Introduction
Chronic rhinosinusitis (CRS) is a common disease of
the nasal cavity and sinuses that affects millions of people
worldwide (1). CRS can be divided
into two subtypes: CRS with nasal polyps (CRSwNP) and CRS without
nasal polyps (CRSsNP) (2). Compared
with CRSsNP, CRSwNP has a more complex pathogenesis and usually
requires surgery (3). At present,
the exact origin of CRSwNP remains unclear. Inappropriate
therapeutic options lead to a high recurrence rate (4).
According to the type of inflammatory cell
infiltration, CRSwNP can be divided into two subgroups:
Eosinophilic and non-eosinophilic or neutrophilic (5). Non-eosinophilic CRSwNP always presents
with neutrophil-predominant inflammation (6). The subgroups have a variety of
pathogeneses and may require different therapeutic options.
Eosinophilic subgroup is considered to be induced by Th2 cells,
with interleukin (IL)-5 as major cytokine, resulting in increased
eosinophil survival and an eosinophilic type of inflammation. The
predominant T-effector cell in non-eosinophilic subgroup is the
Th17 cell, and resulting in a predominance of neutrophils (7,8). In a
previous study, the authors suggested that Caucasian patients with
CRSwNP usually manifest with eosinophilic CRSwNP, whereas Chinese
patients always show non-eosinophilic inflammation (9). However, this view remains
controversial. In the present study, the levels of eosinophil
cationic protein (ECP), which is released by active eosinophils,
and myeloperoxidase (MPO) were tested to detect the subtype of
CRSwNP in Chinese patients.
Staphylococcus aureus and S. aureus
enterotoxins (SEs) are common pathogens of CRS. However, the exact
underlying pathogenesis remains unclear. SEs are composed mainly of
SEA, SEB, SEC, SED and shock syndrome toxin (TSST-1) (10). In Caucasian patients with CRSwNP, SEs
have been suggested to act as classic allergens or superantigens,
which are able to activate millions of T and B lymphocytes
(11). Superantigens elicit the
production of high levels of allergen-specific immunoglobulin
(Ig)E, and are usually associated with the elevation of total IgE
and eosinophilic inflammation (12).
However, evidence is lacking concerning the role of superantigens
for SEs in Chinese patients with CRSwNP. In non-eosinophilic
CRSwNP, SEs may act as an infection factor; this hypothesis was
tested in the current study.
Materials and methods
Subjects
A total of 74 patients with CRSwNP who had undergone
functional endoscopic sinus surgery at the First Affiliated
Hospital of the College of Medicine, Zhejiang University (Hangzhou,
China) from July to December 2013 (50 males and 24 females; mean
age, 42.35 years) were included in the present study. The diagnosis
of CRSwNP was based on European Paediatric Ophthalmological Society
guidelines (13) and was confirmed
by pathological diagnosis after surgery. Patients who used
corticosteroids or antihistamines for the previous 4 weeks were
excluded from this study, as were patients with asthma. The
duration of CRSwNP in the patients ranged from 3 months to 20
years. Six patients who underwent septoplasty or sinus cystectomy
were designated as the control group.
The present research was approved by the Ethics
Committee of the First Affiliated Hospital of Zhejiang University.
Each patient supplied written informed consent.
Atopy status detection
Atopy status was measured in vitro by testing
the serum using the Phadiatop test and UniCAP100 automated system
(Phadia AB; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. The test included the
most common aeroallergens. The cut-off value was set at 0.35
KU/l.
Homogenate supernatant of the nasal
tissues
All of the nasal tissues (polyps or turbinates) were
collected at the time of surgery, were placed immediately in liquid
nitrogen, and then stored at −80°C. Specimens were first thawed in
saline (0.1 g in 1 ml) and processed in a tissue homogenizer. The
suspensions were then centrifuged for 5 min at 1,006.2 × g.
The supernatants were collected and stored at −80°C prior to
analysis.
