Introduction
The spine is perhaps the most important component of
the human skeletal system, as weight, impact or pressure in any
area of the body is applied to it (1). Spinal cord injuries most frequently
occur in thoracic vertebra 12 to lumbar vertebra 1 of the spine in
young adults, accounting for 4.3% of all body fractures (1). Thoracolumbar spine compression
fractures account for >90% of all spinal lord fractures and are
one of the primary causes of long-term chronic lumbar spinal pain,
which severely impacts the quality of life of those suffering from
it (2). Furthermore, traumatic
spinal cord fracture and dislocation may be fatal (3), with the majority of patients
experiencing sensory disturbance of limbs and incontinence
(4). Symptoms of paralysis following
spinal cord injuries are difficult to recover from and require
adequate and appropriate nursing, posing a challenge for clinical
treatment (5).
In spinal cord injuries induced by compression
fracture, changes in the expression of microRNAs (miR) and mRNAs
have been detected; therefore, they may be beneficial in the
clinical diagnosis and treatment of spinal cord injuries (6,7).
Transforming growth factor (TGF)-β1 is a member of the TGF
superfamily, which is generated by various types of cells. In the
bones, TGF-β1 is produced by osteocytes, osteoblasts, osteoclasts
and chondrocytes. TGF-β1 produced by osteoblasts is immediately
bound to the bone matrix (8). It has
been demonstrated that TGF-β1, which is a potent chemokine,
stimulates the synthesis of collagen. TGF-β1 increases the growth
of extracellular bone matrix and has important regulatory effects
on the formation of bones and cartilage (9). During early repair of spinal cord
injuries, endogenous TGF-β1 expression is rapidly upregulated in
the spinal cord, exerting its effect by activating glial cells and
phagocytes. As a result, connective tissues are formed,
angiogenesis is promoted, extracellular matrix is deposited and
collagen is synthesized (10–12).
These procedures serve important roles in the repair of nervous
tissue lesions. However, the effect of TGF-β1 in spinal cord
injuries induced by thoracolumbar spine compression fractures
remains unclear. In the present study, the expression of TGF-β1 and
miR-185, and the regulation of TGF-β1 by miR-185 were evaluated in
patients with spinal cord injuries induced by thoracolumbar spine
compression fractures.
Materials and methods
Patients
A total of 44 patients with spinal cord injuries
induced by thoracolumbar spine compression fractures, hospitalized
at Luoyang Orthopedic-Traumatological Hospital between June 2012
and February 2015, were enrolled in the present study. Among them,
18 patients underwent surgery between 1 and 7 days post-fracture
(Group A), and 26 underwent surgery between 8 and 14 days
post-fracture (Group B). These patients had no prior history of
spinal cord injury and had no history of receiving hormonal
treatment, traditional Chinese medicine or radiotherapy. Among the
44 patients, 20 had thoracic spine compression fractures, 18 had
lumbar spine compression fractures and 6 had thoracolumbar spine
compression fractures. Regarding the reasons for injury, high
falling caused eight cases of thoracic spine compression fractures,
nine cases of lumbar spine compression fractures and three cases of
thoracolumbar spine compression fractures; traffic accidents caused
ten cases of thoracic compression fractures, eight cases of lumbar
compression fractures and one case of thoracolumbar spine
compression fracture; extreme blunt trauma caused two cases of
thoracic spine compression fractures, one case of lumbar spine
compression fracture and one case of thoracolumbar spine
compression fracture. Three types of sample were harvested from
patients: i) Bone tissue at the fracture was harvested during
surgery and stored in liquid nitrogen at −80°C; ii) fasting
peripheral blood was harvested on the morning of the day of surgery
and subsequently stored in ethylene diamine tetraacetic acid tubes
at −20°C; iii) a total of 2 ml cerebrospinal fluid was harvested
during surgery, followed by centrifugation (1,500 × g) at
−4°C for 10 min, and was stored at −80°C. All procedures were
approved by the Ethics Committee of Luoyang
Orthopedic-Traumatological Hospital. Written informed consent was
obtained from all patients or their families.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (cat. no. 10606ES60; Yi Sheng
Biotechnology Co., Ltd., Shanghai, China) was used to extract total
RNA following the manufacturer's protocol. Ultraviolet
spectrophotometry (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) was used to determine the purity of RNA,
by measuring A260/A280. cDNA was subsequently obtained via reverse
transcription using the TIANScript II RT kit (cat. no. KR107;
Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturer's protocol. RT-qPCR was performed using iQ5 optical
system software (version 2.1; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) with SuperReal PreMix (SYBRGreen; cat. no. FP204; Tiangen
Biotech, Co., Ltd.) according to the manufacturer's protocol. The
primer sequences used were as follows: TGF-β1, forward
5′-GGACACCAACTATTGCTTCAG-3′ and reverse 5′-TCCAGACTCCAAATGTAG-3′;
β-actin forward 5′-TTCCAGCCTTCCTTCCTGG-3′ and reverse
5′-TTGCGCTCAGGAGGAGGAAT-3′. PCR amplification conditions were as
follows: Initial denaturation at 95°C for 2 min; 30 cycles of
denaturation at 94°C for 45 sec, annealing at 55°C for 55 sec and
elongation at 72°C for 1 min; final extension at 72°C for 10 min.
