MedCalc Software bvba, Ostend, Belgium).
Results
Patients
During the enrollment period, a total of 703
patients were found to be eligible for inclusion in the current
study. Patient characteristics are shown in Table I. The majority of patients enrolled
into the present study were male (483/703 patients; 68.70%). The
most common etiology of liver cirrhosis was infection with
hepatitis B/C virus and/or alcohol consumption (500/703 patients;
71.12%). In addition, the mean D-dimer level was 0.80±1.48 µg/ml
(reference range, 0–0.3 µg/ml). The mean MELD score was 8.63±8.14.
The mean Child-Pugh score was 7.66±2.20. In-hospital mortality was
found to be 5.4% (38/703 patients). The major causes of mortality
included the following: Gastrointestinal bleeding (n=13),
progressive liver failure (n=10), refractory hepatic encephalopathy
(n=6), multiple organ failure (n=4), severe infection (n=3),
spontaneous bacterial peritonitis and hepatic encephalopathy (n=1)
and pulmonary embolism (n=1).
 | Table I.Patient characteristics (n=703). |
Table I.
Patient characteristics (n=703).
| Variables | Values | Reference range |
|---|
| Mean age, years |
56.42±11.08 | – |
| Gender, n |
|
|
| Male | 483 (68.7%) | – |
| Female | 220 (31.3%) | – |
| Etiology of liver
cirrhosis, n |
|
|
| Viral
hepatitis alone | 266 (37.8%) | – |
| Alcohol
alone | 183 (26.3%) | – |
| Viral
hepatitis + alcohol | 51 (7.3%) | – |
| Other or
unknown | 203 (28.9%) | – |
| Ascites, n |
|
|
|
Absence | 381 (54.2%) | – |
|
Presence | 322 (45.8%) | – |
| Hepatic
encephalopathy, n |
|
|