Total IgE and ECP levels in the serum
and supernatant
Total IgE levels in the serum and nasal tissue
supernatant were detected by enzyme-linked immunosorbent assay
(#BMS209; ELISA; eBioscience, Vienna, Austria). First, the number
of microwell strips required to test the desired number of samples
plus the appropriate number of wells needed to run the blanks and
standards were determined. Each sample, standard, blank and
optional control sample was assayed in duplicate. Next, horseradish
peroxidase (HRP)-conjugated antibody was prepared. The microwell
strips were washed twice with ~400 µl Wash Buffer per well with
thorough aspiration of the microwell contents between washes. Then,
100 µl Assay Buffer (1X) was added in duplicate to the blank wells,
followed by the addition of 90 µl Assay Buffer (1X) and 10 µl each
sample in duplicate to the sample wells. To each well, including
the blank wells, 50 µl diluted HRP-conjugated antibody was then
added. The wells were covered with an adhesive film and incubated
at room temperature (18–25°C) for 1 h on a microplate shaker set at
400 rpm. Next, the adhesive film and the liquid in the wells were
removed. The microwell strips were washed four times with wash
buffer according to the test protocol. Next, 100 µl
Tetramethylbenzidine Substrate Solution was pipetted into each
well. The microwell strips were then incubated at room temperature
(18–25°C) for 30 min, taking care to avoid direct exposure to
intense light. The enzyme reaction was stopped by quickly pipetting
