Introduction
Pterygium surgery has changed over the past decade,
and several techniques are currently available to the ophthalmic
surgeon. Conjunctival autograft (CA) and CA combined with
adjunctive mitomycin C (MMC) are commonly performed procedures for
the treatment of primary and recurrent pterygium (1–3).
Scleral necrosis/melting is a rare complication that
may develop following pterygium surgery. The possible pathological
mechanisms underlying scleral necrosis following pterygium surgery
include infection, hypersensitivity response and ischemia; the
latter may serve an important role, and the use of adjunctive MMC
may increase its likelihood (4).
Treatments include the use of antibiotics, systemic
immunosuppressive drugs and surgical repair of the scleral defect
with a variety of graft materials. In a number of studies, high
doses of systemic immunosuppressive therapy are described as being
necessary for the arrest of the progression of the melting process
(4–7), although they have a number of side
effects.
In the current study, personalized treatment
tailored to the pathogenesis was used as an alternative to high
doses of systemic immunosuppressive therapy. The present study
aimed to investigate the efficacy of tailored treatment for the
management of scleral necrosis following pterygium surgery. Herein,
nine cases of scleral necrosis following pterygium excision are
presented and their pathogenesis and management are discussed.
Subjects and methods
Study design
This retrospective study was performed according to
the principles of the Declaration of Helsinki, informed consent was
obtained from the patients, and the Ninth People's Hospital,
Shanghai JiaoTong University School of Medicine Ethics Committee
(Shanghai, China) approved the study. A series of nine cases of
scleral necrosis following pterygium excision between September
2009 and September 2012 were evaluated retrospectively. The
patients had undergone nasal or temporal pterygium excision in a
number of local hospitals, and were recommended to the Department
of Ophthalmology, Ninth People's Hospital, by their primary
physicians.
Participants
All the individuals included in the present study
underwent a comprehensive ophthalmologic examination and routine
diagnostic evaluation; initial treatment and the degree of scleral
necrosis were recorded.
Laboratory evaluations were performed to search for
an inflamed, autoimmune or infectious etiology of the necrotizing
scleritis. These laboratory evaluations included the following:
Scrapes from the surface of the graft sent for smear and culture
examination; routine blood [blood cell count, erythrocyte
sedimentation rate (ESR), C-reactive protein [CRP] expression level
and serum uric acid estimation]; extensive blood tests for
autoimmune diseases (rheumatoid factor, antinuclear antibodies,
anti-DNA antibodies, antinuclear antigen antibodies, and complement
and thyroid antibodies); serology (human immunodeficiency virus 1
and 2, varicella-zoster virus, hepatitis B virus, hepatitis C
virus, Venereal Disease Research Laboratory test and Treponema
pallidum haemagglutination assay); chest radiography; and
Mantoux test for tuberculosis.
Scleral melt severity was categorized as follows: A,
scleral thinning without corneal melting; B, scleral thinning with
corneal melting; and C, scleral thinning with uveal exposure.
Treatments were based on ophthalmic examination,
medical history of the patients and laboratory results.
