Introduction
The fruit of the plum tree Prunus mume
Siebold & Zucc. (PM) is used across East Asia, particularly in
Korea and Japan (1), as a
traditional herbal medicine for the relief of digestive problems,
fatigue and fever. PM contains a number of phenolic compounds,
including phenolic acids and flavonoids (1,2), which
have antioxidant and free radical scavenging activities in
vivo (3–6). Furthermore, PM extracts exhibit many
pharmacological activities, including antimicrobial (7–10),
immune enhancing (11), anti-cancer
(1,12,13), and
anti-fatigue (14) effects, and have
been demonstrated to enhance osteoclast differentiation (15) and improve blood flow (16). Additionally, previous studies have
reported that using PM extracts with probiotics inhibits the
development of atopic dermatitis (17) and enhances immunity (18).
Interstitial cells of Cajal (ICCs) are the
pacemakers of the gastro-intestinal tract and generate rhythmic
responses in cell membrane electrical potentials (19,20),
thus serving important roles in the regulation of GI motility
(21). Additionally, endogenous
agents are able to regulate GI motility function via ICCs (22–25).
Furthermore, transient receptor potential melastatin (TRPM) 7
(26) or Cl− channels,
such as anoctamin1 (ANO1) (27–29), are
associated with pacemaker potentials in the GI tract. Therefore,
TRPM7 and ANO1 may be therapeutic targets for the treatment of GI
motility disorders.
It has been reported previously that PM is able to
enhance the propulsive motion and motility of the small intestine
(7) and promote the frequency of
defecation and colon contraction in rats, which supports the
potential role of PM as a therapeutic agent for the treatment of
constipation (30). However, little
is known about the effect of PM on ICC clusters in the GI tract.
The aims of the present study were to evaluate the effects of the
methanoic extract of PM (m-PM) on the electrical pacemaker
potentials of cultured ICCs and characterize m-PM-mediated
signaling pathways.
Materials and methods
Preparation of m-PM
PM fruits were harvested in the Wondong area,
(Yangsan, Geongnam, Korea) in June 2012 and were authenticated by
Professor Hyungwoo Kim (School of Korean Medicine, Pusan National
University, Yangsan, Korea). A standard extraction process was
performed to obtain m-PM, as previously described (24). Briefly, 50 g PM fruit was immersed in
0.5 l methanol, sonicated for 15 min and allowed to stand for 24 h.
The extract obtained was filtered through No. 20 Whatman filter
paper and lyophilized using a freeze dryer (Labconco Corp., Kansas
City, MO, USA). A total of 2.42 g of lyophilized powder (m-PM) was
subsequently obtained (yield, 4.84%). A 12.1 g sample of m-PM was
deposited at the School of Korean Medicine, Pusan National
University (voucher no. MH2012-008).
Ethics
Animal care and experiments were conducted in
accordance with the guidelines issued by the ethics committee of
Pusan National University (Busan, Korea; Approval no.
PNU-2014-0725) and the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (31).
Preparation of cells and cell
cultures
A total of 78 BALB/c mice (male:female, 41:37; age,
4–7 days; weight, 2.0–2.2 g; Samtako Bio Korea Co., Ltd., Osan,
Korea) were anesthetized with 0.1% ether (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) and sacrificed using cervical
dislocation. Mice were maintained under controlled conditions
(temperature, 20±2°C; humidity, 50±5%; 12 h light/dark cycles) and
were allowed free access to food and water. Small intestines were
removed and opened along the mesenteric border, and luminal
contents were removed via washing with Krebs-Ringer bicarbonate
solution. Sharp dissection was performed to remove small intestine
mucosae and small strips of intestine muscle were subsequently
equilibrated in Ca2+-free physiological salt solution
(in mmol/l: 125 NaCl, 5.36 KCl, 0.34 NaOH, 0.44
Na2HCO3, 10 glucose, 2.9 sucrose, and 11
HEPES buffer) for 20 min and dispersed using an enzyme solution
containing 1.5 mg/ml collagenase (Worthington Biochemical Corp.,
Lakewood, NJ, USA), 2.5 mg/ml bovine serum albumin (Sigma-Aldrich;
Merck Millipore), 2.5 mg/ml trypsin inhibitor (Sigma-Aldrich; Merck
Millipore) and 0.5 mg/ml adenosine triphosphate (ATP)
(Sigma-Aldrich; Merck Millipore). Cells were plated on glass
coverslips coated with 0.01% poly-L-lysine solution (Sigma-Aldrich;
Merck Millipore) and cultured in an atmosphere containing 95%
O2 and 5% CO2 in smooth muscle basal medium
(Clonetics Corp.; Lonza, Walkersville, MA, USA) supplemented with
stem cell factor (5 ng/ml; Sigma-Aldrich; Merck Millipore) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C.