100 µl Stop Solution into each well. The absorbance of each
microwell was read on a spectrophotometer using 450 nm as the
primary wavelength, and the results were calculated.
ECP levels were also measured by ELISA (#SK00128-01;
Aviscera Bioscience, Inc., Santa Clara, CA, USA), according to the
manufacturer protocol.
SEA, SEB and MPO levels in the
supernatant
SEA [#BIO(TW)-E01(Hu)-00254] and SEB
[#BIO(TW)-E01(Hu)-00253] levels were measured using ELISA (R&D
Systems, Inc., Minneapolis, MN, USA). MPO levels were also detected
using an ELISA kit (#BMS2038INST; eBioscience) according to the
manufacturer's protocol.
Statistical analysis
All data analyses were performed using SPSS for
Windows 20.0 (IBM SPSS, Armonk, NY, USA). Considering the
non-normal distributions of the parameters, median (25–75%
percentile) was used to describe the data. Differences in each
group were compared using the Mann-Whitney U test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Atopy status of CRSwNP patients
Serum testing was conducted for 68 CRSwNP patients
and 6 controls. Among the CRSwNP patients 15 (22.1%) showed
positive results in the Phadiatop test, and none of the controls
exhibited atopy.
ECP and MPO levels in nasal tissue
supernatant
All of the CRSwNP patients and controls underwent
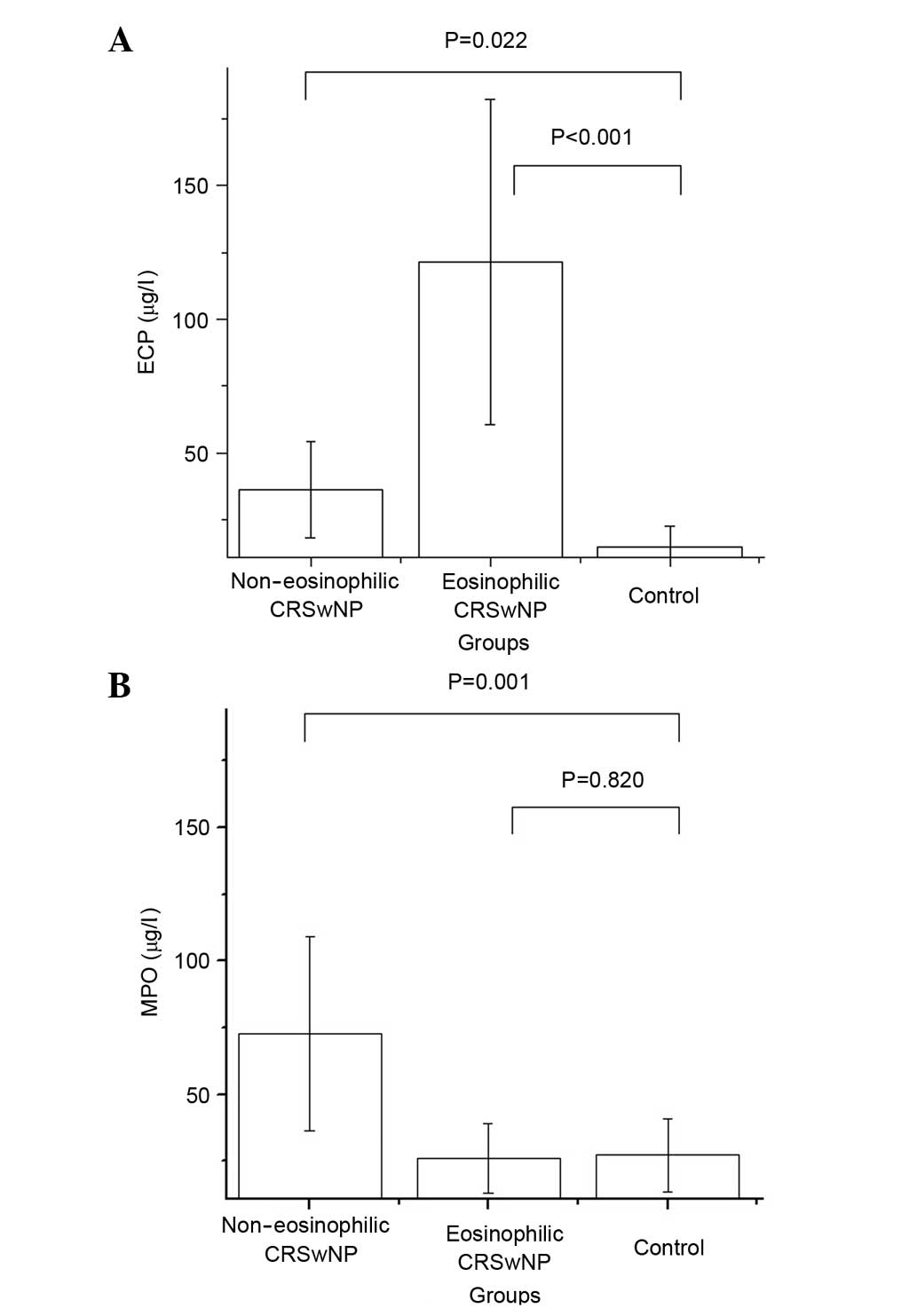
the supernatant tests. As described previously, eosinophilic CRSwNP
is characterized by a supernatant ECP/MPO ratio >2 (14). According to this criterion, 18
patients were assigned to an eosinophilic CRSwNP group, and the