Surgical technique
The surgery was performed under peribulbar
anesthesia [0.75% bupivacaine (Sensorcaine; Harvest Pharmaceutical
Co., Ltd.); 2% lidocaine (Xylocaine; Harvest Pharmaceutical Co.,
Ltd.)]. Surgical techniques were chosen on the basis of the degree
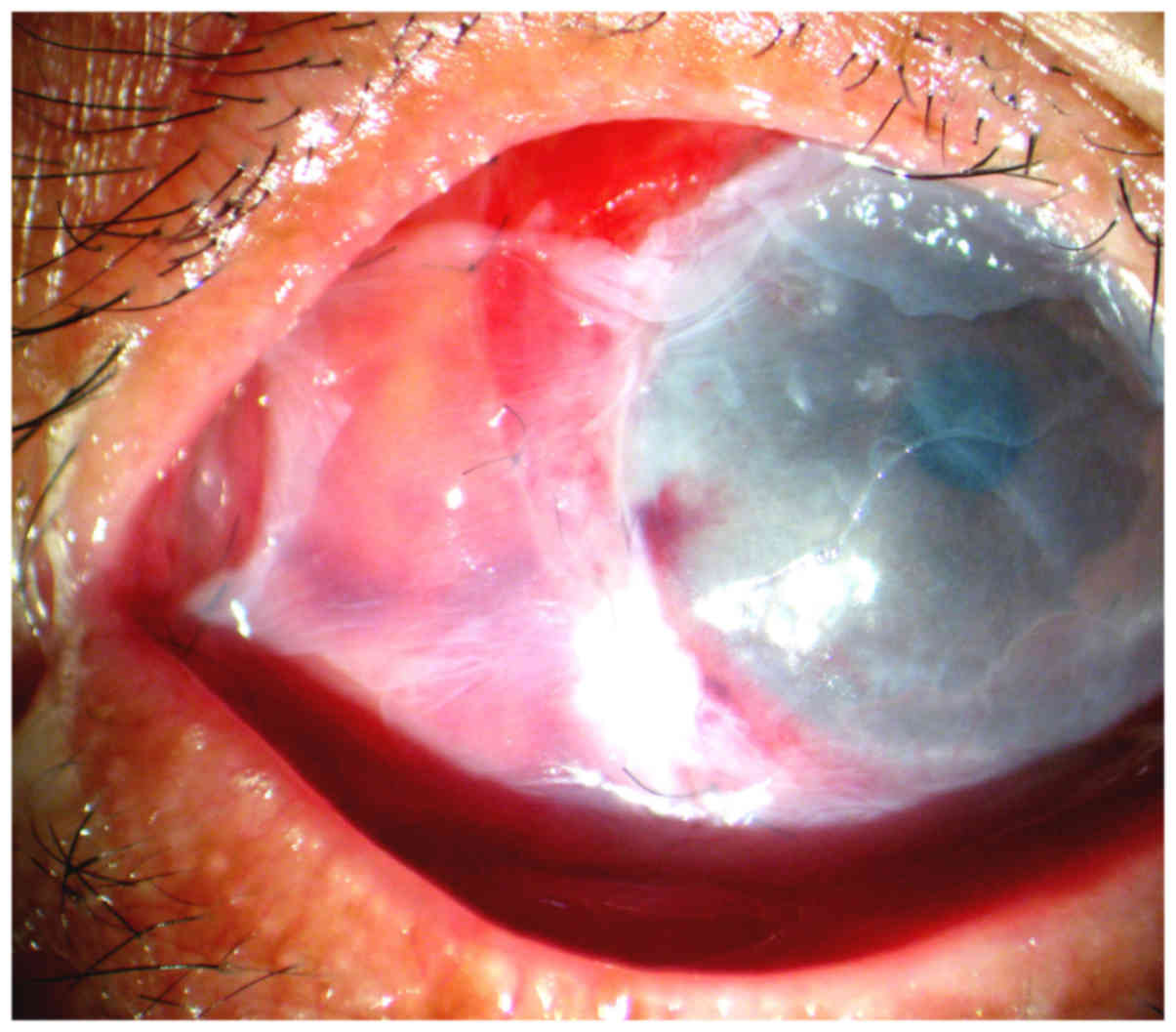
of scleral melting, and included Tenon's membrane covering (TMC)
combined with amniotic membrane transplantation (AMT) and a
corneoscleral patch graft (CPG) combined with AMT.
For TMC, the conjunctiva, Tenon's membrane and
episcleral tissue were dissected carefully to expose the defect in
the sclera. All devitalized, infected and necrotic soft tissue was
debrided and the adjacent unaffected tissue was preserved.
Subsequently, Tenon's membrane was dissected around the lesion area
and extended along the surface of the globe to cover the lesion
completely, followed by closure. If the adjacent Tenon's membrane
could not be mobilized, a rotational Tenon's membrane autograft was
used. Finally, the area was covered with an amniotic membrane
(Fig. 1).
For CPG, the donor sclera was soaked three times in
Ringer's lactate solution (Minsheng Pharmaceutical Group Co., Ltd.,
Hangzhou, China) for 10 min, in betadine (Yongan Pharmaceutical
Group Co., Ltd., Chengdu, China) for 10 min and finally in 20 mg/ml
gentamicin solution (Yaoyou Pharmaceutical Group Co., Ltd.,
Chongqing, China) for 10 min, followed by shaping to fit the defect
area after all devitalized and necrotic tissue had been debrided.
