Introduction
Neurosurgery evolved into an independent medical
specialty more than one hundred years ago. However, the rapid
technological advancement and the quantum leap in our understanding
of the human nervous system has reshaped this field in the past few
decades. With the most sophisticated medical instrumentation,
surgeries that were previously deemed impossible can now be
performed.
A good example of this medical evolution is the
surgical management of pituitary adenomas. The earliest trial dates
back to the late 19th century when Canton and Horsley performed the
first transcranial pituitary surgeries (1,2). Due to
significant mortality rates associated with this approach,
Schloffer explored a transphenoidal route to the sellar region in
the early 1900s (3). Following the
introduction of the operating microscope into transphenoidal
surgery in the 1960s (4), there was
a marked reduction in postoperative morbidity and mortality
(5–8). This microsurgical technique is still
considered to be the gold standard for pituitary tumor management
(9). As for the endoscope, it was
used as an adjunct to the operative microscope when first
introduced (10). With the
increasing emphasis on minimally invasive surgeries, endoscopic
surgery has increased in popularity since the mid-1990s (11,12), and
may have the potential to replace microscopic surgery as the new
standard technique (13). However,
no matter which technique or approach is used, pituitary surgery is
associated with significant complications, including
endocrinopathies, vision impairment, and cerebrospinal fluid leak
(14). In a recent large
meta-analysis, Ammirati et al (15) reported that 11.6% of patients
undergoing pituitary surgery presented with postoperative
hypopituitarism and 4.3% with permanent diabetes insipidus (DI).
Therefore, the central question in the surgical management of
pituitary adenomas is how to achieve maximal tumor resection whilst
preserving pituitary functions and minimizing postoperative
complications.
In the present study, 153 continuous microsurgical
cases performed between 2010 and 2014 were reviewed and the results
were compared with the existing literature in order to elucidate
any techniques that may facilitate the improved identification and
preservation of the gland and stalk during surgery without
compromising the extent of tumor resection, which is essential to
minimize postoperative morbidity and mortality.
Materials and methods
Ethics statement
Ethical approval was granted by the institutional
review boards of Xiangya Hospital Central South University
(Changsha, China). Informed consent was obtained from patients
scheduled for surgery following an explanation of the study aims
and protocol.
Patients
A total of 153 continuous microscopic surgeries
(Table I) for pituitary adenomas
were performed by a senior author of the present study (QL) at
Xiangya Hospital, Central South University between August 2010 and
December 2014. Among these patients were 87 males and 66 females
(mean age, 44.4±30 years). A total of 138 patients underwent
transnasal transphenoidal surgery and 15 received transcranial
surgery (subfrontal or pterional craniotomy), depending on their
individual tumor characteristics. All surgeries were performed
using Zeiss Pentero microscopes (Zeiss AG, Oberkochen, Germany).
Magnetic resonance imaging (MRI) was performed prior to and
following surgery. Diagnoses were further confirmed by pathology
using a modified Hardy's grading system (16).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. of patients |
|---|
| Age | 44.4±30 |
| Gender |
|
| Male | 87 (56.9%) |
|
Female | 66 (43.1%) |
| Approach |
|
|
Transnasal transphenoidal | 138 (90.2%) |
|
Subfrontal | 10 (6.5%) |
|
Pterional | 5 (3.3%) |
Follow-up
Pre- and postoperative MRI were analyzed to
determine the extent of resection, including gross-total resection
(GTR), near-total resection (NTR) and partial resection. Pituitary
hormone levels were measured before the operation and on the
post-operative days 1 and 3. Fasting venous blood samples were
used. All samples were processed in the Clinical Endocrinology
Research Center at Xiangya Hospital Central South University. The
hormone levels were measured by radioimmunoassay using the standard
commercially available kits. Hypopituitarism was defined as
impairment in at least one axis. Hormone levels were monitored one
week after discharge, monthly for 3 months, and then semiannually.
MRI follow-ups were scheduled every six months within the first
year, and were extended to annually after this period. Patients who
received non-GTR underwent further radiotherapy for one month after
surgery.
Results
Extent of tumor resection
As outlined, a modified Hardy's grading system
(17) was used for tumor
classification. Pre- and postoperative MRI were analyzed to
determine the extent of resection, including GTR, NTR and partial
resection. As shown in Table II,
all patients with grade I and II tumors underwent transphenoidal
surgery, with a GTR rate of 94.3%. For grade III and IV tumors, GTR
was achieved in 72.9% of the patients in the transphenoidal group
and 46.7% of the patients in the transcranial group. The overall
GTR rate was 81.2% for the transphenoidal approach, and 46.7% for
the transcranial approach.
 | Table II.Association between tumor size,
surgical approach and extent of resection. |
Table II.