others were assigned to the non-eosinophilic CRSwNP group. The
total ECP/MPO ratio was 0.572, with a notable bias toward
neutrophilic inflammation. Table I
lists the ECP and MPO levels in the supernatants of the three
groups. The supernatant ECP level was significantly elevated in the
non-eosinophilic and eosinophilic CRSwNP groups compared with that
in the control group (P<0.022 and P<0.001, respectively). The
supernatant MPO level was significantly elevated in the
non-eosinophilic CRSwNP group compared with the control group
(P=0.001) (Fig. 1).
 | Table I.ECP and MPO levels in the nasal tissue
supernatant. |
Table I.
ECP and MPO levels in the nasal tissue
supernatant.
| Group | ECP (µg/l) | P-value | MPO (µg/l) | P-value |
|---|
| Non-eosinophilic
CRSwNP | 36.31
(20.70–69.15) | 0.022 | 72.64
(34.65–178.37) | 0.001 |
| Eosinophilic
CRSwNP | 121.38
(63.59–164.57) | <0.001 | 26.09
(18.14–49.57) | 0.820 |
| Control | 15.00
(13.21–31.32) |
| 27.34
(14.92–30.26) |
|
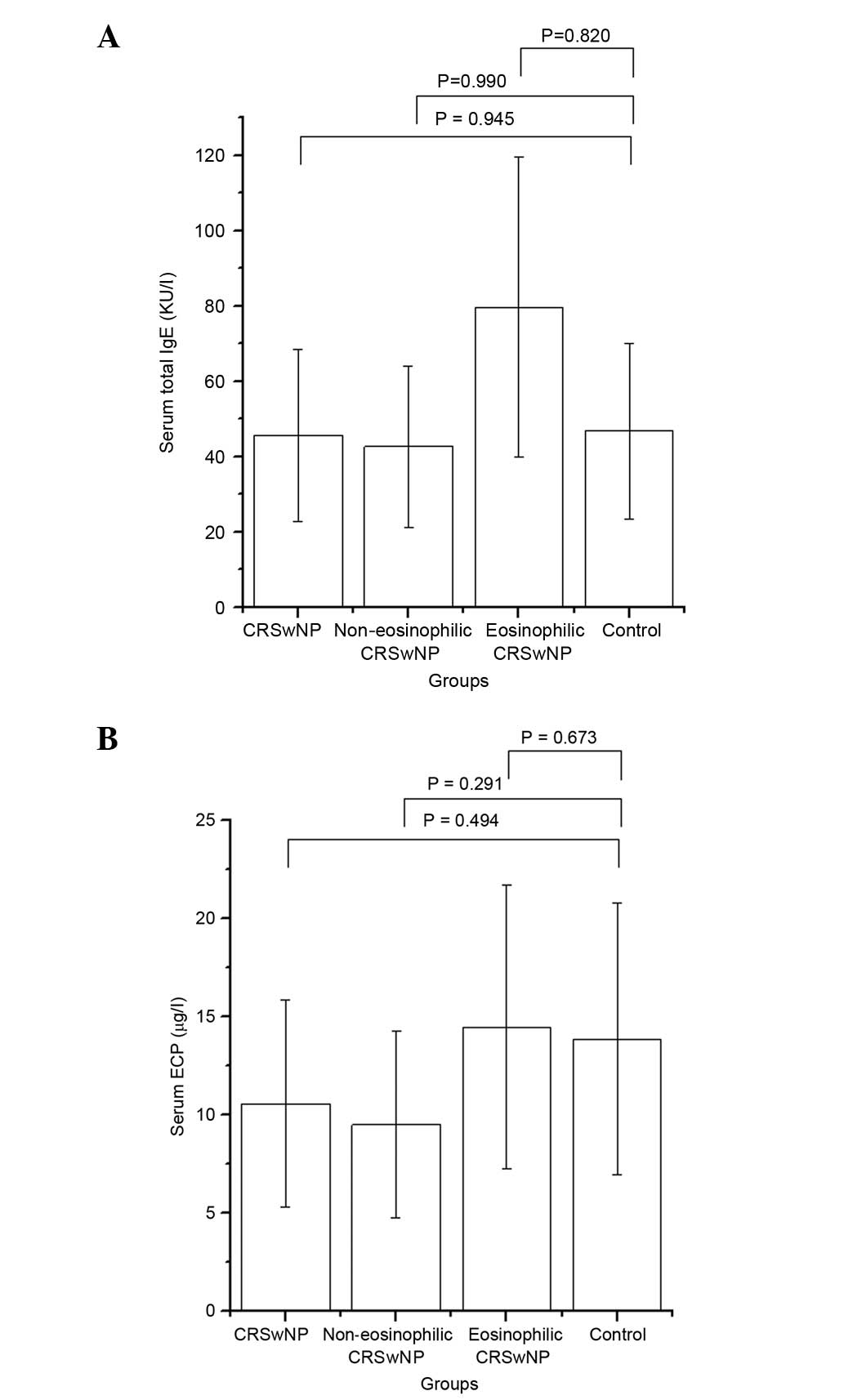
Total IgE and ECP levels in the
serum
In the CRSwNP group, the ECP level in the serum was
10.55 µg/l (range, 6.92–22.60 µg/l). Compared with the control
group, the CRSwNP group, including eosinophilic and
non-eosinophilic subgroups, showed no significant elevation in
serum ECP level. In the CRSwNP group, the total IgE level in the
serum was 45.70 KU/l (20.00–152.75 KU/l). Compared with the control
group, the CRSwNP group showed no significant difference in the