The 2−ΔΔCq method (13)
was used to calculate TGF-β1 levels, and β-actin was used as a
reference gene. Using online prediction websites, including miRanda
(http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org), PicTar
(http://pictar.mdc-berlin.de/) and
BibiServ (http://bibiserv.techfak.uni-bielefeld.de/), miR-185
was predicted to regulate TGF-β1. The forward primers for miR-185
and U6 small nuclear RNA (internal control) were
5′-TGGAGAGAAAGGCAGTTCCTGA-3′ and 5′-GCTTCGGCAGCACATATACTAAAAT-3′,
respectively. The common downstream primer for miR-185 and U6 was
5′-CGCTTCACGAATTTGCGTGTCAT-3′. PCR amplification conditions were as
follows: Initial denaturation at 95°C for 3 min; 40 cycles of
denaturation at 95°C for 12 sec, annealing at 62°C for 35 sec and
elongation at 62–95°C for 15 sec. The miRcute miRNA qPCR detection
kit (SYBR Green) was used to perform qPCR (cat. no. FP401; Tiangen
Biotech Co., Ltd.). The 2−ΔΔCq method (13) was used to calculate miR-185/U6
levels.
Western blotting
Proteins were extracted and a bicinchoninic acid
protein concentration determination kit [cat. no. RTP7102;
Real-Times (Beijing) Biotechnology Co., Ltd., Beijing, China] was
used to determine protein concentration. Protein samples (20 µg per
lane) were separated by 10% SDS-PAGE and resolved proteins were
subsequently transferred to polyvinylidene difluoride membranes on
ice (100 V, 2 h) and blocked for 1 h with 5% skimmed milk at room
temperature. Membranes were subsequently incubated at 4°C overnight
with polyclonal rabbit anti-human TGF-β1 primary antibody (1:500;
cat. no. ab92486; Abcam, Cambridge, MA, USA) and rabbit anti-human
β-actin primary antibody (1:5,000; cat. no. ab129348; Abcam).
Following extensive washing, the membranes were incubated with
polyclonal goat anti-rabbit secondary antibody conjugated with
horseradish peroxidase (1:3,000; cat. no. ab6721; Abcam) for 1 h at
room temperature. Membranes were developed using an enhanced
chemiluminescence detection kit (cat. no. FDO0142; Oddfoni
Biological Technology Co., Ltd., Nanjing, China) for imaging. To
acquire and analyze imaging signals, Image lab software (version
3.0; Bio-Rad) was used. The content of target protein was expressed
as a relative value against β-actin.