The graft was then secured to the edges of the resection site using
8–0 Vicryl sutures on the scleral side and 10–0 nylon sutures on
the corneal side (if required). The repaired sclera was covered
with an amniotic membrane graft.
For AMT, following TMC or CPG, the corneoscleral
patch or Tenon's membrane patch was covered with amniotic membrane,
with the stromal side facing down; 10/0 monofilament nylon sutures
were used to suture the surrounding conjunctiva.
Medication
Postoperatively, all patients were administered
topical 0.1% dexamethasone (4 times daily; Alcon, Fort Worth, TX,
USA), 0.3% ciprofloxacin (4 times daily; Wujing Pharmaceutical Co.,
Ltd., Wuhan, China) and artificial tears (6 times daily; Alcon) for
1 month. Systemic immunosuppressive therapy, oral prednisolone (30
mg/day for 2 weeks; Lijun Pharmaceutical Co., Ltd.), was
administered prior to tapering of the dosage; however, if
laboratory results confirmed the existence of immune dysfunction,
prednisolone (60 mg/day) was used as the starting dose. Low-dose
maintenance corticosteroid therapy (5 mg/day prednisolone) was
required for 3 months. Antimicrobial treatment (0.3% ciproflaxin)
was administered according to the results of antimicrobial
sensitivity testing.
Results
Patient clinical features
Nine patients (four male and five female) were
included in this study. The right eye was affected in five
patients, and the left eye was affected in four. Their age ranged
between 41 and 71 years old, with an average age of 57 years.
Six patients had undergone uneventful pterygium
surgery with conjunctival autografting (CA) combined with MMC
(0.02% MMC for 2 min). Case nos. 6, 7 and 9 had undergone nasal
pterygium excision with CA, but did not have adjunctive MMC
intraoperatively. All patients had presented with severe pain and
redness in the eye ~2–8 weeks postoperatively. In the local
hospital, all patients had received administration of steroid and
antibiotic eye drops, two patients had accepted oral nonsteroidal
anti-inflammatory drugs, six had undergone AMT, and one was
administered oral prednisone at 10 mg for 5 days. However, all
these methods were unable to arrest the progression of the
necrotizing process. Three patients presented with degree A of
scleral necrosis; the underlying sclera was necrotic, avascular and
thinning, but with no evidence of active corneal melting (case nos.
2,4 and 5). Four patients presented with degree B of scleral
necrosis (case nos. 1,3,6 and 7) and two presented with degree C
scleral necrosis (case nos. 8 and 9).
Four patients with negative past medical histories
denied fever, night sweats, weight loss, malaise, joint pains, skin
rashes and headaches. Three patients had hypertension. The
remaining two patients had a significant past medical history; one
was a 52-year-old male who had undergone repeated chemotherapy and
radiotherapy because of lung cancer recurrence (case no. 8), and
the other was a 54-year-old female with a medical history of
rheumatoid arthritis (case no. 9).
Laboratory evaluation
Laboratory evaluation revealed that case no. 9 had a
high CRP level (CRP, 24 mg/l; normal levels, <10 mg/l), an ESR
of 90 mm/h (normal levels, <20 mm/h in females or <15 mm/h in
males), rheumatoid factor (+) and anti-nuclear antigen antibodies
(+). In addition, case no. 7 had a high ESR (65 mm/h).
Microbiologic studies for case no. 2 revealed the presence of
Staphylococcus epidermidis, which is typically a saprophyte
of the conjunctiva; in vitro antimicrobial testing showed
that this strain was multiresistant but was sensitive to
ciprofloxacin. The other patients showed no laboratory
abnormalities, and the the microbiological investigations of case
nos. 6, 7 and 9 were negative. These parameters were tabulated,
summarized and analyzed accordingly (Table I).
 | Table I.Summary of patient data. |
Table I.