| Absence | 656 (93.3%) | – |
| Presence | 47 (6.7%) | – |
| Red blood cell count,
1012/l | 3.03±0.85 | 4.3–5.8 |
| Hemoglobin level,
g/l | 91.76±29.19 | 130–175 |
| White blood cell
count, 109/l | 5.71±4.84 | 3.5–9.5 |
| Platelet level,
109/l | 93.61±70.94 | 135–350 |
| Total bilirubin,
µmol/l | 44.87±74.15 | 5.1–22.2 |
| Albumin, g/l | 31.48±7.11 | 40–55 |
| Alanine transaminase,
U/l | 42.69±14.85 | 9–50 |
| Aspartate
aminotransferase, U/l | 76.94±468.48 | 15–40 |
| Alkaline phosphatase,
U/l | 109.79±97.67 |
45–125 |
| γ-glutamyl
transferase, U/l | 102.76±167.21 | 10–60 |
| Creatinine,
µmol/l | 87.31±103.32 | 44–133 |
| Prothrombin time,
sec | 17.36±6.35 | 11.5–14.5 |
| Activated partial
prothrombin time, sec | 44.59±13.55 | 28–40 |
| International
normalized ratio | 1.53±1.91 | – |
| D-dimer, µg/ml | 0.80±1.48 | 0–0.3 |
| Child-Pugh
scorea | 7.66±2.20 | – |
| MELD score | 8.63±8.14 | – |
|
>15 | 570 | – |
| ≤15 | 133 | – |
| In-hospital
mortality, n |
|
|
| No | 665 (94.6%) | – |
| Yes | 38 (5.4%) | – |
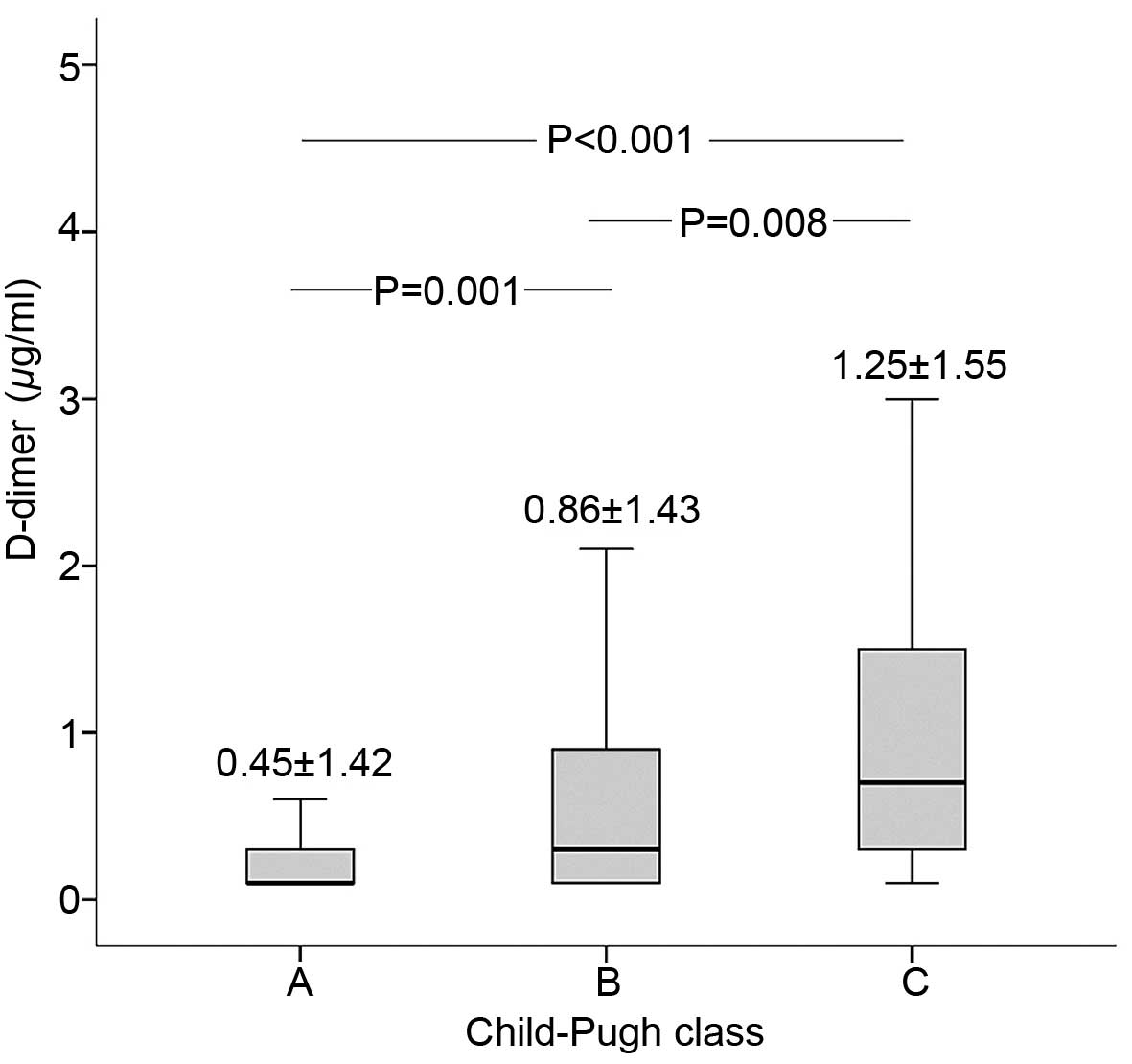
D-dimer and Child-Pugh score
The correlation of D-dimer levels with the
Child-Pugh score of the patients was investigated by Pearson's
χ2 test. The level of D-dimer was positively correlated
with Child-Pugh score (correlation coefficient, 0.219; P<0.001).
As shown in Fig. 1, D-dimer level
was the highest in Child-Pugh class C group, followed by the
Child-Pugh class B and then Child-Pugh class A groups. Furthermore,
significant differences were observed between the different groups
(C vs. B, P=0.008; B vs. A, P=0.001).