serum total IgE level. Data are listed in Table II and Fig. 2.
 | Table II.Serum total IgE and ECP level in
different groups. |
Table II.
Serum total IgE and ECP level in
different groups.
| Group | Serum total IgE
(KU/l) | P-value | Serum ECP (µg/l) | P-value |
|---|
| CRSwNP | 45.70
(20.00–152.75) | 0.945 | 10.55
(6.92–22.60) | 0.494 |
| Non-eosinophilic
CRSwNP | 42.65
(19.88–140.75) | 0.990 | 9.49
(6.18–17.28) | 0.291 |
| Eosinophilic
CRSwNP | 79.65
(24.90–194.00) | 0.820 | 14.45
(9.80–42.80) | 0.673 |
| Control | 46.80
(17.08–296.75) |
| 13.85
(9.56–26.58) |
|
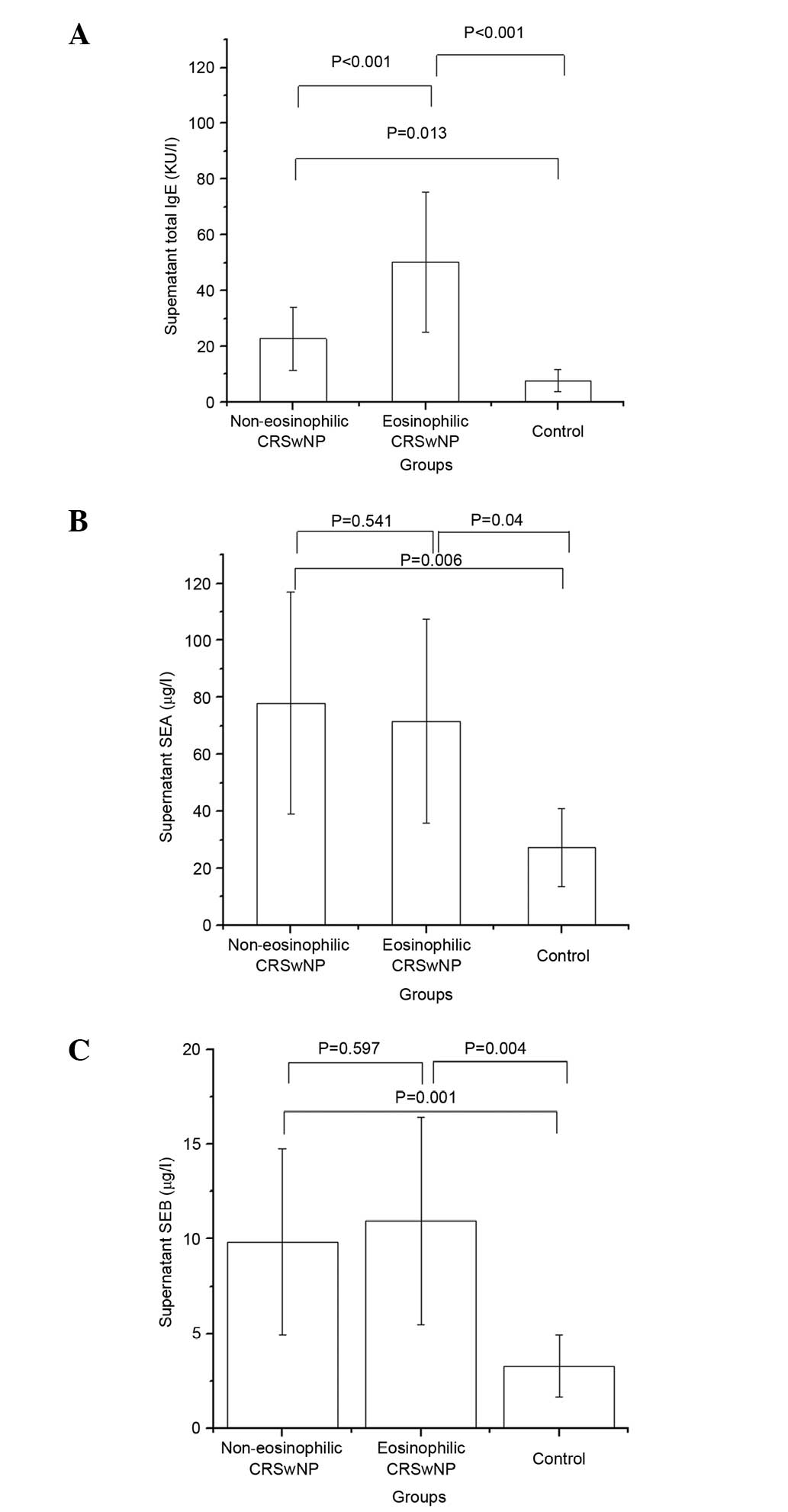
Total IgE and SE levels in the
supernatant
Table III
summarizes total IgE and SE levels in the supernatant. Compared
with the control group, the non-eosinophilic and eosinophilic
CRSwNP groups showed significant elevations in supernatant total
IgE levels (P=0.013 and P<0.001, respectively). The eosinophilic
CRSwNP group also showed a significant elevation in supernatant
total IgE level compared with that in the non-eosinophilic group
(P<0.001). Compared with the control group, the non-eosinophilic
and eosinophilic CRSwNP groups showed significant elevations in the
supernatant SEA levels (P=0.006 and P=0.04, respectively) and SEB
levels (P=0.001 and P=0.004, respectively). The differences in
supernatant SEA and SEB levels between the non-eosinophilic and
eosinophilic CRSwNP groups were not found to be statistically
significant (Fig. 3).
 | Table III.Total IgE, SEA and SEB level in the
supernatant. |
Table III.