Summary of patient data.
|
|
|
|
|
|
|
|
|
|
| Current
intervention |
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
| Scleral necrosis |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Patient no./age,
y | Medical Gender | Eye | history | surgery | Degree | Onset | Previous
intervention | Laboratory
evaluations | Last follow up
(months) | Surgery | Medication +
duration |
|---|
| 1/41 | M | L | None | CA+MMC | B | 4 w | AMT | – | 12 | TMC+AMT | Rt |
| 2/50 | F | L | None | CA+MMC | A | 2 w | N | Staphylococcus
epidermidis (+) | 12 | TMC+AMT | 0.3% Ciprofloxacin 6
times daily |
| 3/53 | M | R | None | CA+MMC | B | 8 w | AMT | – | 14 | TMC+AMT | Rt |
| 4/60 | F | L | Hypertension | CA+MMC | A | 3 w | NSAIDS | – | 15 | TMC+AMT | Rt |
| 5/63 | F | R | None | CA+MMC | A | 4 w | N | – | 12 | TMC+AMT | Rt |
| 6/68 | F | L | Hypertension | CA | B | 4 w | AMT | – | 13 | TMC+AMT | Oral pred 30 mg/d
for 2 wks then taperedb |
| 7/71 | M | R | Hypertension | CA | B | 5 w | Prednisone 10 mgx5
d+AMT | ESR=65mm/h | 15 | TMC+AMT | Oral pred 60 mg/d
for 2 wks then taperedb |
|
| 8/52 | M | R | Lung cancer | CA+MMC | C | 2 w | NSAIDS+AMT | – | 16 | CPG+AMT | Oral pred 30 mg/d
for 2 wks then taperedb |
| 9/54 | F | R | Rheumatoid
arthritis | CA | C | 4 w | AMT | CRP=24mg/ESR
=90mm/h RF (+) anti-nuclear antigen antibodies (+) | 17 | CPG+AMT | Oral pred with
methotrexatea |
Current intervention
Case nos. 1–7 received TMC combined with AMT; among
them, case nos. 1–5 received no systemic immunosuppressive therapy,
case no. 6 was administered oral prednisolone at 30 mg/day for 2
weeks followed by tapering postoperatively, and case no. 7 was
administered oral prednisolone 60 mg/day because of his high ESR.
For case nos. 8 and 9, because of the marked scleral thinning and
uveal exposure, a CPG procedure was performed in combination with
AMT. In addition, case no. 8 was administered oral prednisolone at
30 mg/day for 2 weeks before tapering, and case no. 9 was
administered 60 mg/day for 2 weeks because of their history of
rheumatoid arthritis and high CRP and ESR. Antibiotic treatment for
case no. 2 comprised 0.3% ciprofloxacin 6 times daily.
Postoperative results
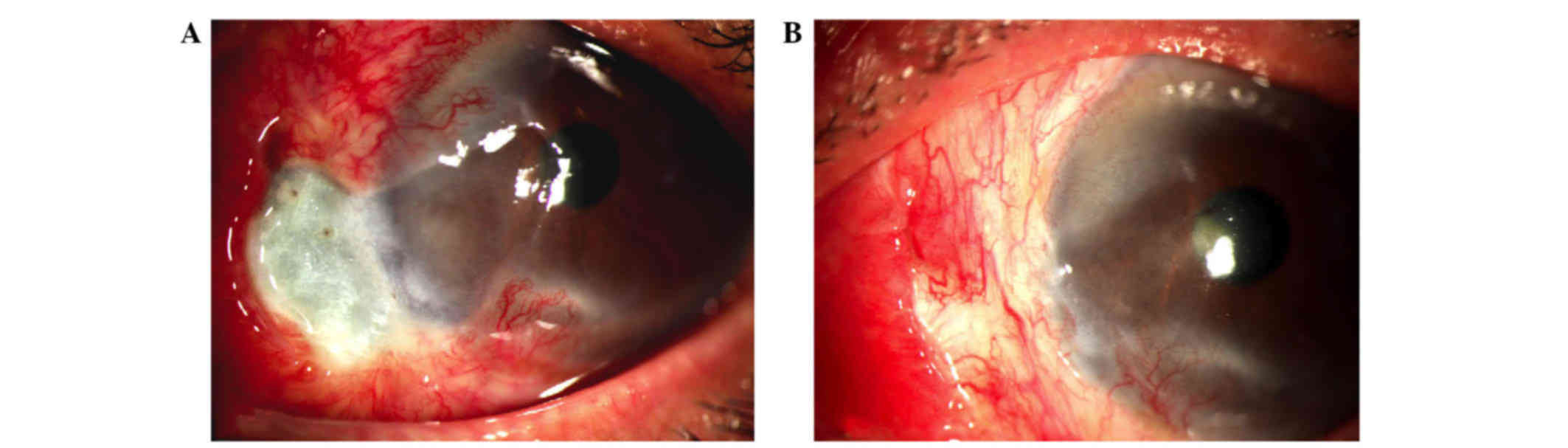
All patients experienced uncomplicated postoperative
courses except case no. 9. In the patients, the scleral necrosis
disappeared progressively within 3–8 weeks accompanied with
normalization of the sclera, conjunctival reepithelialization and
neoangiogenesis (Fig. 2). There were
no relapses over a period of one-year follow-up.