Association between tumor size,
surgical approach and extent of resection.
|
| Number of
patients | Transphenoidal
approach | Transcranial
approach |
|---|
|
|
|
|
|
|---|
| Grade | Transphenoidal | Transcranial | GTR | NTR | Other | GTR | NTR | Other |
|---|
| I | 7 | – | 6 (85.7%) | 1 (14.3%) | – | – | – | – |
| II | 46 | – | 44 (95.7%) | 2 (4.3%) | – | – | – | – |
| III | 73 | 7 | 59 (80.8%) | 11 (15.1%) | 3 (4.1%) | 2 (28.6%) | 4 (57.1%) | 1 (14.3%) |
| IV | 12 | 8 | 3 (25.0%) | 6 (50.0%) | 3 (25.0%) | 5 (62.5%) | 2 (25.0%) | 1 (12.5%) |
| Total | 138 | 15 | 112 (81.2%) | 20 (14.5%) | 6 (4.3%) | 7 (46.7%) | 6 (40.0%) | 2 (13.3%) |
Complications
Postoperative complications are listed in Tables III and IV. The present study predominantly focused
on the pituitary functions of patients, including hypopituitarism
and DI, which indicate the integrity of the pituitary gland and the
stalk.
 | Table III.Pre- and post-operative cases of
hypopituitarism. |
Table III.
Pre- and post-operative cases of
hypopituitarism.
|
|
| Post-operative |
|---|
|
|
|
|
|---|
|
| Pre-operative | New/worsened | Recovered | Improved | Unchanged |
|---|
| TS | 69 | 0 | 18 (26.1%) | 24 (34.8%) | 27 (39.1%) |
| TC | 12 | 0 | 1 (8.3%) | 5 (41.7%) | 6 (50.0%) |
 | Table IV.Other postoperative
complications. |
Table IV.
Other postoperative
complications.
|
| TS | TC |
|---|
| Permanent diabetes
insipidus | 0 | 0 |
| Transient diabetes
insipidus | 6 (4.3%) | 4 (26.7%) |
| CSF leak | 3 (2.2%) | 0 |
| Intracranial
hemorrhage | 0 | 1 (6.7%) |
| Intracranial
infection | 0 | 1 (6.7%) |
| Perioperative
mortality | 0 | 0 |
None of the 153 patients examined developed new
anterior pituitary insufficiency. A total of 69 (50.0%) patients
undergoing transsphenoidal surgery presented with hypopituitarism
prior to surgery. Among those, 18 (26.1%) patients exhibited normal
pituitary functions following the procedure. In the transcranial
group, 12 (80%) patients demonstrated preoperative hypopituitarism;
the pituitary functions of one patient (8.3%) returned to normal
following surgery. Partial recovery of the pituitary functions was
achieved in 24 (34.8%) patients in the transsphenoidal group and 5
(41.7%) patients in the transcranial group. Pituitary functions
remained unchanged in the remaining patients.
No patients presented with DI prior to surgery;
however, 4.3% patients in the transsphenoidal group and 26.7%
patients in the transcranial group exhibited transient
postoperative DI. Their symptoms resolved quickly with or without
vasopressin treatment prior to discharge.
Follow-up
The follow-up period ranged from 6 months to 5 years
with a mean of 2.2 years. No patients exhibited clear tumor
recurrence in the GTR group. Among those who received non-GTR,
three patients (8.8%) were found to exhibit regrowth and underwent
subsequent radiotherapy or surgery.
Patients with abnormal pituitary functions after
surgery routinely received hormone replacement therapy. In the case
of post-operative hypothyroidism, oral levothyroxine tablets were
prescribed. For patients with adrenocortical insufficiency,
hydrocortisone was administered. Patients were followed in the
endocrinology clinic. Pituitary hormone levels were measured and
the drug doses were adjusted accordingly. The hormone levels of
6.5% of patients (4/62) returned to normal and no longer required
hormone replacement during the follow-up period.