ELISA
Blood and cerebrospinal fluid underwent
determination of TGF-β1 concentration by ELISA according to the
manufacturer's protocol (TGF-β1 ELISA kit; cat. no. ab100674;
Abcam). Briefly, 50 µl control and 10 µl tissue samples were seeded
onto wells in an assay plate, followed by 40 µl sample dilution
reagent. Horseradish peroxidase-labeled antibody (100 µl) was
subsequently added to all wells except for the blank, followed by
incubation at 37°C for 1 h prior to five plate washes. Substrates
(50 µl) were added prior to incubation at 37°C for 15 min. Finally,
stop solution (50 µl) was added to each well and the determination
of optical density at 450 nm was performed within 15 min using a
Multiskan FC Microplate Photometer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Statistical analyses
Results were analyzed using SPSS version 18.0 (SPSS,
Inc., Chicago, IL, USA). The data were presented as mean ± standard
deviation. Multi-group measurements were subjected to one-way
analysis of variance. In cases of homogeneity of variance, least
significant difference and Student-Newman-Keuls methods were used;
in cases of heterogeneity of variance, Dunnett's T3 or Tamhane's T2
method was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of TGFβ1 mRNA in patients
with spinal cord injuries induced by thoracolumbar spine
compression fractures increases with time following injury
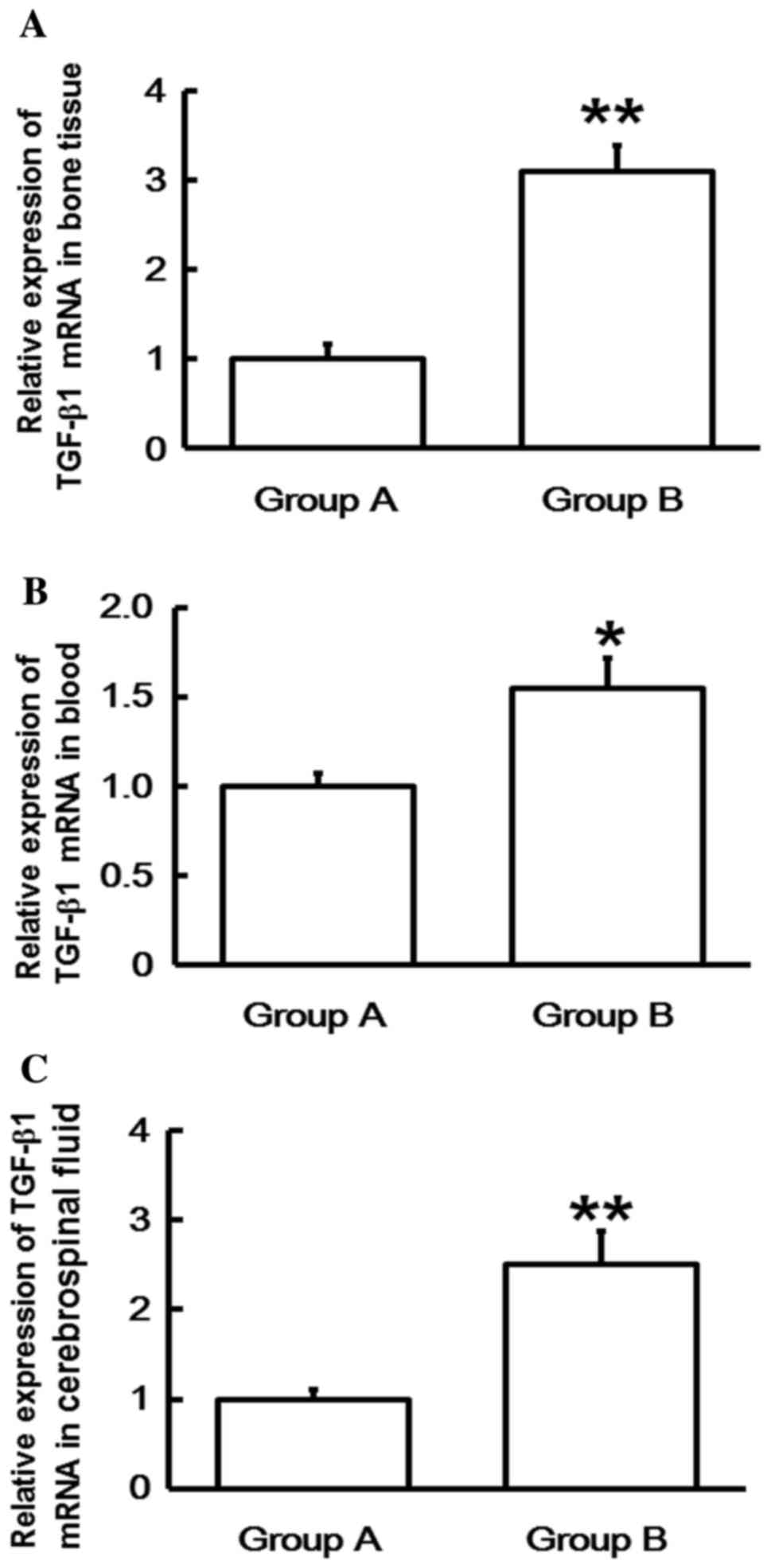
RT-qPCR was performed in order to determine levels
of TGF-β1 mRNA. The data indicated that TGF-β1 mRNA levels in the
bone tissues, blood and cerebrospinal fluid of Group B were
significantly higher than those in Group A (P<0.05; Fig. 1). This suggests that the expression
of TGF-β1 mRNA in patients with spinal cord injuries induced by
thoracolumbar spine compression fractures increases with time
following injury.