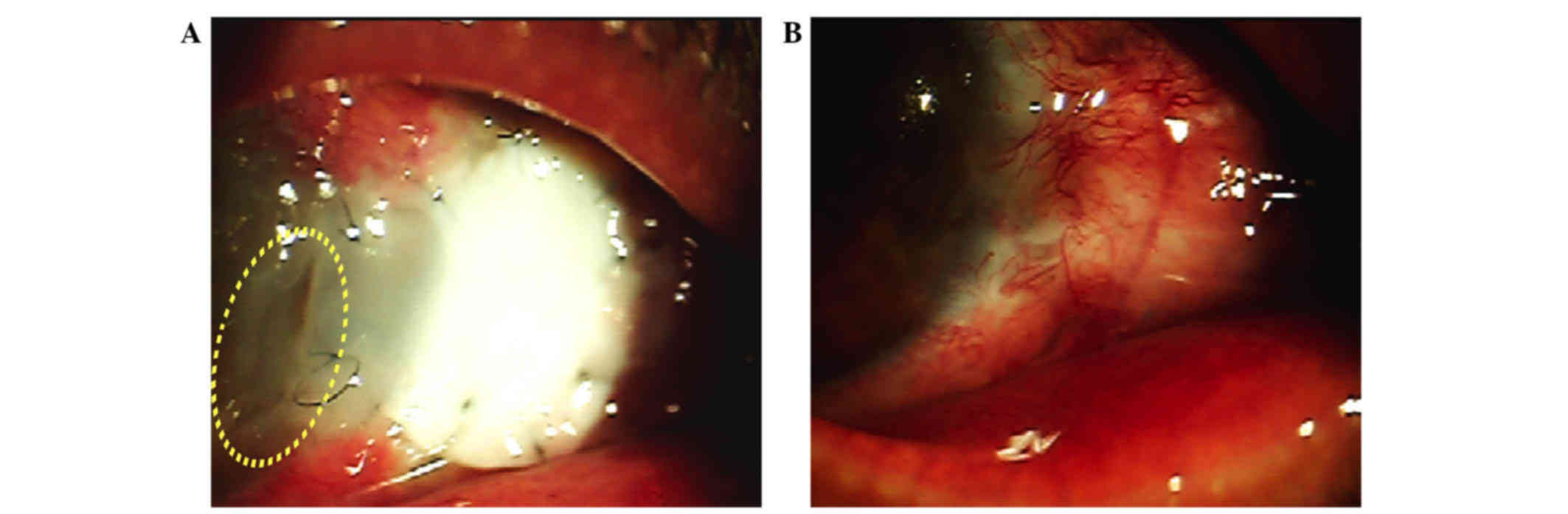
In case no. 9, ~2 weeks after surgery, the corneal
margin of the graft started to melt and the remainder of the graft
appeared softened but intact over the defect, without uveal
exposure (Fig. 3A). At this stage,
following rheumatology consultation, the prednisolone dose was
increased to 80 mg/day for 3 weeks, and then tapered gradually to
the maintenance dose (8 mg/day) combined with oral methotrexate
(7.5 mg/week; Xinyi, Shanghai, China) and topical cyclosporine
(0.5% twice daily; Novartis International AG, Basel, Switzerland)
for 12 months. The area of scleral necrosis resolved after 8 weeks;
meanwhile, the perforated area was sealed with vascularized
epithelialization (Fig. 3B). The
immunosuppressive drug doses remained unchanged during the one-year
follow-up period, and no relapses occurred.