Typical cases
Case 1
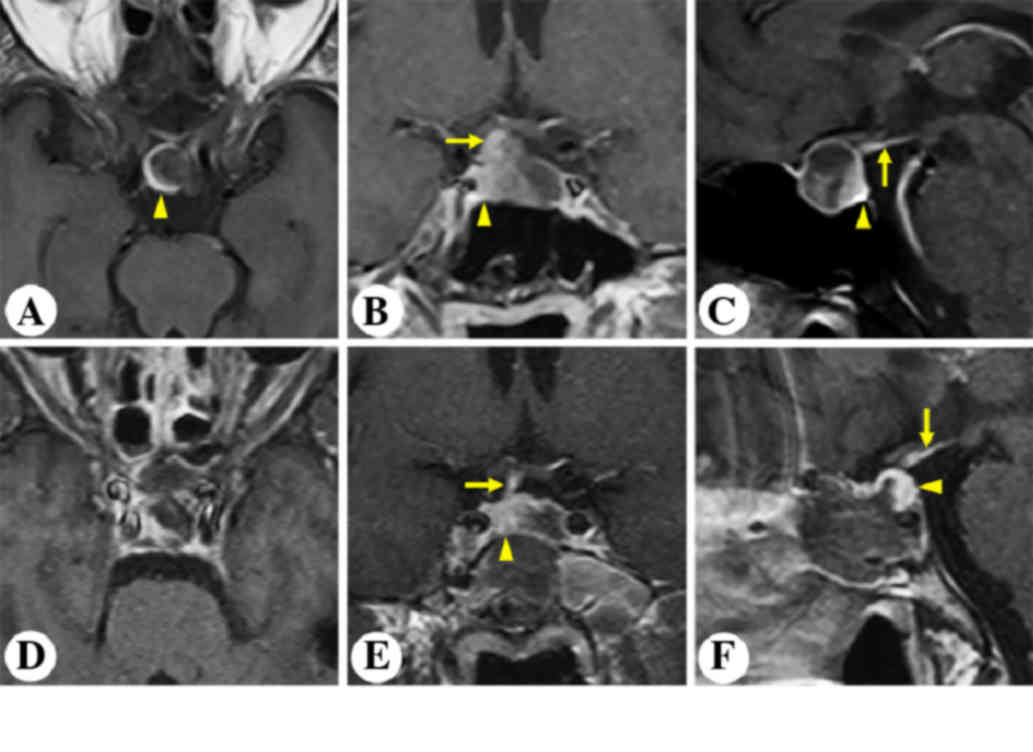
A 63-year-old male complained of severe intermittent
headache for the past three years. MRI revealed an intrasellar
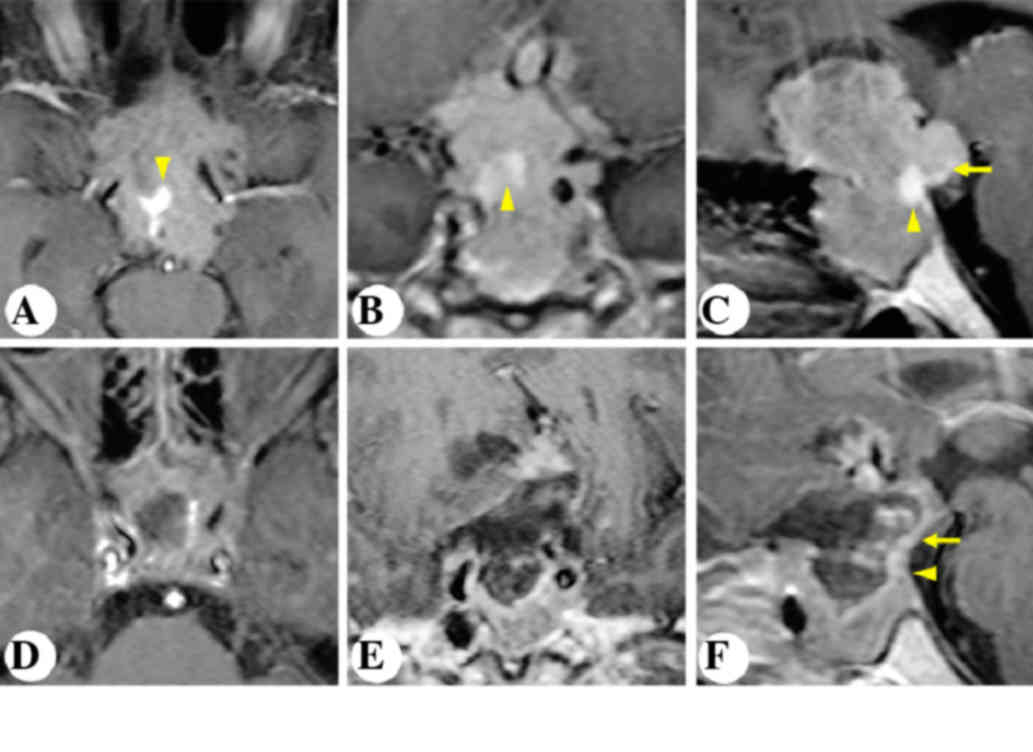
non-enhancing lesion (Fig. 1). The
pituitary gland and stalk was stretched and displaced
posterolaterally to the right (Fig.
1A-C). The patient underwent microscopic transphenoidal surgery
(Fig. 2). Postoperative MRI
demonstrated gross-total resection of the tumor and an anatomically
intact gland and stalk (Fig. 1D-F).
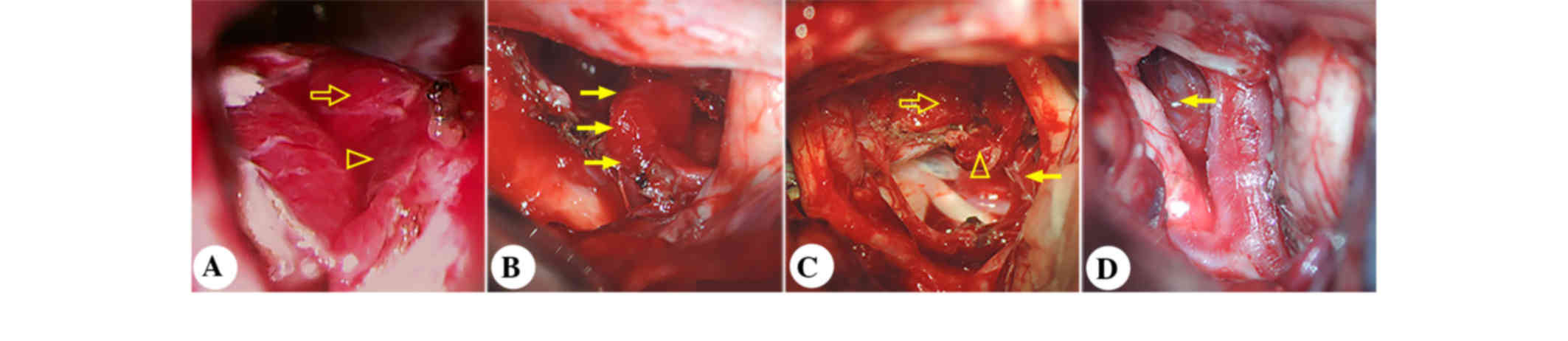
An intraoperative view of the pituitary gland (Fig. 2) is shown in Fig. 2A after tumor removal.