Whole cell patch-clamp
experiments
The Na+-Tyrode solution used in bath
solution contained 135 mM NaCl, 5 mM KCl, 135 mM NaCl, 2 mM
CaCl2, 10 mM glucose, 1.2 mM MgCl2 and 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer,
adjusted to pH 7.4 with NaOH. A pipette solution was also used,
which contained 140 mM KCl, 5 mM MgCl2, 2.7 mM
K2ATP, 0.1 mM Na guanosine triphosphate (GTP), 2.5 mM
creatine phosphate disodium, 5 mM HEPES buffer and 0.1 mM ethylene
glycol bis (2-aminoethyl ether)-N,
N,N',N'-tetraacetic acid (EGTA), adjusted to
pH 7.2 with KOH. The whole-cell patch-clamp technique was performed
to record the membrane electrical potentials in cultured ICCs and
membrane potentials were amplified using an Axopatch 1-D (Molecular
Devices, LLC, Sunnyvale, CA, USA). Command pulses were applied
using a Samsung-compatible personal computer and pClamp software
(ver. 9.0; Molecular Devices). Data were filtered at 1 kHz and
displayed on a computer monitor. pClamp and Origin software (ver.
8.0; MicroCal, Northampton, MA, USA) were used for statistical
analysis. All experiments were performed at 30°C.
TRPM7 overexpression
Human embryonic kidney (HEK)-293 cells (American
Type Culture Collection, Manassas, VA, USA) were transfected with
Flag-murine LTRPC7/pCDNA4-TO construct and subsequently cultured in
Dulbecco's Modified Eagle medium (Thermo Fisher Scientific, Inc.)
supplemented with 5 µg/ml blasticidin, 0.4 mg/ml zeocin and 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.). Adding 1 µg/ml
tetracycline to the medium for 24 h induced TRPM7 overexpression.
HEK293 cells overexpressing TRPM7 were bathed in a solution
containing 145 mM NaCl, 2.8 mM KCl, 2 mM CaCl2, 10 mM
glucose, 1.2 mM MgCl2 and 10 mM HEPES buffer, adjusted
to pH 7.4 with NaOH. The pipette solution contained 145 mM
Cs-glutamate, 8 mM NaCl, 10 mM Cs-2-bis
(2-aminophenoxy)-ethane-N,N,N',N'-tetraacetic
acid, and 10 mM HEPES-CsOH, adjusted to pH 7.3 with CsOH.
Ca2+ activated
Cl− channel overexpression
HEK-293 cells were transfected with the
pEGFP-N1-mANO1 construct for 24 h and these cells were cultured on
glass coverslips in Dulbecco's Modified Eagle medium, which was
supplemented with 10% fetal bovine serum. The bath solution
contained 146 mM HCl, 10 mM HEPES, 10 mM glucose, 1 mM
MgCl2, 1 mM CaCl2 and 150 mM
N-methyl-D-glucamine (NMDG), adjusted to pH 7.4. The pipette
solution contained 150 mM NMDG-Cl, 1 mM MgCl2, 3 mM
MgATP, 10 mM EGTA, 5 mM CaCl2 and 5 mM HEPES buffer at
pH 7.2 (titrated with NMDG). WEBMAX-C STANDARD software (C. Patton,
Stanford University, www.stanford.edu/~cpatton/maxc.html) was used to fix
the free calcium concentration at 200 nM.
Pharmacological agents
Pharmacological agents, including methoctramine,
4-diphenylacetoxy-N-methyl-piperidine methiodide (4-DAMP),
guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S), thapsigargin,
PD98059, SB203580 and SP600125, were purchased from Sigma-Aldrich
(Merck Millipore). They were dissolved in dimethyl sulfoxide (DMSO)