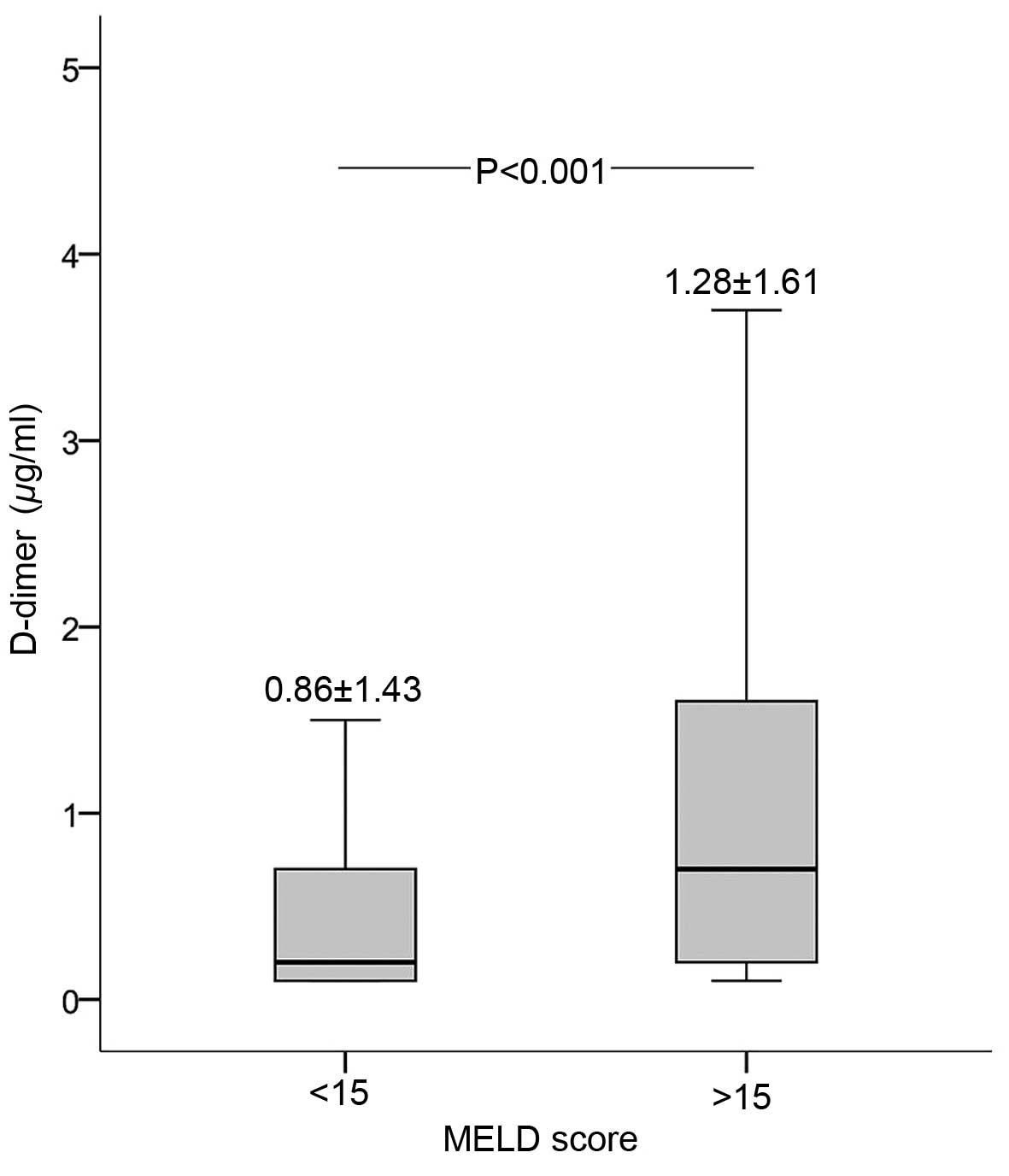
D-dimer and MELD score
The correlation of D-dimer levels with the
Child-Pugh score of the patients was investigated by Pearson's
χ2 test. As shown in Fig.
2, D-dimer level was positively correlated with the MELD score
(correlation coefficient, 0.207; P<0.001). In addition, D-dimer
level was significantly higher in the MELD score >15 group
compared with that in the MELD score <15 group (Fig. 2).