Case 2
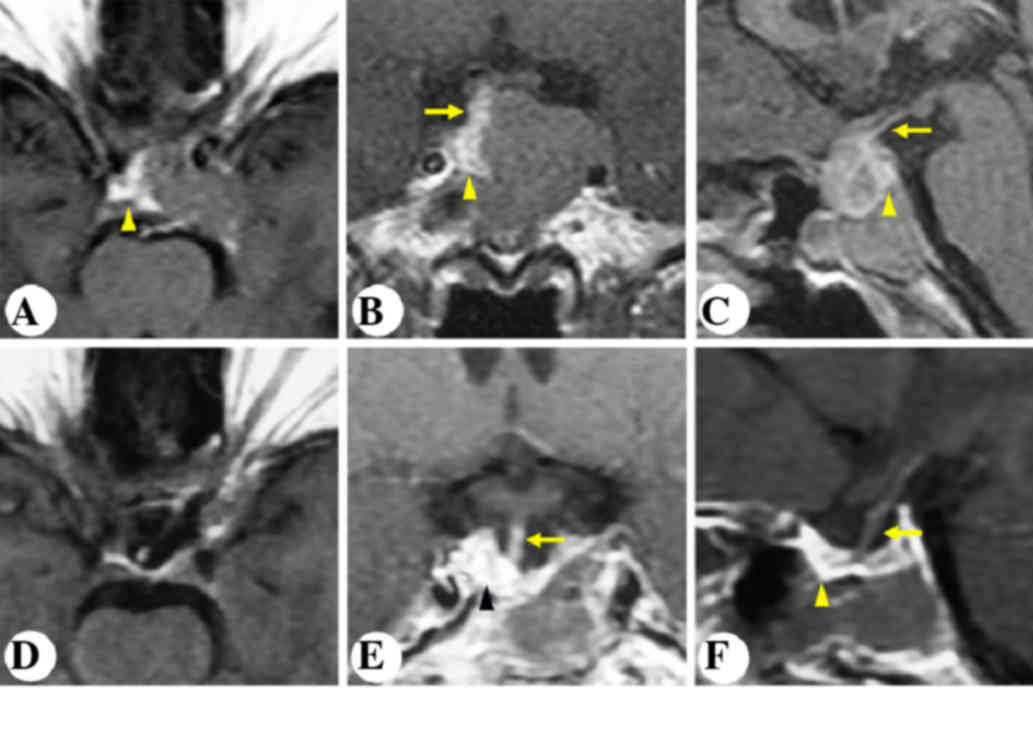
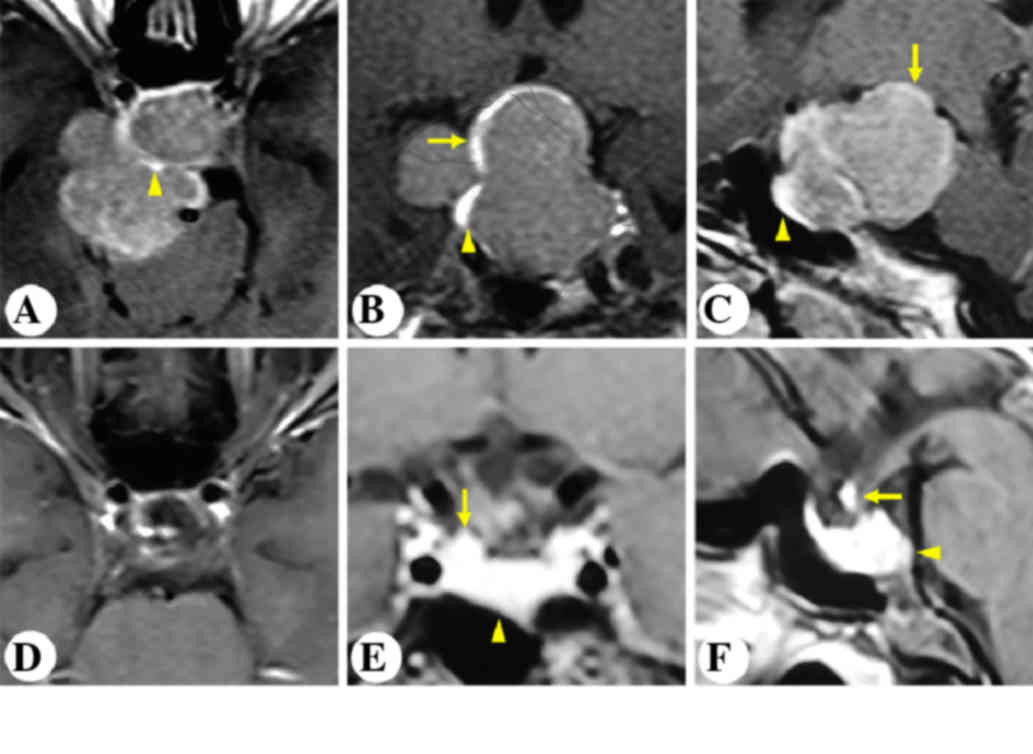
A 48-year-old female complained of progressive
intermittent headache and dizziness for the past nine years. MRI
revealed an intrasellar and suprasellar non-enhancing lesion,
invading the left cavernous sinus and the sphenoidal sinus. The
stalk was displaced posterolaterally to the right (Fig. 3A-C). The patient underwent
microscopic transphenoidal surgery. Postoperative MRI outlined GTR
of the tumor and an anatomically intact gland and stalk (Fig. 3D-F).