or distilled water and stored at −20°C. The final concentration of
DMSO in the bath solution was maintained at <0.1%.
Statistical analysis
Results are expressed as mean ± standard error of
the means. Student's t-test for unpaired data was performed
to compare control and experimental groups. Origin software
(version 8.0; OriginLab, Northampton, MA, USA) was used to perform
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of m-PM on pacemaker electrical
potentials in cultured ICCs
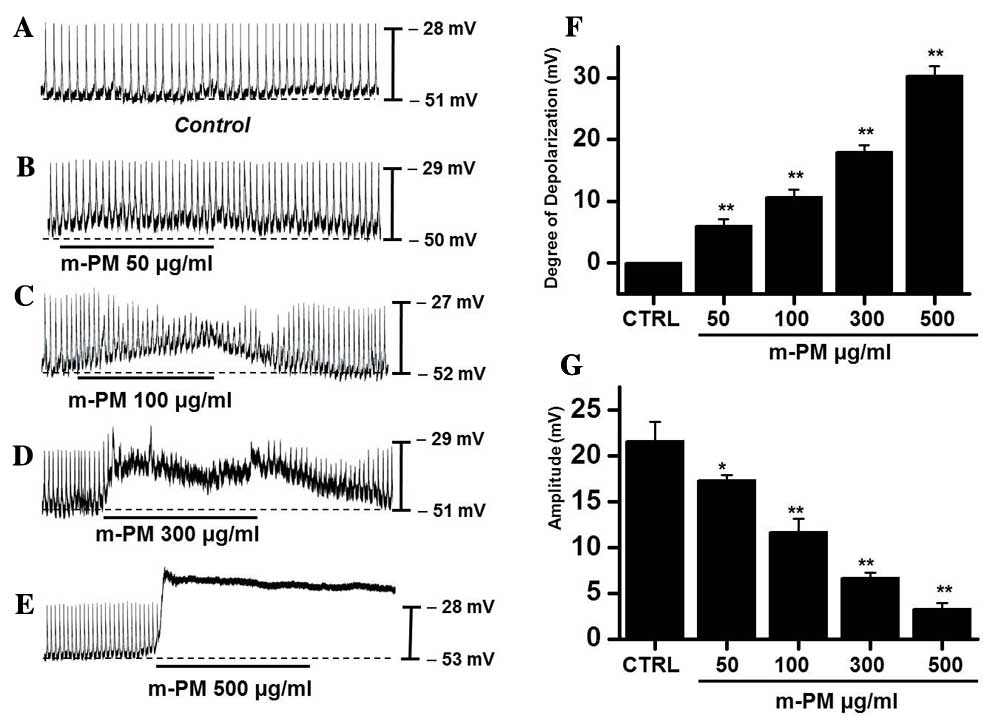
The effect of m-PM on pacemaker electrical
potentials in cultured ICCs was investigated. Mean resting
electrical potential of membranes was −51.7±2.4 mV and the
electrical amplitude was 21.6±2.3 mV. Following m-PM administration
(50–500 µg/ml), mean membrane electrical potentials were
depolarized to 6.2±1.3 (50 µg/ml), 10.6±1.2 (100 µg/ml), 18.5±1.5
(300 µg/ml) and 30.3±1.6 mV (500 µg/ml; Fig. 1A-E), and corresponding amplitudes
decreased to 17.3±0.6, 11.4±1.5, 6.7±0.8, and 3.3±0.5 mV,
respectively (Fig. 1B-E). The
effects of m-PM on pacemaker electrical potentials are presented in
Fig. 1F and G (n=7). These results
suggest that m-PM modulates the pacemaker potentials of ICCs.
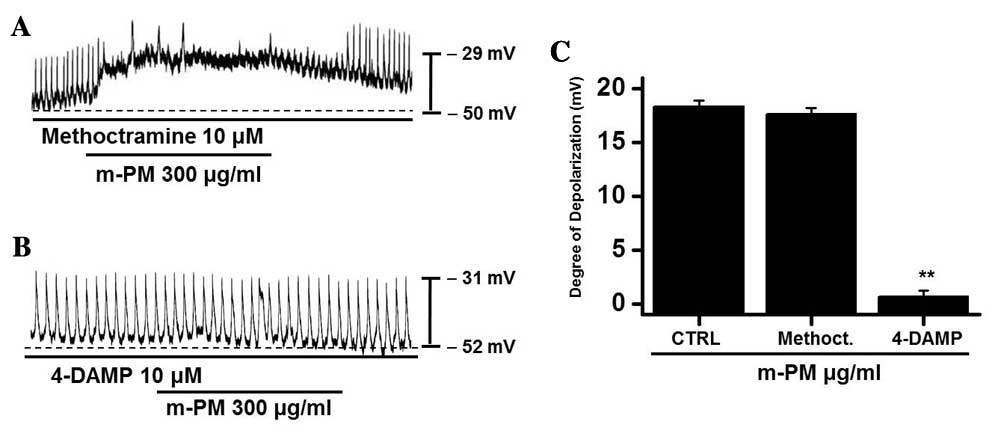
m-PM receptors in cultured ICCs
To study the m-PM receptors on ICCs, muscarinic
receptors were investigated as they mediate membrane electrical
depolarization in the GI tract (32,33).
Furthermore, it has been demonstrated that ICCs express
M2 and M3 muscarinic receptors in the GI
tract (34). Pretreatment with
muscarinic receptor antagonists was performed to identify which
muscarinic receptor was associated with the response. Membranes
were pretreated with 10 µm methoctramine, which is a muscarinic
M2 receptor antagonist, or 4-DAMP, which is a muscarinic
M3 receptor antagonist, for 5 min prior to m-PM (300
µg/ml) administration. Neither antagonist had any effect on
pacemaker potentials. Methoctramine did not inhibit the effect of
m-PM (Fig. 2A), whereas 4-DAMP was
able to inhibit m-PM-induced membrane depolarization (Fig. 2B). The mean membrane electrical
depolarization by m-PM following pretreatment with methoctramine or
4-DAMP was 17.5±0.7 and 0.9±0.4 mV, respectively (n=5 in each;
Fig. 2C). These results indicate
that m-PM affects ICCs through M3 receptors, not
M2 receptors.
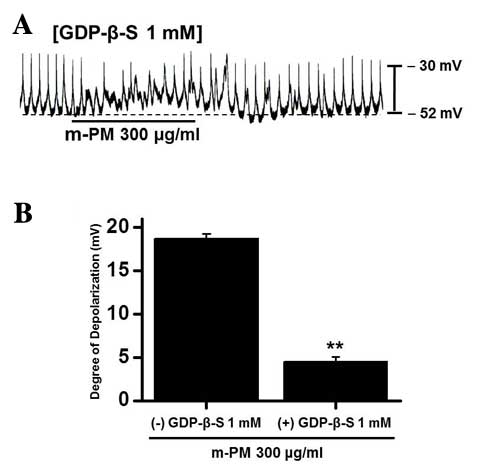
Association between G proteins and
m-PM-induced pacemaker electrical potentials in cultured ICCs
GDP-β-S, which permanently inactivates G-protein
binding proteins (35,36), was administered to determine whether
G-proteins are associated with the effects of m-PM on cultured
ICCs. m-PM (300 µg/ml) induced ICC membrane depolarization
(Fig. 1D); however, when GDP-β-S (1
mM) was present in the pipette solution, m-PM-induced
depolarization was markedly reduced (n=5; Fig. 3). These results suggest that
G-proteins have a role in the m-PM-induced pacemaker depolarization
of ICCs.
