Introduction
Neuropathic pain results from lesions to the
peripheral nervous system (PNS) caused by infection, mechanical
trauma, neurotoxic chemicals, metabolic diseases or tumor invasion
(1). It involves a number of
pathophysiological changes that occur within the central and
peripheral nervous systems (1).
Afferent discharge may emanate from the injury site and neuronal
somata in the dorsal root ganglion (DRG), or even elsewhere along
the injured axons in response to certain physical or chemical
stimuli. This may lead to peripheral and central sensitization, an
important causative factor for the onset and maintenance of
neuropathic symptoms, including spontaneous pain, hyperalgesia and
allodynia (1,2). Following peripheral nerve injury, the
ipsilateral injured side contains injured and intact sensory axons
and neurons.
L5 spinal nerve ligation (SNL) is an
experimental neuropathic pain model exhibiting a clear separation
between injured and non-injured cell bodies (3). It has been demonstrated that almost all
L5 DRG neurons were axotomized following transection of
the L5 spinal nerve, while the L4 ganglionic
cells infiltrated the degenerating L5 axonal
environments. The contribution of injured and intact sensory
neurons to the onset and maintenance of neuropathic pain is
controversial (4). Sapunar et
al (5) observed that distinct
electrophysiological changes occur in injured and adjacent DRG
membranes. The afterhyperpolarization (AHP) duration in
L5 neurons with C fibers shortened following axotomy,
while adjacent L4 neurons showed no change in action
potential duration, AHP dimensions or excitability following SNL.
However, an in vivo study demonstrated that, following SNL,
electrophysiological changes occurring in large- and medium-sized
somata of intact (L4) as well as axotomized
(L5) DRG neurons were similar and included a longer
action potential (AP) duration, slower AP rise and falling rates, a
lower current and voltage threshold, and a higher input resistance
(6).
Following peripheral nerve lesion, the distribution
and properties of transmembrane ion channels in injured axons and
DRG neurons change, leading to alterations in the excitability and
conductive properties of peripheral afferent fibers (7). DRG neurons express a variety of VACCs,
a family of transmembrane proteins widely distributed in excitable
cells and also detected in a number of non-excitable cells. When
the plasma membrane becomes depolarized, the VACC channels open,
mediating Ca2+ entry in response to sub-threshold
depolarizing signals and APs (8).
α2δ1 is the most abundant VACC sub-type in the spinal cord and DRG,
and co-expression of α2δ1 with α1 and β VACC sub-units in
vitro results in accelerated activation, inactivation kinetics
and increased calcium current density (9). Increased α2δ1 sub-unit expression and
calcium channel activity in the dorsal horn have been observed in
neuropathic pain (10). Furthermore,
it has been demonstrated that gabapentin (GBP), the first-line drug
for the treatment of neuropathic pain, specifically binds to the
α2δ1 sub-unit of N-type VDCCs and exerts various actions
responsible for pain attenuation (11).
In the present study, nerve injury-induced changes
in the electrophysiological properties of DRG high-voltage
activated (HVA)-Ca2+ currents were examined, as well as
the influence of GBP on these changes in injured-side axotomized
and adjacent uninjured DRGs in model rats subjected to SNL.
Materials and methods
Surgical procedure
A total of 96 healthy male Sprague-Dawley (SD) rats
(age, 4–6 weeks; weight, 120–150 g) were purchased from the Model
Animal Research Center of Nanjing University (Nanjing, China). They
were kept under a temperature of 22–24°C, a humidity of 50–60% and
a 12 h light-dark cycle. All rats had ad libitum access to
food and water. Rats were randomly divided into two groups: An SNL
group (n=60) and a sham group (n=36). The 60 rats in the SNL group
received a unilateral, tight ligation and transection of the left
L5 spinal nerve using a modification of the surgical
procedure previously described by Kim and Chung (12). In brief, under anesthesia with 2%
pentobarbital sodium administered intraperitoneally (i.p., 50
mg/kg; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and
following exposure of the left L5 spinal nerve, the left
L5 and L4 spinal nerves were identified. The
left L5 spinal nerve was separated and tightly ligated
with 5–0 silk sutures between the DRG and the conjunction to form
the sciatic nerve and transected just distal to the ligature. The
36 sham rats underwent exposure, while they did not undergo
ligation or transection of the spinal nerve. The present study was
performed in strict accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals of the National
Institutes of Health. The experimental animal protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
Nanjing University (Nanjing, China).