Discussion
Scleral necrosis is a rare but severe form of
scleritis, which threatens the vision and integrity of the eye. It
has been reported following all types of ocular surgery, but most
commonly following pterygium excision (4,5,8). Postoperative infection is one possible
reason for the occurrence of scleritis following surgery. However,
as with many reported cases described in the literature, smear
examinations and cultures were negative in this study, except for
case no. 2, whose microbiologic studies revealed the presence of
Staphylococcus epidermidis which is typically a saprophyte
of the conjunctiva. A hypersensitivity response to surgical trauma
and scleral ischemia resulting from disruption of episcleral
vasculature and manipulation of the previous surgical scar may be
possible alternative explanations (9). Various methods have been proposed for
the treatment of noninfectious scleral necrosis, but there is no
consensus in the medical literature regarding its appropriate
management. As described in the literature, besides reinforcement
of the thinning or perforated sclera (9), high doses of systemic immunosuppressive
therapy are typically recommended (7,10–12).
In the present study, case nos. 1–5 accepted the
administration of 0.02% MMC intraoperatively to prevent recurrence.
MMC is an antineoplastic antibiotic alkylating agent that
selectively inhibits DNA replication by forming covalent linkages
with guanosine residues in DNA (2,13);
therefore, preventing proliferation of Tenon's fibroblasts
(2). However, the delay in wound
healing results in vascular compromise to the surgical site, and
this, coupled with the permanent inhibition of fibroblast
proliferation, exposes the sclera to avascular necrosis (14). This may be one explanation for the
cases in the present study experiencing scleral necrosis. Excessive
cauterization and conjunctival graft inversion during surgery are
other possible causes.
As described above, ischemia is likely to be
responsible for the occurrence of scleral necrosis. Therefore, if
provision of vascular tissue to cover the defect can optimize the
conditions for repair of the sclera, and allow the maintenance of
blood flow to the grafted area, then relatively unaggressive
therapy may be sufficient. Two options have been proposed to deal
with this situation; the tarsoconjunctival pedicle flap (12) and conjunctiva-Müller muscle pedicle
flap (15), but these methods are
complicated and may cause problems such as ptosis, retraction of
the eyelid, fornix shortening and eyelid margin deformities.
According to the basic surgical principle of ‘simpler is better’, a
Tenon's membrane flap is preferable to cover the lesion before the
conventional procedure of scleral repair. The loose connective
tissue of Tenon's capsule is highly vascularized; these vessels
show a specialized morphology characterized by numerous
arteriovenous anastomoses, and a muscle-rich venous network. Good
ductility and good blood supply mean that the Tenon's membrane is
suitable for repair of defects. In the present study, without any
systemic immunosuppressive therapy, case nos. 1–5 had good
postoperative courses, which confirms the feasibility of this
method.
Reinforcement of the sclera is important,
particularly when the uveal tract is exposed, to prevent prolapse
of ocular contents and secondary infection. Amniotic membrane
grafts and corneoscleral patch grafts are tissues commonly used for
scleral reconstruction. In the present study, seven patients
received TMC combined with AMT, but two patients with a risk of
uveal exposure received CPG combined with AMT. Use of the amniotic
membrane has a number of advantages, such as its easy availability,
nonantigenic nature and lack of risk of immunologic rejection,
which makes it an excellent graft and a substrate for ocular
surface reconstruction (16). In
addition, amniotic membrane contains many growth factors, promotes
reepithelialization, and reduces fibrosis and inflammation, which
benefit epithelialization. Covering the corneoscleral patch graft
or Tenon's membrane with AMT is important, because it may help the
ocular surface to reepithelialize rapidly to achieve a viable
graft.
It is noteworthy that three of the patients in the
present study developed scleral necrosis without the application of
adjunctive MMC or intraoperative irradiation, and microbiological
investigations were negative. Given the absence of these potential
causes, the diagnosis of surgically induced necrotizing scleritis
(SINS) was applied. Classical SINS is thought to be a delayed-type
hypersensitivity response whereby mild surgical trauma or ischemia
exposes tissue antigens, to which the immune system becomes
sensitized (5,17,18).