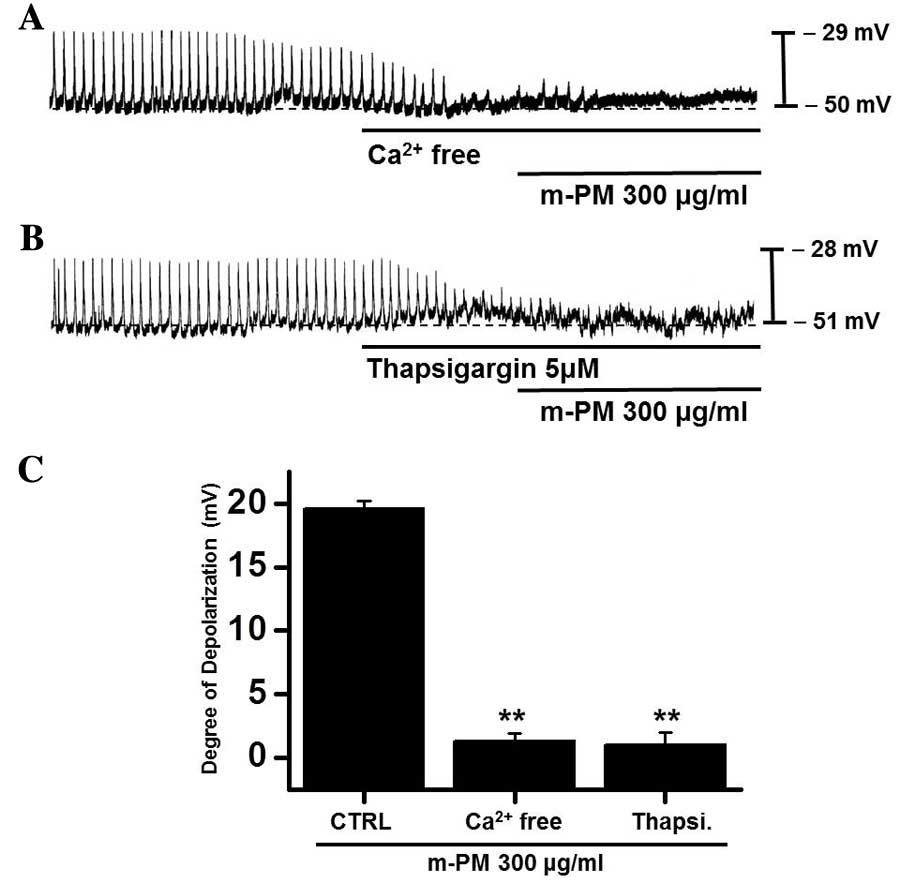
Effects of external
Ca2+-free solution and Ca2+-ATPase inhibitor
of endoplasmic reticulum on m-PM-induced pacemaker electrical
potentials of cultured ICC
An influx of external Ca2+ is required
for GI contractions and pacemaker electrical depolarizations in
ICCs (37). Furthermore, pacemaker
electrical depolarizations are regulated by intracellular
Ca2+ modulations (37).
To investigate the roles of external and internal Ca2+
on m-PM-induced pacemaker depolarizations, m-PM was applied in the
absence of external Ca2+ and in the presence of
thapsigargin (a Ca2+-ATPase inhibitor in endoplasmic
reticulum). When exposed to the external Ca2+-free
condition, pacemaker potentials were abolished and were unaffected
by the administration of m-PM (Fig.
4A). Pretreatment with thapsigargin (5 µM) also suppressed
pacemaker electrical potentials and in these conditions, m-PM had
no effect on pacemaker electrical potentials (Fig. 4B). The effects of m-PM on pacemaker
electrical potentials are presented in Fig. 4C (n=6). These results suggest
external Ca2+ or internal Ca2+ regulations
modulate m-PM-induced pacemaker electrical potentials in cultured
ICCs.