Case 3
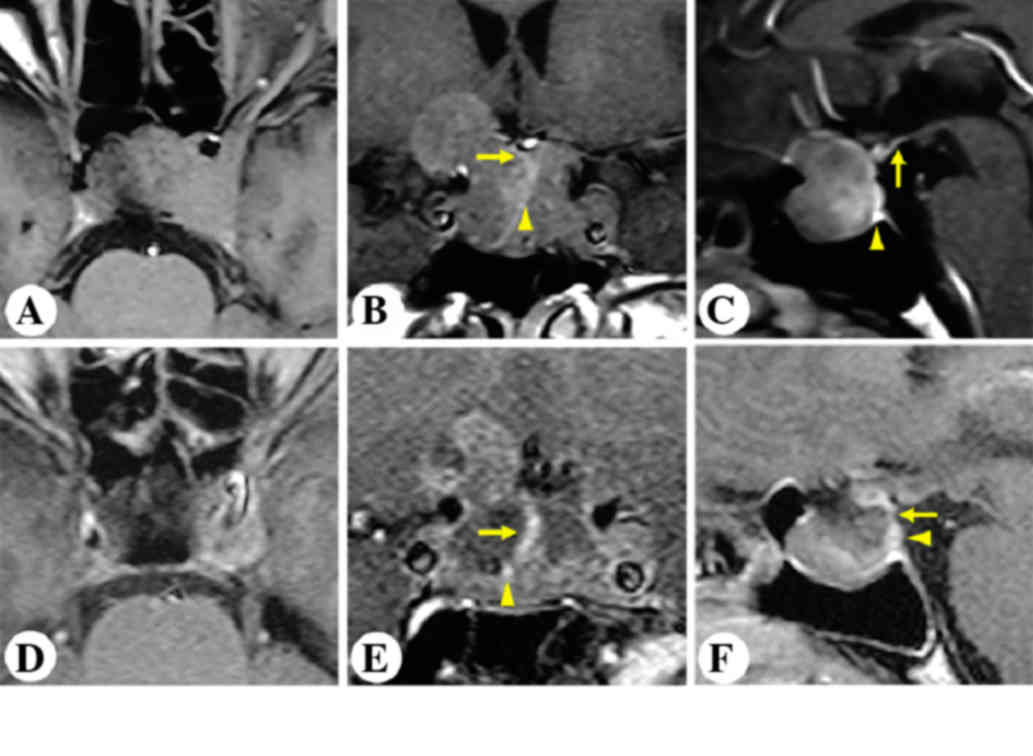
A 20-year-old male presented with severe
intermittent headache and progressive vision impairment in the
right eye for two years. Preoperative MRI is presented in Fig. 4A-C. MRI revealed an intrasellar and
suprasellar non-enhancing lesion, invading the left cavernous
sinus. The patient underwent unilateral subfrontal surgery. As
shown in Fig. 2B, the pituitary
stalk was visualized between the optic nerves, and the gland
extended distally into the sella turcica. Postoperative MRI
revealed gross-total resection of the tumor and an anatomically
intact gland and stalk (Fig.
4D-F).
Case 4
A 50-year-old male complained of progressive visual
disturbance in both eyes for 10 years. MRI demonstrated a large
intra- and suprasellar lesion, invading the sphenoidal sinus
inferiorly and the third ventricle superiorly, compressing the
optic chiasm and encasing the left internal carotid arteries. The
stalk was buried deep inside the tumor, and the gland was displaced
posteriorly (Fig. 5A-C). Lab tests
suggested a nonfunctioning pituitary adenoma. The patient
subsequently underwent unilateral subfrontal craniotomy. Following
tumor resection, the pituitary gland and the stalk were visualized
in the posteromedial side of the right optic nerve (Fig. 2C). Postoperative MRI showed
near-total resection (Fig.