Such hypotheses are supported by the success of systemic
immunosuppressive therapy (5,17,18),
the presence of immune complexes in episcleral vessel walls
(17,19) and the high prevalence of vasculitis
among such patients (4,18,20–22).
In the present study, laboratory evaluation showed
that case no. 9 had a high level of CRP and high ESR, and was
positive for rheumatoid factor and antinuclear antigen antibodies.
Case no. 7 had high ESR, suggesting the presence of immune
dysfunction. In case no. 6, although there were no obvious
abnormalities identified in extensive blood tests for autoimmune
diseases, SINS was suspected because of the absence of the common
pathogenetic mechanisms described above. For these patients with
suspected SINS, systemic immunosuppressive therapy was administered
based on the severity of the illness. Case no. 6 was administered
oral prednisolone at 30 mg/day for 2 weeks before the symptoms and
signs allowed postoperative tapering, and cases no. 7 and 9 were
administered prednisolone at 60 mg/day for 2 weeks. In case nos. 6
and 7, besides a hypersensitivity reaction, the necrosis was
attributable to the fragile vasculature attributable to their age
and the presence of hypertension; therefore, TMC was applied to
improve the blood supply. Although case no. 8 had received MMC
previously, his rapid and severe disease progression suggested the
presence of a hypersensitivity reaction, and therefore was
administered prednisolone at 30 mg/day. All the patients had
uncomplicated postoperative courses except case no. 9. Case no. 9,
a patient with rheumatoid arthritis, developed graft necrosis
within 2 weeks following the scleral patch graft. In this
particular case, the graft was covered with amniotic membrane that
had retracted within 5 days, which may have delayed the
epithelialization of the graft. Furthermore, the conjunctiva and
Tenon's membrane were severely affected by the vasculitic process
and they may not have provided sufficient vascular supply to the
graft (5,15). The current recommendation for this
situation is the concomitant use of systemic immunosuppressive
drugs, which reduces the severity of vasculitis and increases the
chance of survival of the patch graft in such patients (4,9). In this
case, the high level of ESR and CRP and the melting graft
postoperatively indicated the presence of active scleritis.
Following consultation with a rheumatologist, more aggressive
immunosuppressive therapy was adopted, which prevented the graft
from melting.
Limitations of the present study included the
relatively small number of patients and the retrospective data
collection, primarily because of its infrequent collection
following pterygium surgery. In addition, delayed type
hypersensitivity and SINS are possible explanations for the cases
described. Therefore, further observations and research concerning
the underlying mechanisms of pathogenesis are required.
In conclusion, the possible pathogenetic mechanisms
underlying scleral necrosis following surgery include infection, a
hypersensitivity response and ischemia. By using different
treatments tailored to the pathogenesis of a particular case, the
present study was able to use relatively unaggressive therapy. For
scleral necrosis caused by ischemia, Tenon's membrane flap may be a
simple and safe treatment that avoids the need for high-dose
immunosuppression, while for those with suspected SINS or infected
cases, high-dose immunosuppression or appropriate antibiotics are
necessary.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170876, 31271029
and 81320108010) and the Science and Technology Commission of
Shanghai (grant nos. 13ZR1423600 and 12ZR1417300).