Association of mitogen-activated
protein kinase (MAPKs) with m-PM-induced pacemaker potentials of
cultured ICCs
To evaluate the mechanisms involved in the
interaction between m-PM and M3 receptors, the role of
mitogen-activated protein kinases (MAPKs) was investigated. It has
been demonstrated that muscarinic receptors are able to activate
MAPKs in various cell types (38,39);
therefore, the potential role of MAPKs in regulating the effects of
m-PM was determined by administrating a p42/44 MAPK inhibitor
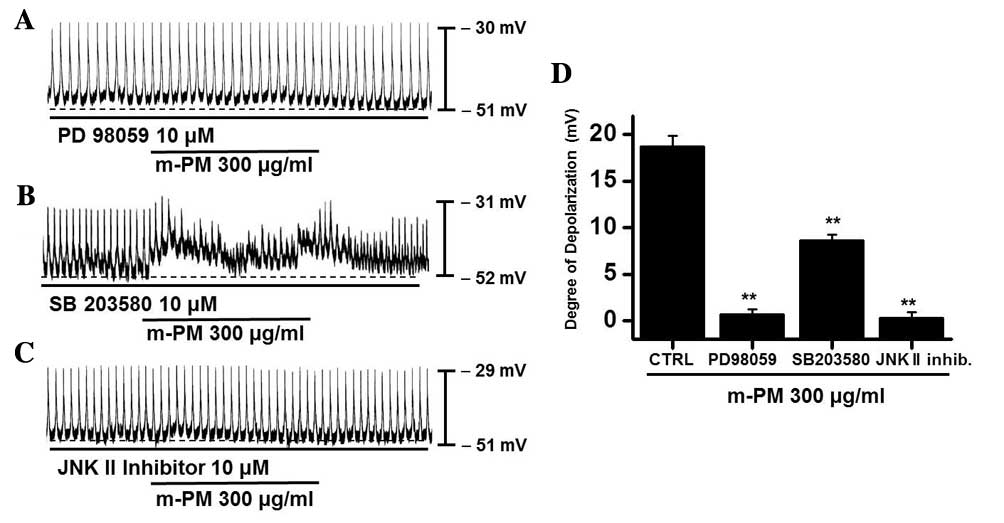
(PD98059), a p38 MAPK inhibitor (SB203580) or a c-jun NH2-terminal
kinase (JNK) II inhibitor (SP600125). PD98059 (10 µM), m-PM did not
induce membrane electrical depolarization (Fig. 5A). m-PM-induced membrane electrical
depolarization was partially blocked by the administration of
SB203580 (Fig. 5B) and completely
blocked by the administration of SP600125 (Fig. 5C). The effects of m-PM on pacemaker
electrical potentials are presented in Fig. 5D (n=5). These results suggest that
MAPKs modulate m-PM-induced pacemaker electrical potentials in
cultured ICCs.
Association of TRPM7 and
Ca2+-activated Cl− channels with m-PM-induced
pacemaker potentials in cultured ICCs
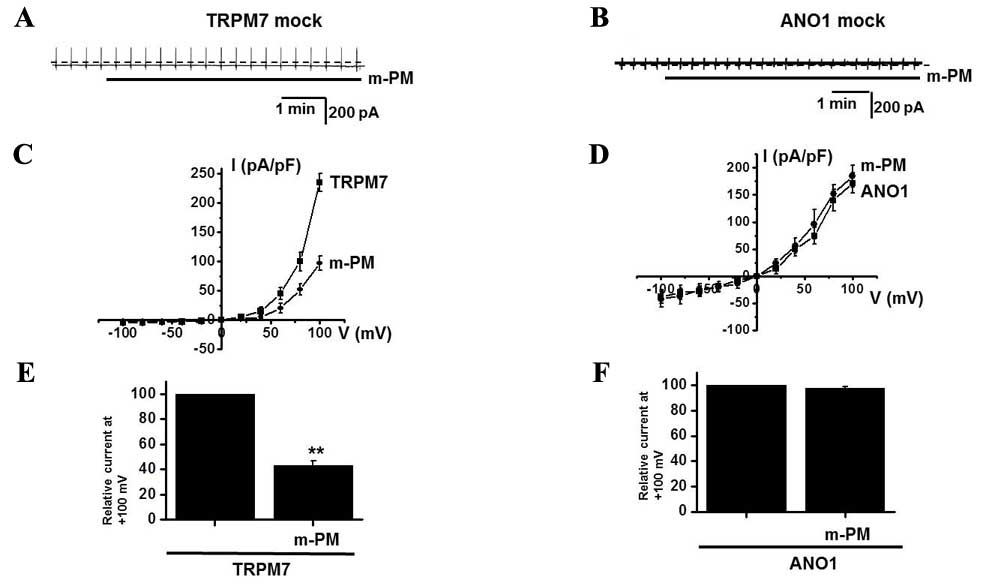
In the murine small intestine, pacemaker potentials
are predominantly induced by the activation of non-selective cation
channels (25,26) or Cl−channels (27–29). To
determine which channel is associated with the m-PM-induced
depolarization of pacemaker potentials, the effects of m-PM on
TRPM7 and Ca2+-activated Cl− channels were
examined. Membranes were transfected with the FLAG-murine
TRPM7/pCDNA4/TO construct using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.) and ~90% of cells were transfected. In a previous
study, TRPM7-transfected HEK293 cells induced by tetracycline
produced a flag-reactive band with a relative molecular mass of 220
kilodaltons (21). Another study
revealed that ANO1 channels were overexpressed in HEK293 cells
transfected with an ANO1 construct (40) and whole cell currents were recorded
using patch-clamp techniques. In the present study, TRPM7 and ANO1
currents were activated in mock transfected cells (Fig. 6A and B) and it was demonstrated that
m-PM inhibited the activities of TRPM7 channels, but did not affect
the Ca2+-activated Cl− ANO1 channels (n=4;
Fig. 6C-F), thus indicating that the
effects of m-PM are attributable to TRPM7 channels.
Discussion
In the present study, it was demonstrated that
administration of m-PM induced depolarization of ICC pacemaker
potentials through muscarinic M3 receptor signaling
pathways in a G protein-MAPK dependent manner. Furthermore, m-PM
was able to inhibit TRPM7 currents, indicating that TRPM7 is
associated with the m-PM-induced membrane depolarization of
ICCs.