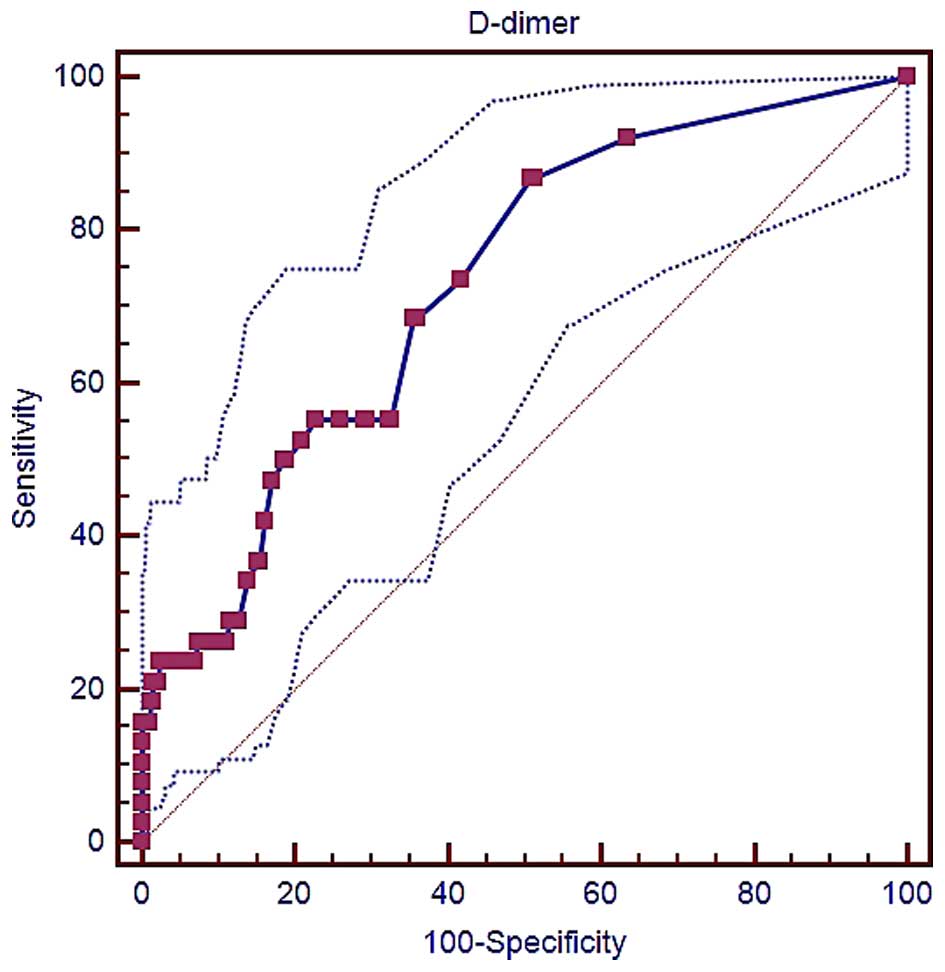
D-dimer and in-hospital mortality
The specificity and sensitivity of D-dimer levels
for predicting the in-hospital mortality of liver cirrhosis
patients were determined, and the AUROC was calculated. As shown in
Fig. 3, the AUROC of D-dimer level
for this prediction was 0.729 (95% CI, 0.695–0.762; P<0.0001).
The best cut-off D-dimer value was 0.28 µg/ml, with a sensitivity
of 86.84% and a specificity of 49.17%.