Glossary
Abbreviations
Abbreviations:
|
CA
|
conjunctival autograft
|
|
MMC
|
mitomycin C
|
|
ESR
|
erythrocyte sedimentation rate
|
|
CRP
|
C-reactive protein
|
|
NSAID
|
nonsteroidal anti-inflammatory
drug
|
|
TMC
|
Tenon's membrane covering
|
|
AMT
|
amniotic membrane transplantation
|
|
CPG
|
corneoscleral patch graft
|
|
SINS
|
surgically induced necrotizing
scleritis
|
References
|
1
|
Kheirkhah A, Hashemi H, Adelpour M, Nikdel
M, Rajabi MB and Behrouz MJ: Randomized trial of pterygium surgery
with mitomycin C application using conjunctival autograft versus
conjunctival-limbal autograft. Ophthalmology. 119:227–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Díaz L, Villegas VM, Emanuelli A and
Izquierdo NJ: Efficacy and safety of intraoperative mitomycin C as
adjunct therapy for pterygium surgery. Cornea. 27:1119–1121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandes M, Sangwan VS, Bansal AK,
Gangopadhyay N, Sridhar MS, Garg P, Aasuri MK, Nutheti R and Rao
GN: Outcome of pterygium surgery: Analysis over 14 years. Eye
(Lond). 19:1182–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain V, Shome D, Natarajan S and Narverkar
R: Surgically induced necrotizing scleritis after pterygium surgery
with conjunctival autograft. Cornea. 27:720–721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morley AM and Pavesio C: Surgically
induced necrotising scleritis following three-port pars plana
vitrectomy without scleral buckling: A series of three cases. Eye
(Lond). 22:162–164. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de la Maza M Sainz, Molina N,
Gonzalez-Gonzalez LA, Doctor PP, Tauber J and Foster CS: Scleritis
therapy. Ophthalmology. 119:51–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young AL, Wong SM, Leung AT, Leung GY,
Cheng LL and Lam DS: Successful treatment of surgically induced
necrotizing scleritis with tacrolimus. Clin Experiment Ophthalmol.
33:98–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamazoe K, Shimazaki-Den S, Otaka I, Hotta
K and Shimazaki J: Surgically induced necrotizing scleritis after
primary pterygium surgery with conjunctival autograft. Clin
Ophthalmol. 5:1609–1611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sangwan VS, Jain V and Gupta P: Structural
and functional outcome of scleral patch graft. Eye (Lond).
21:930–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gregory ME, Weir CR and Ramaesh K:
Excision of granulation tissue and free conjunctival autograft in
the management of necrotizing scleritis. Cornea. 29:577–579. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rachitskaya A, Mandelcorn ED and Albini
TA: An update on the cause and treatment of scleritis. Curr Opin
Ophthalmo. 21:463–467. 2010. View Article : Google Scholar
|
|
12
|
Davidson RS, Erlanger M, Taravella M,
Gregory DG and Durairaj VD: Tarsoconjunctival pedicle flap for the
management of a severe scleral melt. Cornea. 26:235–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dadeya S and Fatima S: Comeoscleral
perforation after pterygium excision and intraoperative mitomycin
C. Ophthalmic Surg Lasers Imaging. 34:146–148. 2003.PubMed/NCBI
|
|
14
|
Solomon A, Kaiserman I, Raiskup FD, Landau
D and Frucht-Pery J: Long-term effects of mitomycin C in pterygium
surgery on scleral thickness and the conjunctival epithelium.
Ophthalmology. 111:1522–1527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yazici B: Use of conjunctiva-Müller muscle
pedicle flap in surgical treatment of necrotizing scleritis.
Ophthal Plast Reconstr Surg. 24:19–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim BH: Surgical treatment of necrotic
scleral calcification using combined conjunctival autografting and
an amniotic membrane inlay filling technique. Eye (Lond).
25:1484–1490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Díaz-Valle D, Benítez del Castillo JM,
Castillo A, Sayagués O, Bañares A and García-Sánchez J: Immunologic
and clinical evaluation of postsurgical necrotizing sclerocorneal
ulceration. Cornea. 17:371–375. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Donoghue E, Lightman S, Tuft S and
Watson P: Surgically induced necrotising sclerokeratitis
(SINS)-precipitating factors and response to treatment. Br J
Ophthalmol. 76:17–21. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fong LP, de la Maza M Sainz, Rice BA,
Kupferman AE and Foster CS: Immunopathology of scleritis.
Ophthalmology. 98:472–479. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh RP and McCluskey P: Scedosporium
prolificans sclerokeratitis 10 years after pterygium excision with
adjunctive mitomycin C. Clin Experiment Ophthalmol. 33:433–434.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai T, Leibovitch I, Zadeh R, Chehade M,
Tamblyn D and Selva D: Surgically induced necrotizing scleritis
occurring 48 years after strabismus surgery. J Pediatr Ophthalmol
Strabismus. 42:180–182. 2005.PubMed/NCBI
|
|
22
|
Akpek EK, Thorne JE, Qazi FA, Do DV and
Jabs DA: Evaluation of patients with scleritis for systemic
disease. Ophthalmology. 111:501–506. 2004. View Article : Google Scholar : PubMed/NCBI
|