Expression of TGF-β1 protein in
patients with spinal cord injuries induced by thoracolumbar spine
compression fractures is increased with time following injury
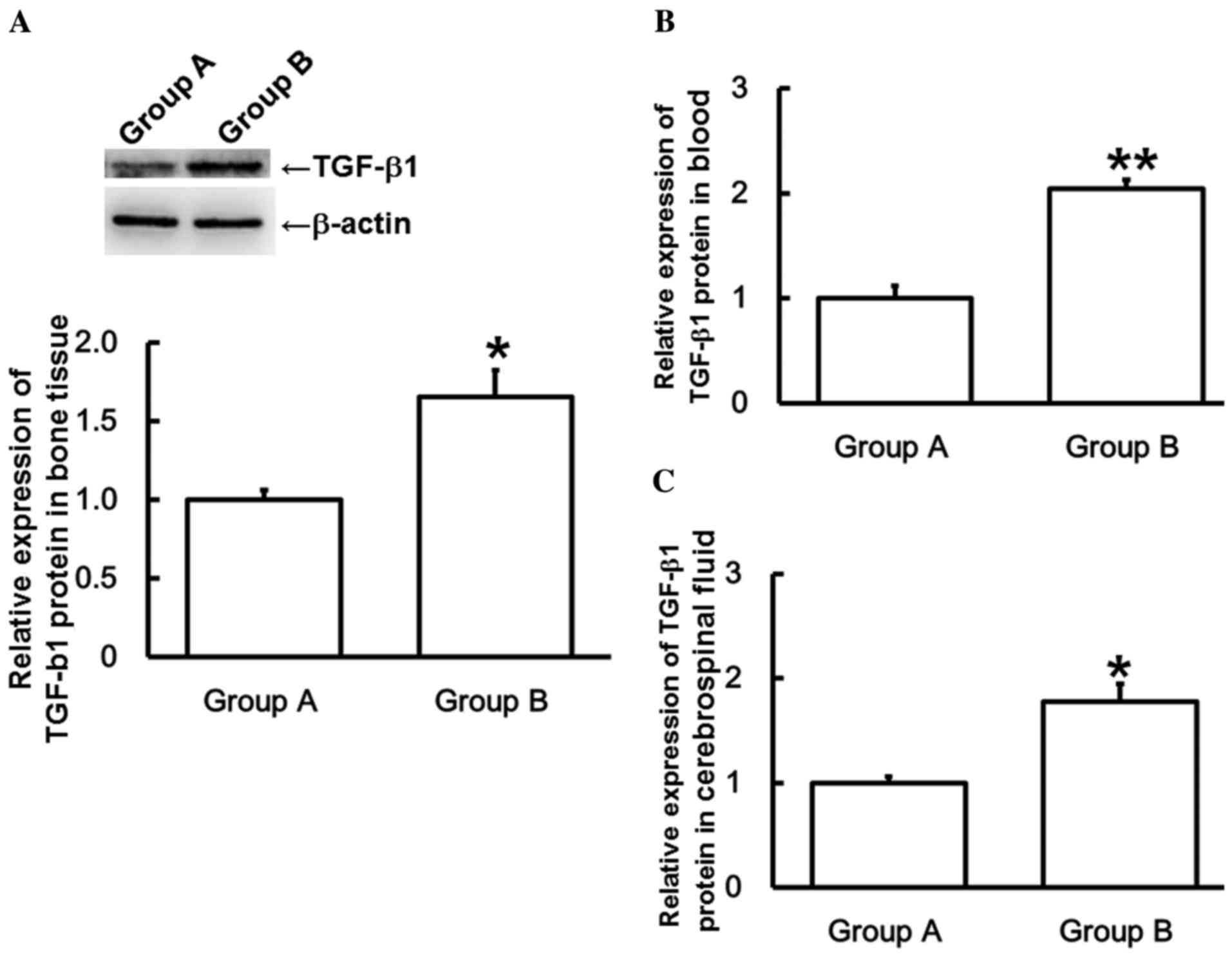
To measure TGF-β1 protein expression, western
blotting and ELISA were used. Results from western blotting
indicated that TGF-β1 protein expression was significantly higher
in the bone tissue of Group B than those in Group A (P<0.05;
Fig. 2A), which is consistent with
the trend of TGF-β1 mRNA expression in bone tissues. ELISA
indicated that TGF-β1 protein expression levels were significantly
higher in the blood and cerebrospinal fluid of Group B than those
in Group A (P<0.05; Fig. 2B and
C), which is consistent with the trends of TGF-β1 mRNA
expression in blood and cerebrospinal fluid. These results indicate
that the expression of TGF-β1 protein in patients with spinal cord
injuries induced by thoracolumbar spine compression fractures is
increased with time following injury.