Discussion
The present study identified that the D-dimer level
of liver cirrhosis patients was significantly correlated with the
Child-Pugh and MELD scores. This finding was largely consistent
with several previous studies. For instance, an Egyptian study by
El-Sayed et al investigated the D-dimer levels in 67
patients with chronic liver diseases and 30 healthy controls
(17). The study observed that
cirrhotic patients with Child-Pugh class A and B had significantly
higher D-dimer levels compared with the non-cirrhotic patients and
healthy controls (class B, 147.32±114.16 ng/ml; class A,
115.3±138.4 ng/ml; non-cirrhotic liver disease, 28.86±40.03 ng/ml;
healthy controls, 17.6±11.7 ng/ml). In addition, a Chinese study by
Cong et al analyzed the D-dimer levels of 43 cirrhotic
patients classified according to the Child-Pugh scores, as well as
of 16 healthy controls (18). The
D-dimer levels were demonstrated to gradually increase among
Child-Pugh class A, B and C. In an Italian study, Violi et
al also identified that the median D-dimer levels were 95.5,
113 and 1,453 ng/ml in patients with Child-Pugh class A, B and C,
respectively (19). Furthermore,
another Italian study by Primignani et al enrolled 43
cirrhotic patients with esophageal variceal bleeding and 43
cirrhotic patients without bleeding (20). In the patients with bleeding, the
mean D-dimer levels were 127.38±2.13, 155.89±3.29 and 432.3±2.9
ng/ml for Child-Pugh class A, B and C, respectively. By contrast,
in the patients without bleeding, the mean D-dimer levels were
25.6±2.4, 97.58±3.38 and 246.36±2.65 ng/ml for Child-Pugh class A,
B and C, respectively. Additionally, the mean D-dimer levels were
significantly higher patients with bleeding that had a MELD score
>17 compared with those having a MELD score <17 (486.5±3.22
vs. 161.2±3.10, respectively; P=0.01) (20). However, the authors did not observe
any significant association of D-dimer levels with MELD score in
patients without bleeding (20).
Collectively, the aforementioned studies supported the activation
of fibrinolysis according to the severity of liver dysfunction.
However, it must be acknowledged that the correlation between
D-dimer and the degree of liver dysfunction was relatively weak in
the present study (correlation coefficient, <0.3).
Another finding of the current study was that higher
D-dimer levels were able to significantly predict the in-hospital
mortality in cirrhotic patients. Therefore, D-dimer testing may be
used for the prognostic stratification of liver cirrhosis.
Similarly, Primignani et al also compared the association of
D-dimer levels with the 6-week mortality rate of cirrhotic patients
with esophageal variceal bleeding (20). They identified that the mean D-dimer
level was 172.9±2.70 and 525.6±3.29 ng/ml in survivors and
non-survivors, respectively. The proportion of hyperfibrinolysis,
defined as a D-dimer level of >483 ng/ml, was 11 and 67% in
survivors and non-survivors, respectively. In addition, the odds
ratio of D-dimer level for predicting the 6-week mortality was 16
(20). These findings further
supported the prognostic value of D-dimer levels in cirrhotic
patients. By comparison, the current study further identified the
accurate cut-off value in a more generalized population (with and
without bleeding). However, considering that the AUROC was 0.729 in
the present study, the prognostic value of D-dimer levels may be
moderate.
A major limitation of the current study was its
retrospective nature, which results in potential patient selection
bias. However, considering that a relatively large number of
patients were included in the study, the bias was weak.
In conclusion, the D-dimer levels of liver cirrhosis
patients were found to be significantly associated with the degree
of liver dysfunction. Furthermore, higher D-dimer levels predicted
an increased risk of in-hospital mortality as a result of liver
cirrhosis. Further prospective cohort studies are thus warranted to
confirm the present findings.
Acknowledgements
This study was partially supported by a grant from
the Natural Science Foundation of Liaoning Province (grant no.
2014020059) for Dr Hongyu Li.
References
|
1
|
Le Gal G, Righini M and Wells PS: D-dimer
for pulmonary embolism. JAMA. 313:1668–1669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodger MA, Le Gal G, Wells P, Baglin T,
Aujesky D, Righini M, Palareti G, Huisman M and Meyer G: Clinical
decision rules and D-Dimer in venous thromboembolism: Current
controversies and future research priorities. Thromb Res.
134:763–768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geersing GJ, Janssen KJ, Oudega R, Bax L,
Hoes AW, Reitsma JB and Moons KG: Excluding venous thromboembolism
using point of care D-dimer tests in outpatients: A diagnostic
meta-analysis. BMJ. 339:b29902009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palareti G, Legnani C, Cosmi B, Guazzaloca
G, Pancani C and Coccheri S: Risk of venous thromboembolism
recurrence: High negative predictive value of D-dimer performed
after oral anticoagulation is stopped. Thromb Haemost. 87:7–12.
2002.PubMed/NCBI
|
|
5
|
Palareti G, Cosmi B, Legnani C, Antonucci
E, De Micheli V, Ghirarduzzi A, Poli D, Testa S, Tosetto A, Pengo
V, et al: D-dimer to guide the duration of anticoagulation in
patients with venous thromboembolism: A management study. Blood.