Behavioral tests
All experimental rats were subjected to behavioral
tests at 1 day prior to as well as 1, 3, 7 and 14 days following
surgery. All tests were repeated three times.
To assess tactile allodynia, rats in clear plastic
cages with a wire mesh bottom were tested using an up-down method
as described by Choi et al (13). A filament (Supertips™ flexible Von
Frey hairs, IITC Life Science, Woodland Hills, CA, USA) with a
suitable, calibrated buckling weight was applied to the plantar
surface of the hind paw with pressure causing the filament to bend.
Absence of paw lifting after 4 sec led to the use of the next
filament with increased weight, whereas paw lifting indicated a
positive response and led to the use of the next weaker filament.
This paradigm continued until the withdrawal threshold was
estimated as the smallest fiber size that evoked at least three
withdrawal responses during five consecutive applications with the
same fiber.
A von Frey mechanical pain threshold detector (Model
2390 Electric von Frey aesthesiometer; IITC Life Science) was used
to assess mechanical hyperalgesia. A paw-flick response was
elicited by applying increasing force (in g) using rigid tips (400
g; Model 2391; IITC Life Science) focused on the plantar surface of
the hindpaw. The force applied that elicited a reflex removal of
the hindpaw was recorded.
Thermal hyperalgesia was determined by comparison of
paw withdrawal latency to thermal stimuli between operated and
non-operated sides. Rats were exposed to a radiant source generated
and controlled by an IITC Model 390 Analgesia Meter (10 V; 30 W;
spot dimensions, 4×6 mm; IITC Life Science). The light radiation
intensity was set at 50% with intervals of 30 sec (in order to
avoid extensive damage).
Dissociation of DRG neurons
At 15 days after surgery, rats in the SNL or sham
group were anesthetized with 2% pentobarbital sodium (50 mg/kg
i.p., Sigma-Aldrich; Merck Millipore) for the dissociation of DRG
neurons. The L4 and L5 DRGs and attached
dorsal roots were removed and the connective tissue capsule was
dissected using microdissection scissors in ice-cold, oxygenated,
buffered Dulbecco's modified Eagle's medium (DMEM, High Glucose,
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
3.7 g/l NaHCO3 (pH 7.2). DRG fragments were rinsed with
DMEM three times and subsequently transferred into 1.5 ml DMEM
containing 2 mg/ml type I collagenase (Sigma-Aldrich; Merck
Millipore) and 1.2 mg/ml type I trypsin (Sigma-Aldrich; Merck
Millipore) and aerated with 5% CO2 and 95% O2
at 36.5°C for 25 min until complete dissociation was confirmed
microscopically. DRG neurons were rinsed in an oxygenated, standard
extracellular solution containing 145 mmol/l tetraethylammonium
chloride, 0.8 mmol/l MgCl2, 5 mmol/l CaCl2,
20 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
(HEPES; Sigma-Aldrich, Merck Millipore) and 10 mmol/l D-glucose
(the pH was adjusted to 7.3 with Tris base) to terminate the enzyme
reactions. Individual neurons were dissociated by passing DRG
fragments through a set of fire-polished glass pipettes. Finally,
neurons were plated onto culture dishes consisting of extracellular
solution through the coated metallic screen (250 mesh, WS Tyler,
Mentor, OH, USA). Cells were allowed to attach to the culture
dishes for 2–3 h at room temperature.
Whole-cell patch-clamp recording
Neurons were studied 8–10 h after dissociation.
Cells were visualized with an inverted microscope and their soma
diameter was determined as small (<30 µm), medium (30–40 µm) or
large (>40 µm) with the aid of a calibrated microscope eyepiece
reticle (Olympus Corporation, Tokyo, Japan). The subsequent
analysis focused on the medium-diameter neurons, which were
considered as the nociceptive afferent (Aδ and C fibres,
slow-conducting velocities) and low-threshold sensory afferent
(14).
Whole-cell configuration of the patch-clamp
technique was used for current clamp and voltage-clamp
electrophysiological recordings using the EPC-9 patch-clamp
amplifier (HEKA Elektronik, Lambrecht, Germany) with an AgCl
reference electrode. Electrical stimuli pulses and current
recording were controlled and performed using PULSE+PULSEFIT ver.
8.79 (HEKA Elektronik). Channel currents were sampled using the
ITC-16 data acquisition system (InstruTech; HEKA Elektronik).