Total IgE, SEA and SEB level in the
supernatant.
| Group | Total IgE (KU/l) | P-value | SEA (µg/l) | P-value | SEB (µg/l) | P-value |
|---|
| Non-eosinophilic
CRSwNP | 22.72
(9.22–36.22) | 0.013a | 77.99
(43.00–132.45) | 0.006a | 9.85
(4.73–17.04) | 0.001a |
| Eosinophilic
CRSwNP | 50.15
(34.28–90.74) |
<0.001b | 71.56
(29.82–109.21) | 0.541b | 10.97
(5.00–27.99) | 0.597b |
| Control | 7.71 (5.25–8.79) |
<0.001c | 27.38
(12.58–53.41) | 0.04c | 3.28
(1.23–5.13) | 0.004c |
Discussion
CRSwNP is a complex disease that presents challenges
to rhinologists. Its pathogenesis remains unclear, with allergy,
infection, inflammation, anatomic abnormality, bacterial
superantigen and biofilm being suggested (15). The recurrence rate for CRSwNP is high
after surgery or drug treatment in certain patients (16). In the past, allergy was considered to
be an important pathogeny of CRSwNP (17). Allergic CRSwNP showed a worse
therapeutic response than non-allergic CRSwNP in patients treated
with budesonide nasal spray (18).
However, its underlying pathogenesis remains unclear (19). In the present study, 15 (22.1%)
CRSwNP patients showed atopy, representing a higher incidence than
that for healthy people. This finding suggests that atopy remains a
common pathogenesis of CRSwNP, a viewpoint that is shared with two
previous studies (20,21).
Previously, CRSwNP was regarded as being associated
with T helper (Th)2-predominant inflammation, with increased
eosinophil infiltration and IgE elevation (22). In the present study, the serum total
IgE and ECP levels were not observed to be increased in the CRSwNP
groups compared with those in the control group. It is thus
suggested that these indicators in local tissues or nasal lavage
might be more sensitive than those in the serum.
Currently, CRSwNP is subdivided into two subgroups:
Eosinophilic and non-eosinophilic. Different CRS endotypes can be
characterized by the differences in responsiveness to treatments
(23). Thus, the exact diagnosis of
endotypes is important. Eosinophilic CRSwNP is an inflammatory
disease characterized by increased numbers of eosinophils, in
particular, fibroblasts, mast cells, goblet cells and Th2
lymphocytes (24). Eosinophilic
CRSwNP is usually associated with IgE IL-5 elevation, classic
allergy and superantigens (25).
Eosinophilic CRSwNP has been found in the majority of CRSwNP
patients who are Caucasian (9).
Non-eosinophilic CRSwNP exhibits neutrophilic inflammation in which
neutrophil recruitment into the sinus effusion is mediated by the
upregulation of adhesion molecules of the vascular endothelium
induced by IL-17 and by the enhanced secretion of IL-8 from
epithelial cells and neutrophils (26,27).
Additionally, non-eosinophilic CRSwNP is usually characterized by
Th1/Th17-shifted immunity (14).
Non-eosinophilic inflammation is common in Chinese CRSwNP patients
(28). The difference in endotypes
is not associated with only racial differences; in a previous
study, the cytokine profiles in Japanese patients with CRSwNP were
found to be similar to those of European patients with CRS
(29).
In the present study, 56 (75.7%) of the CRSwNP
patients were considered to have non-eosinophilic CRSwNP, and the
others were considered to have eosinophilic CRSwNP. This suggests
that non-eosinophilic inflammation plays an important role in
CRSwNP in Chinese patients, which is in agreement with the findings
of previous research (30,31). In the current study, the supernatant
ECP and MPO levels were elevated in the eosinophilic CRSwNP groups.
It may be inferred that both eosinophils and neutrophils are
involved in the pathogenesis of eosinophilic CRSwNP, with
eosinophils being the main functional cells in the eosinophilic
group.
Many studies have suggested a potential role for SEs
in the etiology and pathogenesis of CRSwNP (32,33). SEs
usually act as superantigens or classic allergens, which are able
to stimulate the elevation of local total IgE and allergen-specific
IgE levels and eosinophilic inflammation (34). In the current study, the supernatant
total IgE, SEA and SEB levels were upregulated in both the
eosinophilic and non-eosinophilic CRSwNP groups. The supernatant
total IgE level was significantly higher in the eosinophilic group
compared with that in the non-eosinophilic group. These results
implied that SEs are involved in the allergic pathogenesis of the
eosinophilic CRSwNP endotype. However, in the non-eosinophilic
endotype, SEs may play an alternative role in the pathogenesis of
this disease. Consistent with the hygiene hypothesis, SEs may act
as infection factors, but not allergens, due to the frequent
exposure to bacteria and viruses.
In conclusion, allergy remains a common factor in
the pathogenesis of CRSwNP. Neutrophilic inflammation is present in
the majority of Chinese CRSwNP patients. Additionally, local
indicators appear to reflect the inflammatory status more
accurately than do serum indicators. SEs may act as an infection
factor rather than as a superantigen in Chinese patients with
non-eosinophilic CRSwNP. Thus, long-term antibiotic therapy may be
an option for Chinese patients with non-eosinophilic CRSwNP.