Expression of miR-185 in patients with
spinal cord injuries induced by thoracolumbar spine compression
fractures is reduced with time following injury
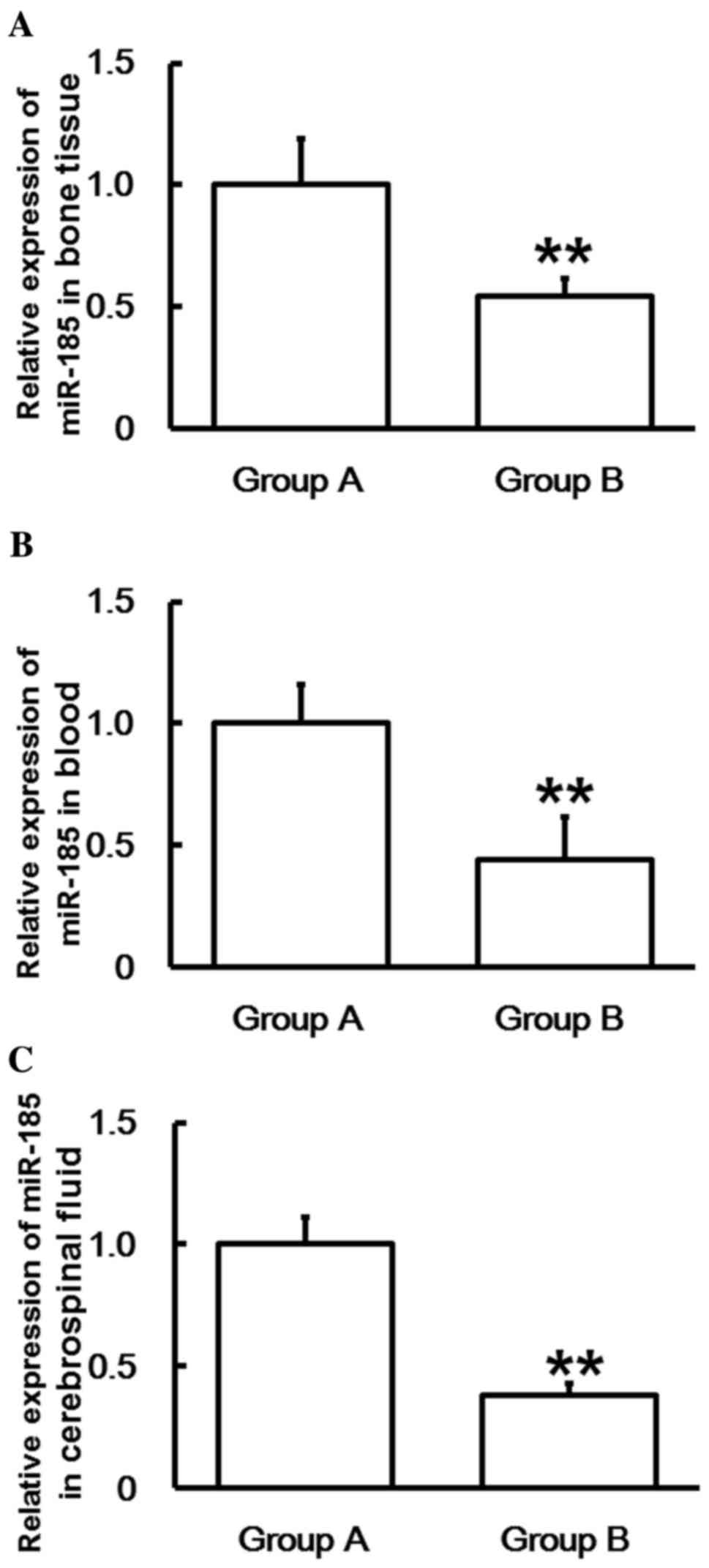
To measure miR-185 expression, RT-qPCR was
performed. The data indicated that the miR-185 levels in bone
tissues, blood and cerebrospinal fluid of Group B were
significantly lower than those in Group A (P<0.01; Fig. 3). These results suggest that the
expression of miR-185 in patients with spinal cord injuries induced
by thoracolumbar spine compression fractures decreases with time
following injury.