5D-F).
Case 5
A 33-year-old female was diagnosed with acromegaly
three years ago. Pituitary hormone panel suggested a growth hormone
secreting adenoma. Preoperative MRI showed a homogenously enhancing
intra-, supra-, and parasellar lesion with a multilobular
configuration (Fig. 6A-C). The stalk
was compressed, distorted and displaced posterolaterally to the
right. The gland was also displaced anterolaterally to the same
side (Fig. 6A-C). The patient
underwent pterional craniotomy. Following tumor excision, the
pituitary stalk was visualized between the right optic nerve and
the right internal carotid artery (Fig.
2D). Postoperative MRI showed gross total removal of the tumor
and an anatomically intact gland and stalk (Fig. 6D-F).
Discussion
Pituitary adenomas account for 10–15% of all
intracranial tumors (17,18). Surgery is the primary treatment of
choice for all symptomatic patients, with the exception of those
with prolactinomas (19). It remains
still controversial whether microscopic or endoscopic surgery is
the gold standard (9,13). The present study predominantly
focused on the microscopic approach. The majority of pituitary
tumors (with or without suprasellar extension) are removed via
transnasal transphenoidal microscopic surgery. However,
transcranial microscopic surgery is recommended in up to 10% of
patients (5,6,20). In
the present patient series, 138 patients (90.2%) underwent
transphenoidal surgeries, and 15 patients (9.8%) received
transcranial operations. Regardless of the surgical approach, the
treatment goals were four-fold: i) To achieve GTR whenever
possible, ii) reduce intracranial pressure if present, iii) relieve
neurological and endocrine manifestations, and iv) preserve normal
pituitary anatomy. It is important to preserve both the gland and
stalk in order to minimize new postoperative endocrinopathies and
improve quality of life (5,15,21).
Preoperative pituitary MRI provides invaluable
information to facilitate the localization and protection of the
gland and stalk during surgery. The imaging characteristics of the
normal gland (22) and the pituitary
adenomas (23–25) have been well studied. The anterior
lobe is isointense on both T1 and T2 images, and the posterior lobe
is hyperintense on T1 and hypointense on T2 sequences. The gland
(seen as a bright spot in the sella) is diffusely enhanced on post
contrast T1 images, which can be distinguished from the pituitary
adenomas (26). The stalk is wider
near the hypothalamus, and smoothly tapers as it travels caudally.
It is relatively hypointense compared with the optic chiasm and the
neurohypophysis on T1 images, and is also diffusedly enhanced after
IV contrast administration (27).
For microadenomas, the stalk may slightly deviate away from the
tumor (28). For macroadenomas, the
longitudinal axis of the stalk may point directly toward the normal
gland (28).
As mentioned previously, the transphenoidal approach
is the preferred approach for the majority of pituitary adenomas
due to postoperative low morbidity and mortality (6–8,20). During transphenoidal surgery,
adequate dura opening on the sellar floor is essential to allow
sufficient exposure of the tumor. Following removal of the inferior
pole of the tumor, the posterior lobe can be identified as a red
and firm structure, separated from the tumor by a pseudocapsule.
The tumor is then carefully excised from next to the pituitary
gland. Every effort should be made to protect the anterior lobe.
Special attention should be paid to the color, the texture and the
pseudocapsule, and excessive electrocoagulation in its vicinity
should be avoided to preserve the blood supply. Then we can resect
the tumor lateral to the cavernous sinus, and finally explore the
suprasellar region and remove any residual tumor above the
diaphragm. In the present patient series, the anatomical
relationship was relatively straightforward between the
microadenoma and the pituitary gland. For macro- and giant
adenomas, particularly when tumors invade the cavernous sinus, the
anterior lobe and the stalk are usually displaced to the opposite
side, while the location of the posterior lobe is constant in the
sella, as in cases 1 and 2
In contrast to the transphenoidal approach, the
transcranial approach is associated with significant morbidity and
mortality (6–8,29).