Acknowledgements
The study was supported by Zhejiang Province Public
Welfare Funds (grant no. 2014C33203).
The English in this document has been checked by at
least two professional editors, both native speakers of English.
For a certificate, please see: http://www.textcheck.com/certificate/LMh1Rb.
References
|
1
|
Bhattacharyya N and Lee LN: Evaluating the
diagnosis of chronic rhinosinusitis based on clinical guidelines
and endoscopy. Otolaryngol Head Neck Surg. 143:147–151. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polzehl D, Moeller P, Riechelmann H and
Perner S: Distinct features of chronic rhinosinusitis with and
without nasal polyps. Allergy. 61:1275–1279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benninger MS, Ferguson BJ, Hadley JA,
Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe
JD, Stankiewicz JA, et al: Adult chronic rhinosinusitis:
Definitions, diagnosis, epidemiology, and pathophysiology.
Otolaryngol Head Neck Surg. 129:(3 Suppl). S1–S32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tosun F, Arslan HH, Karslioglu Y, Deveci
MS and Durmaz A: Relationship between postoperative recurrence rate
and eosinophil density of nasal polyps. Ann Otol Rhinol Laryngol.
119:455–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirotsu M, Kikuchi K, Kusunoki T, Kase K,
Ono N and Ikeda K: Comparison of bacterial examinations between
eosinophilic and neutrophilic chronic rhinosinusitis with nasal
polyps. Acta Otolaryngol. 131:997–1001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ba L, Zhang N, Meng J, Zhang J, Lin P,
Zhou P, Liu S and Bachert C: The association between bacterial
colonization and inflammatory pattern in Chinese chronic
rhinosinusitis patients with nasal polyps. Allergy. 66:1296–303.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fokkens W, Lund V and Mullol J: European
Position Paper on Rhinosinusitis and Nasal Polyps Group: EP3OS
2007: European position paper on rhinosinusitis and nasal polyps
2007. A summary for otorhinolaryngologists. Rhinology. 45:97–101.
2007.PubMed/NCBI
|
|
8
|
Meltzer EO, Hamilos DL, Hadley JA, Lanza
DC, Marple BF, Nicklas RA, Adinoff AD, Bachert C, Borish L,
Chinchilli M, et al: Rhinosinusitis Initiative: Rhinosinusitis:
Developing guidance for clinical trials. J Allergy Clin Immunol.
118:(5 Suppl). S17–S61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bachert C, Zhang N, van Zele T and Gevaert
P: Chronic rhinosinusitis: From one disease to different
phenotypes. Pediatr Allergy Immunol. 23:(Suppl 22). S2–S4. 2012.
View Article : Google Scholar
|
|
10
|
Patou J, Gevaert P, Van Zele T, Holtappels
G, van Cauwenberge P and Bachert C: Staphylococcus aureus
enterotoxin B, protein A, and lipoteichoic acid stimulations in
nasal polyps. J Allergy Clin Immunol. 121:110–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bernstein JM and Kansal R: Superantigen
hypothesis for the early development of chronic hyperplastic
sinusitis with massive nasal polyposis. Curr Opin Otolaryngol Head
Neck Surg. 13:39–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Zele T, Gevaert P, Holtappels G, van
Cauwenberge P and Bachert C: Local immunoglobulin production in
nasal polyposis is modulated by superantigens. Clin Exp Allergy.
37:1840–1847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: EPOS 2012: European position paper on rhinosinusitis and nasal
polyps 2012. A summary for otorhinolaryngologists. Rhinology.
50:1–12. 2012.PubMed/NCBI
|
|
14
|
Zhang N, Van Zele T, Perez-Novo C, Van
Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P and Bachert
C: Different types of T-effector cells orchestrate mucosal
inflammation in chronic sinus disease. J Allergy Clin Immunol.
122:961–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheahan P, Ahn CN, Harvey RJ, Wise SK,
Mulligan RM, Lathers DM and Schlosser RJ: Local IgE production in
nonatopic nasal polyposis. J Otolaryngol Head Neck Surg. 39:45–51.
2010.PubMed/NCBI
|
|
16
|
Bonfils P, Badoual C, Bonfils NA, Gallas D
and Malinvaud D: Eosinophil infiltration of nasal polyps in
patients with nasal polyposis Role in clinical evolution after
medical and surgical treatment. J Laryngol Otol. 123:509–516. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asero R and Bottazzi G: Nasal polyposis: A
study of its association with airborne allergen hypersensitivity.