Discussion
Thoracic spine compression fractures typically
induce spinal cord injuries (14).
Deformation of the spinal canal, which is where the spinal cord is
located, induced by thoracolumbar spine compression fractures may
directly or indirectly damage spinal nerves. At present, there is
no effective method to directly repair damaged spinal nerves
(15). Notably, intervening in
cellular and molecular mechanisms following spinal cord injury may
inhibit secondary injuries to the spinal cord and serve a role in
its repair.
TGF-β1, an important coupling agent in the process
of bone reconstruction, has an important regulatory effect on bone
reconstruction (16). In the present
study, TGF-β1 mRNA and protein levels were significantly higher in
the bone tissues of patients who underwent surgery 8–14 days
following fracture compared with those that underwent surgery 1–7
days following fracture. In the bone tissue, osteoblasts are one of
the most sensitive cell lines to the mitogenetic effect of TGF-β1
(17). Pfeilschifter et al
(18) showed that TGF-β is
positively correlated with human bone remodeling and formation.
Additionally, Joyce et al (19) demonstrated that subperiosteal
injection of various concentrations of TGF-β1 induces osteogenesis
and cartilage formation. Furthermore, Centrella and McCarthy
(20) suggested that osteogenic or
platelet-derived TGF-β1 is able to bidirectionally stimulate DNA
synthesis in the osteoblasts of fetal mouse skulls and fetal bovine
bones, and the synthetic rate of DNA increases, reaches its peak
and then decreases as TGF-β1 levels steadily increase. The results
of the present study are similar to the aforementioned observations
(18–20), therefore, it has been demonstrated
that upregulation of TGF-β1 serves important roles in skeletal
repair and regeneration.
Similar to the results from bone tissues, TGF-β1
mRNA and protein levels in the blood or cerebrospinal fluid of
patients who underwent surgery 8–14 days following fracture were
significantly higher than those who underwent surgery 1–7 days
following fracture. A previous study has reported that TGF-β1
expression in motor neurons of the spinal cord is associated with
the regulation of secondary injuries in the spinal cord (21). Tyor et al (22) identified that inhibition of TGF-βl
following spinal cord injury may reduce the number of secondary
injuries and the accumulation of monocyte-macrophages in injury
regions. Intravenous injection of TGF-β1 in rats following spinal
cord injury reduces the injury area by 50% 48 h post-injection,
compared with a control group (23).
Romão et al (24) also
observed that TGF-β1 mRNA expression increases with time following
spinal cord injury, reaching its peak on day 7. Additionally, Gudi
et al (25) determined that
TGF-β1 is important in the regulation of neuronal survival and that
TGF-β1 selectively upregulates the expression of endogenous
neurotrophic factors with synergistic effects. Furthermore, an
association between TGF-β1 and various central nervous system
diseases has been identified. In ischemic brain damage, TGF-β1
exerts its neuroprotective effects by reducing the concentration of
calcium ions, activating the endothelial cells in ischemic areas
and promoting the proliferation of blood capillaries (26). These results suggest that injuries in
the spinal cord may stimulate the upregulation of TGF-β1 in the
body to try to activate the repair and regeneration of the spinal
cord, and to reduce inflammation.
miRNAs may interfere with mRNAs and affect their
translation (27). Regulation by
miRNA increases or decreases mRNA expression to mediate the
activities of coding genes of proteins and serves an important role
in the occurrence and development of tumors (28,29). In
the present study, bioinformatics was used to predict the upstream
genes that regulate TGF-β1 and it was determined that miR-185 may
be closely associated with TGF-β1. A recent study has demonstrated
that miR-185 upregulates the TGF-β1 signaling pathway in chronic
benzene poisoning (30). In
addition, Kim et al (31)
reported that miR-185 inhibits cardiac hypertrophy via multiple
signaling pathways. Ma et al (32) demonstrated that miR-185 inhibits
proliferation and induces the apoptosis of clear cell renal cell
carcinoma cells. Furthermore, Bao et al (33) reported that miR-185 is able to target
suppressors of cytokine signaling 3 and thus inhibit the functional
disorder of β cells induced by diabetes. Fu et al (34) have recently indicated that miR-185
targets c-met and inhibits human breast cancer cell proliferation
and metastasis, whereas Wang et al (35) have reported that miR-185 has similar
effects in breast cancer cells and targets the vascular endothelial
growth factor A gene.