Therefore, strict guidelines must be followed. Various factors
should be taken into consideration prior to surgery, including the
patient's age, current health condition, visual function, and
imaging characteristics (29). This
approach is reserved for tumors extending superolaterally to the
supraclinoid internal carotid artery, reaching the foramen of Monro
or encasing the subarachnoid arteries, and tumors with asymmetric
subfrontal extension or predominantly cavernous sinus invasion
(5,29,30). It
is also indicated in patients with small sellae, diaphragmatic
constriction, fibrous pituitary adenomas, or postoperative apoplexy
of the residual suprasellar tumor (5,29,30). All
of the present patients who underwent transcranial procedures had
grade III or IV tumors. The majority of these cases received
unilateral subfrontal craniotomy combined with trans lamina
terminalis approach when necessary, as in cases 3 and 4). Pterional
craniotomy was indicated for the tumors with dominant parasellar
extension, particularly with significant cavernous sinus invasion,
as in case 5). The majority of tumors with regular contours exhibit
distinct anatomical relationships with the gland and stalk. During
surgery, the intrasellar tumor was excised through the
prechiasmatic space. Using similar criteria to identify the
pituitary gland (color, texture and pseudocapsule), the cavernous
sinus was subsequently explored and as much of the tumor as
possible was removed. Finally, the residual tumor was meticulously
dissected away from the optic pathway and the anterior
communicating artery complex, and the suprasellar tumor was
resected. Care must be taken to identify and protect the stalk and
gland along the dilated diaphragmatic foramen. For those large or
giant pituitary multilobular adenomas, the anatomical relationships
are not clear. The tumor can encase the stalk and/or the anterior
lobe, as observed in cases 3–5. The anatomy of the region in
question must be carefully assessed in the preoperative MRI in
order to establish an appropriate surgical plan. We propose that it
is preferable to excise the intrasellar tumor first so that the
tumor blood supply can be restricted to facilitate subsequent tumor
removal and normal anatomy preservation. When dealing with
intrasellar tumors, the residual anterior lobe was identified based
on its color, texture and the pseudocapsule. Excessive traction and
electrocoagulation should be avoided. Following sufficient tumor
decompression, the stalk was identified and preserved using
landmarks, including the dorsum sellae and diaphragma sellae. Care
must be taken to protect bilateral superior hypophyseal arteries.
For tumors with excessive cavernous sinus invasion, the epidural or
subdural approach may be attempted. However, particular attention
should be paid to protect the oculomotor nerve and the cavernous
segment of the internal carotid artery. The residual tumor
extending into the third ventricle can be safely removed after
sufficient decompression due to its loose attachment to the
ventricular floor, supported by the surgeon's knowledge of the
individual suprasellar anatomy during surgery.
New-onset postoperative hypopituitarism and
permanent DI indicate possible intraoperative damage to the
pituitary gland and/or stalk. Excessive use of the aspirator, rough
handling and excessive electrocoagulation (31) near the pituitary gland and stalk can
lead to these potentially fatal endocrinopathies. It has been
reported that new-onset hypopituitarism and permanent DI occurred
in 11.6 and 4.3% of patients undergoing transphenoidal surgeries
(15), and 15 and 3.2% of patients
after transcranial surgery (5). In
the present patient series, preoperative pituitary insufficiency
was identified in 50.0 and 80.0% of the patients undergoing
transsphenoidal or transcranial craniotomy, respectively. Pituitary
functions returned to normal in 26.1 and 8.3% of those patients
following surgery. No new-onset hypopituitarism or worsening of the
preexisting pituitary dysfunctions was detected. Postoperative DI
occurred in 4.3 and 26.7% of the patients undergoing
transsphenoidal and transcranial surgery, respectively, and all
these patients were fully recovered prior to discharge. In our
experience, it was more difficult to preserve the integrity of the
gland and stalk during the transcranial surgery as all the present
transcranial cases were grade III and IV tumors). Fatemi et
al (21) reported that the tumor
diameter (>20 mm) is the single most important predictor of
new-onset hypopiuitarism. In addition, if hypopituitarism presents
prior to surgery, full recovery is less likely following surgery.
However, if the patient does not present with preoperative
pituitary dysfunction or DI, new-onset postoperative
hypopituitarism or permanent DI is not expected to occur.
Pituitary tissue preservation is not a novel concept
(21,26,27,21).
However it is difficult to achieve in cases of large pituitary
tumors that displace or even encase the gland and the stalk. Under
these conditions, normal structures cannot be clearly identified in
the images, which renders pituitary protection difficult during
surgery. The present surgical observations may allow surgeons to
better identify and preserve the gland and stalk without
compromising the extent of resection, which is essential to
minimize postoperative morbidity and mortality.
In conclusion, based on preoperative imaging
characteristics and intraoperative observations, surgeons should
try all possible means to preserve the pituitary stalk and gland
during surgery, in order to minimize postoperative endocrinopathies
and improve the patient's quality of life.
Acknowledgements
The present study was supported by the National Key
Technology Research and Development Program of the Ministry of
Science and Technology of China (grant no. 2014BAI04B01) and the
Technology Plan of Science and Technology Bureau (grant no.
2013SK2022).
References
|
1
|
Caton R: Notes of a case of acromegaly
treated by operation. Br Med J. 2:1421–1423. 1893. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horsley V: On the technique of operations
on the central nervous system. Br Med J. 2:411–423. 1906.
View Article : Google Scholar
|
|
3
|
Schloffer H: Successful surgical treatment
of a pituitary tumor in the nasal cavity. Wien Klin Wochenschr.
20:621–624. 1907.(In German).
|
|
4
|
Hardy J: Surgery of the pituitary gland,
using the trans-sphenoidal approach. Comparative study of 2
technical methods. Union Med Can. 96:702–712. 1967.(In French).
PubMed/NCBI
|
|
5
|
Buchfelder M and Kreutzer J: Transcranial
surgery for pituitary adenomas. Pituitary. 11:375–384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson CB: A decade of pituitary
microsurgery. The Herbert Olivecrona lecture. J Neurosurg.