In intestinal motility, PM has been reported to
enhance propulsive motion and small intestine motility, as
determined by the coated charcoal method (7). Additionally, it has been demonstrated
that PM has laxative effects in constipation rat models, as it
accelerated the spontaneous contraction of isolated colon (30). Furthermore, citric and malic acid,
the major organic acids in plums, stimulate spontaneous
contractions in the colon (30).
These findings support the commonly held belief that plums help to
prevent constipation and that ICCs function as pacemakers in the
small intestine thus modulating GI motility. In the present study,
it was identified that m-PM depolarizes ICC pacemaker activity.
The authors of the present study have previously
investigated the effects of traditional medicines on pacemaker
electrical potentials in ICCs. It has been determined that
Poncirus fructus (PF) is able to modulate pacemaker
electrical potentials via the 5-hydroxytryptamine
(5-HT)3 and 5-HT4 receptor pathways in a
MAPK-dependent manner (24) and
gintonin-mediated membrane depolarization and
Ca2+-activated Cl− channel activation have
been observed in cultured murine ICCs via a lysophosphatidic acid
1/3 receptor signaling pathway (41). Additionally, it was determined that
San-Huang-Xie-Xin-tang (SHXXT) is able to modulate pacemaker
electrical potentials (42). The
results of in vivo experiments suggested that
SHXXT-regulated GI motility was due to the activities of
Coptidis rhizome and Rhei rhizome (42). Furthermore, Schisandra
chinensis (Turcz.) Baill. extract (SC extract) was determined
to modulate ICC pacemaker potentials via external and internal
Ca2+ regulation, and via G protein and the phospholipase
C (PLC) pathway, in a dose-dependent manner, and increased
intestinal transit rates in mouse models of normal and abnormal GI
motility (43). These studies
indicate that traditional medicines, such as PF, ginseng, SHXXT and
SC may potentially be used as gastroprokinetic agents. The results
of the present study demonstrated that m-PM exhibited the potential
of a prokinetic agent for GI motility dysfunctions.
The MAPK family of protein kinases serve critical
roles in signal transduction (44,45) and
the regulation of various cellular responses, including cell cycle
progression, differentiation, inflammation, protein synthesis and
proliferation (46). There are five
subtypes of acetylcholine muscarinic receptors
(M1-M5), of which three (M1,
M3, and M5) are coupled with PLC through a
Gq protein, whereas the other subtypes (M2
and M4) are able to inhibit adenylate cyclase via
Go or Gi proteins (47). In various cellular systems,
muscarinic receptor stimulation has been reported to activate MAPK
(48,49). In the present study, the effects of
m-PM on ICCs in the murine small intestine were investigated. m-PM
modulated pacemaker activities in ICCs through muscarinic
M3 receptor activation via G protein, PLC and
MAPK-dependent mechanisms. Therefore, ICCs are targets for m-PM and
this interaction may improve intestinal motility.
In conclusion, Prunus mume Siebold &
Zucc. was able to depolarize ICC pacemaker potentials in a G
protein and MAPK-dependent manner by stimulating M3
receptors. These findings suggest that Prunus mume Siebold
& Zucc. may be developed as a potential gastroprokinetic agent
for the treatment of GI motility disorders.
Acknowledgements
The present study was supported by the Research
Institute for Convergence of Biomedical Science and Technology
(grant no. 30-2014-011), Pusan National University Yangsan
Hospital.
References
|
1
|
Jeong JT, Moon JH, Park KH and Shin CS:
Isolation and characterization of a new compound from Prunus mume
fruit that inhibits cancer cells. J Agric Food Chem. 54:2123–2128.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kita M, Kato M, Ban Y, Honda C, Yaegaki H,
Ikoma Y and Moriguchi T: Carotenoid accumulation in Japanese
apricot (Prunus mume Siebold & Zucc.): Molecular analysis of
carotenogenic gene expression and ethylene regulation. J Agric Food
Chem. 55:3414–3420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim BJ, Kim JH, Kim HP and Heo MY:
Biological screening of 100 plant extracts for cosmetic use (II):
Anti-oxidative activity and free radical scavenging activity. Int J
Cosmet Sci. 19:299–307. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai CH, Chang RC, Chiou JF and Liu TZ:
Improved superoxide-generating system suitable for the assessment
of the superoxide-scavenging ability of aqueous extracts of food
constituents using ultraweak chemiluminescence. J Agric Food Chem.
51:58–62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuda H, Morikawa T, Ishiwada T, Managi
H, Kagawa M, Higashi Y and Yoshikawa M: Medicinal flowers. VIII.
Radical scavenging constituents from the flowers of Prunus mume:
Structure of prunose III. Chem Pharm Bull (Tokyo). 51:440–443.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim TK, Cha MR, Kim SJ, Kim SY, Jeon KI,
Park HR, Park EJ and Lee SC: Antioxidative activity of methanol
extract from Prunus mume byproduct. Cancer Prev Res. 10:251–256.
2005.
|
|
7
|
Wang L, Zhang HY and Wang L: Comparison of
pharmacological effects of Fructus Mume and its processed products.