In conclusion, the present study demonstrated that
miR-185 may target TGF-β1 to affect its transcription and
translation and thus serve an important role in spinal cord injury
induced by thoracolumbar spine compression fracture. Bone tissues,
blood and cerebrospinal fluid were harvested as research samples.
The fact that miR-185 and TGF-β1 expression was detected in the
blood circulation demonstrates that miR-185 may be useful as a
diagnostics tool, while the expression of miR-185 and TGF-β1 in
bone tissues and cerebrospinal fluid represents the physiological
status of the respective tissues (36). However, further studies are required
to elucidate direct evidence regarding the association between
TGF-β1 regulation by miR-185 and spinal cord injuries induced by
thoracolumbar spine compression fractures.
Acknowledgements
The present study was supported by Luoyang
Orthopedic-Traumatological Hospital. The authors would also like to
thank Professor Xinmin Pan from the Department of Forensic
Pathology, Henan University of Science and Technology.
References
|
1
|
Silverman SL: The clinical consequences of
vertebral compression fracture. Bone. 13:(Suppl 2). S27–S31. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown DB, Gilula LA, Sehgal M and Shimony
JS: Treatment of chronic symptomatic vertebral compression
fractures with percutaneous vertebroplasty. AJR Am J Roentgenol.
182:319–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kwon BK, Tetzlaff W, Grauer JN, Beiner J
and Vaccaro AR: Pathophysiology and pharmacologic treatment of
acute spinal cord injury. Spine. 4:451–464. 2004. View Article : Google Scholar
|
|
4
|
Hao D, He L and Yuan F: Late complications
in patients with spinal cord injuries and related factors. Zhongguo
Jizhu Jisui Zazhi. 15:267–270. 2005.
|
|
5
|
Lee KZ and Chang YS: Recovery of the
pulmonary chemoreflex and functional role of bronchopulmonary
C-fibers following chronic cervical spinal cord injury. J Appl
Physiol (1985). 117:1188–1198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murata K, Ito H, Yoshitomi H, Yamamoto K,
Fukuda A, Yoshikawa J, Furu M, Ishikawa M, Shibuya H and Matsuda S:
Inhibition of miR-92a enhances fracture healing via promoting
angiogenesis in a model of stabilized fracture in young mice. J
Bone Miner Res. 29:316–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheyn D, Kallai I, Tawackoli W, Yakubovich
D Cohn, Oh A, Su S, Da X, Lavi A, Kimelman-Bleich N, Zilberman Y,
et al: Gene-modified adult stem cells regenerate vertebral bone
defect in a rat model. Mol Pharm. 8:1592–1601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hulth A: Current concepts of fracture
healing. Clin Orthop Relat Res. 249:265–284. 1989.
|
|
9
|
Tatsuyama K, Maezawa Y, Baba H, Imamura Y
and Fukuda M: Expression of various growth factors for cell
proliferation and cytodifferentiation during fracture repair of
bone. Eur J Histochem. 44:269–278. 2000.PubMed/NCBI
|
|
10
|
Kiefer R, Streit WJ, Toyka KV, Kreutzberg
GW and Hartung HP: Transforming growth factor-beta 1: A
lesion-associated cytokine of the nervous system. Int J Dev
Neurosci. 13:331–339. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flanders KC, Ren RF and Lippa CF:
Transforming growth factor-betas in neurodegenerative disease. Prog
Neurobiol. 54:71–85. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiraizumi Y, Fujimaki E, Transfeldt EE,
Kawahara N, Fiegel VD, Knighton D and Sung JH: The effect of the
platelet derived wound healing formula and the nerve growth factor
on the experimentally injured spinal cord. Spinal Cord. 34:394–402.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia L: Modern spinal surgery. 1st.
People's Military Medical Press; Beijing: pp. 848–859. 2007
|
|
15
|
Tian W: Practical Orthopedics (1st).