124:196–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palareti G, Cosmi B, Legnani C, Tosetto A,
Brusi C, Iorio A, Pengo V, Ghirarduzzi A, Pattacini C, Testa S, et
al: D-dimer testing to determine the duration of anticoagulation
therapy. N Engl J Med. 355:1780–1789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gram J, Duscha H, Zurborn KH and Bruhn HD:
Increased levels of fibrinolysis reaction products (D-dimer) in
patients with decompensated alcoholic liver cirrhosis. Scand J
Gastroenterol. 26:1173–1178. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cioni G, Cristani A, Mussini C, Grandi S,
Pentore R, Zeneroli ML, Tizzanini W, Zagni G and Ventura E:
Incidence and clinical significance of elevated fibrin(ogen)
degradation product and/or D-dimer levels in liver cirrhosis
patients. Ital J Gastroenterol. 22:70–74. 1990.PubMed/NCBI
|
|
9
|
Saray A, Mesihovic R, Gornjakovic S, Vanis
N, Mehmedovic A, Nahodovic K, Glavas S and Papovic V: Association
between high D-dimer plasma levels and ascites in patients with
liver cirrhosis. Med Arch. 66:372–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai J, Qi X, Li H and Guo X: Role of
D-dimer in the development of portal vein thrombosis in liver
cirrhosis: A meta-analysis. Saudi J Gastroenterol. 21:165–174.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi X, Li H, Chen J, Xia C, Peng Y, Dai J,
Hou Y, Deng H, Li J and Guo X: Serum liver fibrosis markers for
predicting the presence of gastroesophageal varices in liver
cirrhosis: A retrospective cross-sectional study. Gastroenterol Res
Pract. 2015:2745342015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng Y, Qi X, Dai J, Li H and Guo X:
Child-Pugh versus MELD score for predicting the in-hospital
mortality of acute upper gastrointestinal bleeding in liver
cirrhosis. Int J Clin Exp Med. 8:751–757. 2015.PubMed/NCBI
|
|
13
|
Qi X, Peng Y, Li H, Li H and Guo X:
Diabetes is associated with an increased risk of in-hospital
mortality in liver cirrhosis with acute upper gastrointestinal
bleeding. Eur J Gastroenterol Hepatol. 27:476–477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu C, Qi X, Li H, Peng Y, Dai J, Chen J,
Xia C, Hou Y, Zhang W and Guo X: Correlation of serum liver
fibrosis markers with severity of liver dysfunction in liver
cirrhosis: A retrospective cross-sectional study. Int J Clin Exp
Med. 8:5989–5998. 2015.PubMed/NCBI
|
|
15
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamath PS and Kim WR: The model for
end-stage liver disease (MELD). Hepatology. 45:797–805. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Sayed R, El-Karaksy H, El-Raziky M,
El-Hawary M, El Koofy N, Helmy H and Fahmy M: Assessment of
coagulation and fibrinolysis in children with chronic liver
disease. Blood Coagul Fibrinolysis. 24:113–117. 2013.PubMed/NCBI
|
|
18
|
Cong YL, Wei YX, Zhang LW, Yin ZJ and Bai
J: The relationship between hemostatic changes in liver cirrhosis
patients with different degrees of liver lesions in reference to
Child-Pugh scores. Zhonghua Gan Zang Bing Za Zhi. 13:31–34.
2005.(In Chinese). PubMed/NCBI
|
|
19
|
Violi F, Ferro D, Basili S, Saliola M,
Quintarelli C, Alessandri C and Cordova C: Association between
low-grade disseminated intravascular coagulation and endotoxemia in
patients with liver cirrhosis. Gastroenterology. 109:531–539. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Primignani M, Dell'Era A, Bucciarelli P,
Bottasso B, Bajetta MT, de Franchis R and Cattaneo M: High-D-dimer
plasma levels predict poor outcome in esophageal variceal bleeding.
Dig Liver Dis. 40:874–881. 2008. View Article : Google Scholar : PubMed/NCBI
|