Zhong Yao Cai. 33:353–356. 2010.(In Chinese). PubMed/NCBI
|
|
8
|
Chen Y, Wong RW, Seneviratne CJ, Hägg U,
McGrath C, Samaranayake LP and Kao R: The antimicrobial efficacy of
Fructus mume extract on orthodontic bracket: A monospecies-biofilm
model study in vitro. Arch Oral Biol. 56:16–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seneviratne CJ, Wong RW, Hägg U, Chen Y,
Herath TD, Samaranayake PL and Kao R: Prunus mume extract exhibits
antimicrobial activity against pathogenic oral bacteria. Int J
Paediatr Dent. 21:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enomoto S, Yanaoka K, Utsunomiya H, Niwa
T, Inada K, Deguchi H, Ueda K, Mukoubayashi C, Inoue I, Maekita T,
et al: Inhibitory effects of Japanese apricot (Prunus mume Siebold
et Zucc.; Ume) on Helicobacter pylori-related chronic gastritis.
Eur J Clin Nutr. 64:714–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawahara K, Hashiguchi T, Masuda K,
Saniabadi AR, Kikuchi K, Tancharoen S, Ito T, Miura N, Morimoto Y,
Biswas KK, et al: Mechanism of HMGB1 release inhibition from
RAW264.7 cells by oleanolic acid in Prunus mume Sieb. et Zucc. Int
J Mol Med. 23:615–620. 2009.PubMed/NCBI
|
|
12
|
Mori S, Sawada T, Okada T, Ohsawa T,
Adachi M and Keiichi K: New anti-proliferative agent, MK615, from
Japanese apricot ‘Prunus mume’ induces striking autophagy in colon
cancer cells in vitro. World J Gastroenterol. 13:6512–6517. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kai H, Akamatsu E, Torii E, Kodama H,
Yukizaki C, Sakakibara Y, Suiko M, Morishita K, Kataoka H and
Matsuno K: Inhibition of proliferation by agricultural plant
extracts in seven human adult T-cell leukaemia (ATL)-related cell
lines. J Nat Med. 65:651–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim S, Park SH, Lee HN and Park T: Prunus
mume extract ameliorates exercise-induced fatigue in trained rats.
J Med Food. 11:460–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Youn YN, Lim E, Lee N, Kim YS, Koo MS and
Choi SY: Screening of Korean medicinal plants for possible
osteoclastogenesis effects in vitro. Genes Nutr. 2:375–380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuda Y, Ono H, Ohnishi-Kameyama M,
Matsumoto K, Nagata T and Kikuchi Y: Mumefural, citric acid
derivative improving blood fluidity from fruit-juice concentrate of
Japanese apricot (Prunus mume Sieb. et Zucc). J Agric Food Chem.
47:828–831. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung BG, Cho SJ, Koh HB, Han DU and Lee
BJ: Fermented Maesil (Prunus mume) with probiotics inhibits
development of atopic dermatitis-like skin lesions in NC/Nga mice.
Vet Dermatol. 21:184–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung BG, Ko JH, Cho SJ, Koh HB, Yoon SR,
Han DU and Lee BJ: Immune-enhancing effect of fermented Maesil
(Prunus mume Siebold & Zucc.) with probiotics against
Bordetella bronchi septica in mice. J Vet Med Sci. 72:1195–1202.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huizinga JD, Thuneberg L, Kluppel M,
Malysz J, Mikkelsen HB and Bernstein A: W/kit gene required for
interstitial cells of Cajal and for intestinal pacemaker activity.
Nature. 373:347–349. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanders KM: A case for interstitial cells
of Cajal as pacemakers and mediators of neurotransmission in the
gastrointestinal tract. Gastroenterology. 111:492–515. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY,
Park CS, So I, Stanfield PR and Kim KW: Melastatin-type transient
receptor potential channel 7 is required for intestinal pacemaking
activity. Gastroenterology. 129:1504–1517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JH, Kim SY, Kwon YK, Kim BJ and So I:
Characteristics of the cholecystokinin-induced depolarization of
pacemaking activity in cultured interstitial cells of Cajal from
murine small intestine. Cell Physiol Biochem. 31:542–554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim BJ, Kim SY, Lee S, Jeon JH, Matsui H,
Kwon YK, Kim SJ and So I: The role of transient receptor potential
channel blockers in human gastric cancer cell viability. Can J
Physiol Pharmacol. 90:175–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim BJ, Kim HW, Lee GS, Choi S, Jun JY, So
I and Kim SJ: Poncirus trifoliate fruit modulates pacemaker
activity in interstitial cells of Cajal from the murine small
intestine. J Ethnopharmacol. 149:668–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koh SD, Jun JY, Kim TW and Sanders KM: A
Ca2+-inhibited non-selective cation conductance
contributes to pacemaker currents in mouse interstitial cell of
Cajal. J Physiol. 540:803–814. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BJ, So I and Kim KW: The relationship
of TRP channels to the pacemaker activity of interstitial cells of
Cajal in the gastrointestinal tract. J Smooth Muscle Res. 42:1–7.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huizinga JD, Zhu Y, Ye J and Molleman A:
High-conductance chloride channels generate pacemaker currents in
interstitial cells of Cajal. Gastroenterology. 123:1627–1636. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hwang SJ, Blair PJ, Britton FC, O'Driscoll
KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM and Ward
SM: Expression of anoctamin 1/TMEM16A by interstitial cells of
Cajal is fundamental for slow wave activity in gastrointestinal
muscles. J Physiol. 587:4887–4904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh
SD and Sanders KM: A Ca2+-activated Cl(−) conductance in
interstitial cells of Cajal linked to slow wave currents and
pacemaker activity. J Physiol. 587:4905–4918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Na JR, Oh KN, Park SU, Bae D, Choi EJ,
Jung MA, Choi CY, Lee DW, Jun W, Lee KY, et al: The laxative
effects of Maesil (Prunus mume Siebold & Zucc.) on constipation
induced by a low-fibre diet in a rat model. Int J Food Sci Nutr.