People's Medical Publishing House. Beijing: 562–563. 2008.
|
|
16
|
Wu T, Liu Y, Fan Z, Xu J, Jin L, Gao Z, Wu
Z, Hu L, Wang J, Zhang C, et al: miR-21 Modulates the
Immunoregulatory Function of Bone Marrow Mesenchymal Stem Cells
Through the PTEN/Akt/TGF-β1 Pathway. Stem Cells. 33:3281–3290.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Canalis E, McCarthy T and Centrella M:
Growth factors and the regulation of bone remodeling. J Clin
Invest. 81:277–281. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pfeilschifter J, Diel I, Scheppach B,
Bretz A, Krempien R, Erdmann J, Schmid G, Reske N, Bismar H, Seck
T, et al: Concentration of transforming growth factor beta in human
bone tissue: Relationship to age, menopause, bone turnover and bone
volume. J Bone Miner Res. 13:716–730. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joyce ME, Roberts AB, Sporn MB and
Bolander ME: Transforming growth factor-beta and the initiation of
chondrogenesis and osteogenesis in the rat femur. J Cell Biol.
110:2195–2207. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Centrella M, McCarthy TL and Canalis E:
Transforming growth factor beta is a bifunctional regulator of
replication and collagen synthesis in osteoblast-enriched cell
cultures from fetal rat bone. J Biol Chem. 262:2869–2874.
1987.PubMed/NCBI
|
|
21
|
Unsicker K and Krieglstein K: TGF-betas
and their roles in the regulation of neuron survival. Adv Exp Med
Biol. 513:353–374. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tyor WR, Avgeropoulos N, Ohlandt G and
Hogan EL: Treatment of spinal cord impact injury in the rat with
transforming growth factor-beta. J Neurol Sci. 200:33–41. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Want T and Feng Z: Polypeptide growth
factors and spinal cord injury. Health Science and Technology
Publishing House in Xinjiang; Fitst. Urumqi: pp. 104–105. 2003
|
|
24
|
Romão JE Junior, Haiashi AR, Vidonho AF
Junior, Abensur H, Quintaes PS, Araújo MR, Noronha IL, Santos FR
and Machado MM: Causes and prognosis of acute renal failure in
elderly patients. Rev Assoc Med Bras (1992). 46:212–217. 2000.(In
Portuguese). PubMed/NCBI
|
|
25
|
Gudi V, Škuljec J, Yildiz Ö, Frichert K,
Skripuletz T, Moharregh-Khiabani D, Voss E, Wissel K, Wolter S and
Stangel M: Spatial and temporal profiles of growth factor
expression during CNS demyelination reveal the dynamics of repair
priming. PLoS One. 6:e226232011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim D, Schallert T, Liu Y, Browarak T,
Nayeri N, Tessler A and Murray M Fischer: Transplantation of
genetically modified fibroblasts expressing BDNF in adult rats with
a subtotal hemisection improves specific motor and sensory
functions. Neurorehabil Neural Repair. 15:141–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao X, Mohan R, Özcan S and Tang X:
MicroRNA-30d induces insulin transcription factor MafA and insulin
production by targeting mitogen-activated protein 4 kinase 4
(MAP4K4) in pancreatic β-cells. J Biol Chem. 287:31155–31164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–1037. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai W, Chen Y, Yang J, Niu P, Tian L and
Gao A: Aberrant miRNA profiles associated with chronic benzene
poisoning. Exp Mol Pathol. 96:426–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JO, Song DW, Kwon EJ, Hong SE, Song
HK, Min CK and Kim DH: miR-185 Plays an Anti-Hypertrophic Role in
the Heart via Multiple Targets in the Calcium-Signaling pathways.
PLoS One. 10:e01225092015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma X, Shen D, Li H, Zhang Y, Lv X, Huang
Q, Gao Y, Li X, Gu L, Xiu S, et al: MicroRNA-185 inhibits cell
proliferation and induces cell apoptosis by targeting VEGFA
directly in von Hippel-Lindau-inactivated clear cell renal cell
carcinoma. Urol Oncol. 33:169.e1-112015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bao L, Fu X, Si M, Wang Y, Ma R, Ren X and
Lv H: MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction
in diabetes. PLoS One. 10:e01160672015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu P, Du F, Yao M, Lv K and Liu Y:
MicroRNA-185 inhibits proliferation by targeting c-Met in human
breast cancer cells. Exp Ther Med. 8:1879–1883. 2014.PubMed/NCBI
|
|
35
|
Wang R, Tian S, Wang HB, Chu DP, Cao JL,
Xia HF and Ma X: MiR-185 is involved in human breast carcinogenesis
by targeting Vegfa. FEBS Lett. 588:4438–4447. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang CZ, Wu SC, Lin CL and Kwan AL:
Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear
factor κB (p65) and subarachnoid hemorrhage induced early brain
injury in a rat model. Brain Res. 1608:215–224. 2015. View Article : Google Scholar : PubMed/NCBI
|