61:814–833. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laws ER and Jane JA Jr: Pituitary
tumors-long-term outcomes and expectations. Clin Neurosurg.
48:306–319. 2001.PubMed/NCBI
|
|
8
|
Wilson CB: Surgical management of
pituitary tumors. J Clin Endocrinol Metab. 82:2381–2385. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mortini P: Cons: Endoscopic endonasal
transsphenoidal pituitary surgery is not superior to microscopic
transsphenoidal surgery for pituitary adenomas. Endocrine.
47:415–420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Apuzzo ML, Heifetz MD, Weiss MH and Kurze
T: Neurosurgical endoscopy using the side-viewing telescope. J
Neurosurg. 46:398–400. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jho HD, Carrau RL, Ko Y and Daly MA:
Endoscopic pituitary surgery: An early experience. Surg Neurol.
47:213–222; discussion 222–223. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alfieri A: Endoscopic endonasal
transsphenoidal approach to the sellar region: Technical evolution
of the methodology and refinement of a dedicated instrumentation. J
Neurosurg Sci. 43:85–92. 1999.PubMed/NCBI
|
|
13
|
Mamelak AN: Pro: Endoscopic endonasal
transsphenoidal pituitary surgery is superior to microscope-based
transsphenoidal surgery. Endocrine. 47:409–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dehdashti AR, Ganna A, Karabatsou K and
Gentili F: Pure endoscopic endonasal approach for pituitary
adenomas: Early surgical results in 200 patients and comparison
with previous microsurgical series. Neurosurgery. 62:1006–1015;
discussion 1015–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ammirati M, Wei L and Ciric I: Short-term
outcome of endoscopic versus microscopic pituitary adenoma surgery:
A systematic review and meta-analysis. J Neurol Neurosurg
Psychiatry. 84:843–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shou XF, Li SQ, Wang YF, Zhao Y, Jia PF
and Zhou LF: Treatment of pituitary adenomas with a transsphenoidal
approach. Neurosurgery. 56:249–256; discussion 249–256. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kovacs K and Horvath E: Pathology of
pituitary tumors. Endocrinol Metab Clin North Am. 16:529–551.
1987.PubMed/NCBI
|
|
18
|
Scheithauer BW: Surgical pathology of the
pituitary: The adenomas. Part I. Pathol Annu. 19:317–374.
1984.PubMed/NCBI
|
|
19
|
Laws ER and Jane JA Jr: Neurosurgical
approach to treating pituitary adenomas. Growth Horm IGF Res.
15:(Suppl A). S36–S41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wilson CB: Endocrine-inactive pituitary
adenomas. Clin Neurosurg. 38:10–31. 1991.
|
|
21
|
Fatemi N, Dusick JR, Mattozo C, McArthur
DL, Cohan P, Boscardin J, Wang C, Swerdloff RS and Kelly DF:
Pituitary hormonal loss and recovery after transsphenoidal adenoma
removal. Neurosurgery. 63:709–718; discussion 718–719. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirsten Forbes JK and White WL: Imaging of
the pituitary gland. Barrow Quarterly. 18:9–19. 2002.
|
|
23
|
Cottier JP, Destrieux C, Brunereau L,
Bertrand P, Moreau L, Jan M and Herbreteau D: Cavernous sinus
invasion by pituitary adenoma: MR imaging. Radiology. 215:463–469.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaufman B, Kaufman BA, Arafah BM,
Roessmann U and Selman WR: Large pituitary gland adenomas evaluated
with magnetic resonance imaging. Neurosurgery. 21:540–546. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rajaraman V and Schulder M: Postoperative
MRI appearance after transsphenoidal pituitary tumor resection.
Surg Neurol. 52:592–598; discussion 598–599. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oldfield EH: Pituitary adenoma
identification. J Neurosurg. 116:933–934; author reply 934. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simmons GE, Suchnicki JE, Rak KM and
Damiano TR: MR imaging of the pituitary stalk: Size, shape, and
enhancement pattern. AJR Am J Roentgenol. 159:375–377. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cho CH, Barkhoudarian G, Hsu L, Bi WL,
Zamani AA and Laws ER: Magnetic resonance imaging validation of
pituitary gland compression and distortion by typical sellar
pathology. J Neurosurg. 119:1461–1466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Divitiis E and de Divitiis O: Surgery
for large pituitary adenomas: What is the best way? World
Neurosurg. 77:448–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Youssef AS, Agazzi S and van Loveren HR:
Transcranial surgery for pituitary adenomas. Neurosurgery.
57:(Suppl 1). S168–S175; discussion 168–175. 2005.
|
|
31
|
Berker M, Hazer DB, Yücel T, Gürlek A,
Cila A, Aldur M and Onerci M: Complications of endoscopic surgery
of the pituitary adenomas: Analysis of 570 patients and review of
the literature. Pituitary. 15:288–300. 2012. View Article : Google Scholar : PubMed/NCBI
|