64:333–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. National Academies Press (US);
Washington (DC): 2011, PubMed/NCBI
|
|
32
|
Huizinga JD, Chang G, Diamant NE and
El-Sharkawy TY: Electrophysiological basis of excitation of canine
colonic circular muscle by cholinergic agents and substance P. J
Pharmacol Exp Ther. 231:692–699. 1984.PubMed/NCBI
|
|
33
|
Inoue R and Chen S: Physiology of
muscarinic receptor operated nonselective cation channels in
guinea-pig ilieal smooth muscle. EXS. 66:261–268. 1993.PubMed/NCBI
|
|
34
|
Epperson A, Hatton WJ, Callaghan B,
Doherty P, Walker RL, Sanders KM, Ward SM and Horowitz B: Molecular
markers expressed in cultured and freshly isolated interstitial
cells of Cajal. Am J Physiol Cell Physiol. 279:C529–C539.
2000.PubMed/NCBI
|
|
35
|
Komori S, Kawai M, Takewaki T and Ohashi
H: GTP-binding protein involvement in membrane currents evoked by
carbachol and histamine in guinea-pig ileal muscle. J Physiol.
450:105–126. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ogata R, Inoue Y, Nakano H, Ito Y and
Kitamura K: Oestradiol-induced relaxation of rabbit basilar artery
by inhibition of voltage-dependent Ca channels through GTP-binding
protein. Br J Pharmacol. 117:351–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ward SM, Ordog T, Koh SD, Baker SA, Jun
JY, Amberg G, Monaghan K and Sanders KM: Pacemaking in interstitial
cells of Cajal depends upon calcium handling by endoplasmic
reticulum and mitochondria. J Physiol. 525:2355–361. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yagle K, Lu H, Guizzetti M, Möller T and
Costa LG: Activation of mitogen-activated protein kinase by
muscarinic receptors in astroglial cells: Role in DNA synthesis and
effect of ethanol. Glia. 35:111–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sakai H, Otogoto S, Chiba Y, Abe K and
Misawa M: Involvement of p42/44 MAPK and RhoA protein in
augmentation of ACh-induced bronchial smooth muscle contraction by
TNF-alpha in rats. J Appl Physiol (1985). 97:2154–2159. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nam JH, Kim WK and Kim BJ: Sphingosine and
FTY720 modulate pacemaking activity in interstitial cells of Cajal
from mouse small intestine. Mol Cells. 36:235–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim BJ, Nam JH, Kim KH, Joo M, Ha TS, Weon
KY, Choi S, Jun JY, Park EJ and Wie J: Characteristics of
gintonin-mediated membrane depolarization of pacemaker activity in
cultured interstitial cells of Cajal. Cell Physiol Biochem.
34:873–890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim BJ, Kim H, Lee GS, So I and Kim SJ:
Effects of San-Huang-Xie-Xin-tang, a traditional Chinese
prescription for clearing away heat and toxin, on the pacemaker
activities of interstitial cells of Cajal from the murine small
intestine. J Ethnopharmacol. 155:744–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahn TS, Kim DG, Hong NR, Park HS, Kim H,
Ha KT, Jeon JH, So I and Kim BJ: Effects of Schisandra chinensis
extract on gastrointestinal motility in mice. J Ethnopharmacol.
169:163–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garrington TP and Johnson GL: Organization
and regulation of mitogen-activated protein kinase signaling
pathways. Curr Opin Cell Biol. 11:211–218. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Derkinderen P, Enslen H and Girault JA:
The ERK/MAP-kinases cascade in the nervous system. Neuroreport.
10:R24–R34. 1999.PubMed/NCBI
|
|
46
|
Lukacs NW, Strieter RM, Chensue SW, Widmer
M and Kunkel SL: TNF-alpha mediates recruitment of neutrophils and
eosinophils during airway inflammation. J Immunol. 154:5411–5417.
1995.PubMed/NCBI
|
|
47
|
Nathanson NM: A multiplicity of muscarinic
mechanisms: Enough signaling pathways to take your breath away.
Proc Natl Acad Sci USA. 97:6245–6247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Slack BE: The M3 muscarinic acetylcholine
receptor is coupled to mitogen-activated protein kinase via protein
kinase C and epidermal growth factor receptor kinase. Biochem J.
348:2381–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yagle K, Lu H, Guizzetti M, Möller T and
Costa LG: Activation of mitogen-activated protein kinase by
muscarinic receptors in astroglial cells: Role in DNA synthesis and
effect of ethanol. Glia. 35:111–120. 2001. View Article : Google Scholar : PubMed/NCBI
|