Ann Allergy Asthma Immunol. 86:283–285. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kirtsreesakul V and Atchariyasathian V:
Nasal polyposis: Role of allergy on therapeutic response of
eosinophil- and noneosinophil-dominated inflammation. Am J Rhinol.
20:95–100. 2006.PubMed/NCBI
|
|
19
|
Collins MM, Loughran S, Davidson P and
Wilson JA: Nasal polyposis: Prevalence of positive food and
inhalant skin tests. Otolaryngol Head Neck Surg. 135:680–683. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kennedy JL and Borish L: Chronic sinusitis
pathophysiology: The role of allergy. Am J Rhinol Allergy.
27:367–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erbek SS, Erbek S, Topal O and Cakmak O:
The role of allergy in the severity of nasal polyposis. Am J
Rhinol. 21:686–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nguyen LH, Fakhri S, Frenkiel S and Hamid
QA: Molecular immunology and immunotherapy for chronic sinusitis.
Curr Allergy Asthma Rep. 3:505–512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akdis CA, Bachert C, Cingi C, Dykewicz MS,
Hellings PW, Naclerio RM, Schleimer RP and Ledford D: Endotypes and
phenotypes of chronic rhinosinusitis: A PRACTALL document of the
European Academy of Allergy and Clinical Immunology and the
American Academy of Allergy, Asthma & Immunology. JAllergy Clin
Immunol. 131:1479–1490. 2013. View Article : Google Scholar
|
|
24
|
Ouyang Y, Fan E, Li Y, Wang X and Zhang L:
Clinical characteristics and expression of thymic stromal
lymphopoetin in eosinophilic and non-eosinophilic chronic
rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 75:37–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu Y, Cao PP, Liang GT, Cui YH and Liu Z:
Diagnostic significance of blood eosinophil count in eosinophilic
chronic rhinosinusitis with nasal polyps in Chinese adults.
Laryngoscope. 122:498–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki H and Ikeda K: Mode of action of
long-term low-dose macrolide therapy for chronic sinusitis in the
light of neutrophil recruitment. Curr Drug Targets Inflamm Allergy.
1:117–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikeda K, Shiozawa A, Ono N, Kusunoki T,
Hirotsu M, Homma H, Saitoh T and Murata J: Subclassification of
chronic rhinosinusitis with nasal polyp based on eosinophil and
neutrophil. Laryngoscope. 123:E1–E9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bachert C, Zhang N, Holtappels G, De Lobel
L, van Cauwenberge P, Liu S, Lin P, Bousquet J and Van Steen K:
Presence of IL-5 protein and IgE antibodies to staphylococcal
enterotoxins in nasal polyps is associated with comorbid asthma. J
Allergy Clin Immunol. 126:962–968, 968.e1-e6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sejima T, Holtappels G, Kikuchi H,
Imayoshi S, Ichimura K and Bachert C: Cytokine profiles in Japanese
patients with chronic rhinosinusitis. Allergol Int. 61:115–122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao PP, Li HB, Wang BF, Wang SB, You XJ,
Cui YH, Wang DY, Desrosiers M and Liu Z: Distinct immunopathologic
characteristics of various types of chronic rhinosinusitis in adult
Chinese. J Allergy Clin Immunol. 124:478–484, 484.e1-e2. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao PP, Zhang YN, Liao B, Ma J, Wang BF,
Wang H, Zeng M, Liu WH, Schleimer RP and Liu Z: Increased local IgE
production induced by common aeroallergens and phenotypic
alteration of mast cells in Chinese eosinophilic, but not
non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin
Exp Allergy. 44:690–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tripathi A, Conley DB, Grammes LC, Ditto
AM, Lowery MM, Seiberling KA, Yarnold PA, Zeifer B and Kern RC:
Immunoglobulin E to staphylococcal and streptococcal toxins in
patients with chronic sinusitis/nasal polyposis. Laryngoscope.
114:1822–1826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang N, Holtappels G, Claeys C, Huang G,
van Cauwenberge P and Bachert C: Pattern of inflammation and impact
of Staphylococcus aureus enterotoxins in nasal polyps from southern
China. Am J Rhinol. 20:445–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tripathi A, Kern R, Conley DB, Seiberling
K, Klemens JC, Harris KE, Suh L, Huang J and Grammer LC:
Staphylococcal exotoxins and nasal polyposis: Analysis of systemic
and local responses. Am J Rhinol. 19:327–333. 2005.PubMed/NCBI
|