Recordings were acquired at 20 kHz, filtered at 2 kHz and sampled
using a computer with Digidata 1200 A/D acquisition board (HEKA
Elektronik) for offline analyses. Sigmaplot ver. 10.0 (Systat
software, Inc., San Jose, CA, USA) was used for data analysis. The
current-voltage curves were generated using Igor pro 4.0
(WaveMetrics, Inc., Lake Oswego, OR, USA) software.
Micro-electrodes were pulled from glass capillaries
(diameter, 1.6 mm; Beijing Xianqu Weifeng Technology Development
Co., Beijing, China) using a pipette puller (PP-830; Narishige
Scientific Instrument Lab., Tokyo, Japan). The diameter of the
micro-electrode tip was 1–2 mm. The resistance of the internal
solution [CsCl 135 mmol/l, MgCl2 2 mmol/l, EGTA 11
mmol/l, CaCl2 1 mmol/l, HEPES 20 mmol/l,
magnesium-adenosine triphosphate (Mg-ATP) 5 mmol/l, guanosine
5′-triphosphate lithium salt (Li-GTP) 0.4 mmol/l, pH 7.3], filled
into pipettes was 3–6 MΩ. Micropipettes were positioned on the
membrane and gentle suction was applied to obtain a resistant seal
in the GΩ range (>1 GΩ). Following rupture of the membrane with
suction at negative pressure, entering the whole-cell recording
configuration, neurons were allowed to reach adequate equilibration
between the internal pipette solution and the cell interior for 10
min. The slow capacitance, system resistance, series resistance,
capacitance current and leakage current were electronically
compensated with the compensation circuitry of the amplifier during
current recording. Effective data was defined as a steady current
and series resistance <20 MΩ.
Drug application
Drugs were added to extracellular solution, bathing
the dissociated DRG neurons. Fluid changes and continuous
perfusions were achieved through a gravity-dependent flow system.
Stocks of GBP, ω-conotoxin MVIIC, ω-conotoxin MVIIA,
CdCl2 and Li-GTP (Sigma-Aldrich; Merck Millipore) were
prepared in distilled water and stored at −20°C.
Data analysis and statistics
The current density of certain cells was obtained
from the inward current standardized by the membrane capacitance
and compared with those of other cells. The voltage-dependent
current curve was fit according to the Boltzmann equation,
G/Gmax=1/{1+exp[(Va1/2-Vm)/Ka]}, where G is
the conductance calculated as G=I/(Vm-Vr),
where I is the current, Vm is the command voltage,
Vr is the reversal potential, Va1/2 is the
voltage at the half maximal activation current and Ka is the slope
factor describing the voltage dependence of the conductance. The
curve of the steady-state inactivation current was fit according to
the Boltzmann equation, I/Imax = 1 / {1 + exp [(V -
Vi1/2) / Ki]}, where I is the current, V is the
pre-stimulus voltage, Vi1/2 is the voltage at the half
maximal inactivation current and Ki is the slope factor of
inactivation.
SPSS statistical software ver. 16.0 (SPSS Inc.,
Chicago, IL, USA) was used for statistical analysis. Values are
expressed as the mean ± standard deviation. Two-way analysis of
variance was used for comparison among groups and the q test was
used for pairwise comparison. Statistical analysis between any two
groups was performed using the group t-test and a paired
t-test was used for comparison of pre- and post-drug
application within groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Confirmation of establishment of the
rat model of neuropathic pain
All SNL-treated rats that underwent
electrophysiological analysis exhibited clear behavioral
indications of allodynia, mechanical hyperalgesia and thermal
hyperalgesia. Prior to surgery, no significant differences in the
mean foot withdrawal threshold were observed between the
contralateral and ipsilateral feet of the mice in each group as
well as between the SNL and sham groups. Mean withdrawal thresholds
obtained from the feet ipsilateral to the SNL decreased
significantly on the first post-operative day and remained
significantly lower than the pre-operative value for up to 14
post-operative days (P<0.05; Fig. 1A
and B). By contrast, there were no significant changes in the
withdrawal threshold in paws contralateral to the SNL between the
groups or for either foot in the sham-operated group. The latency
for foot withdrawal in SNL rats significantly decreased in response
to thermal stimuli delivered to the ipsilateral foot on the first
post-operative day and remained lower during the entire observation
period, compared with the non-operated side in the SNL rats
(P<0.05; Fig. 1C). However,
latency was not affected by the sham operation.
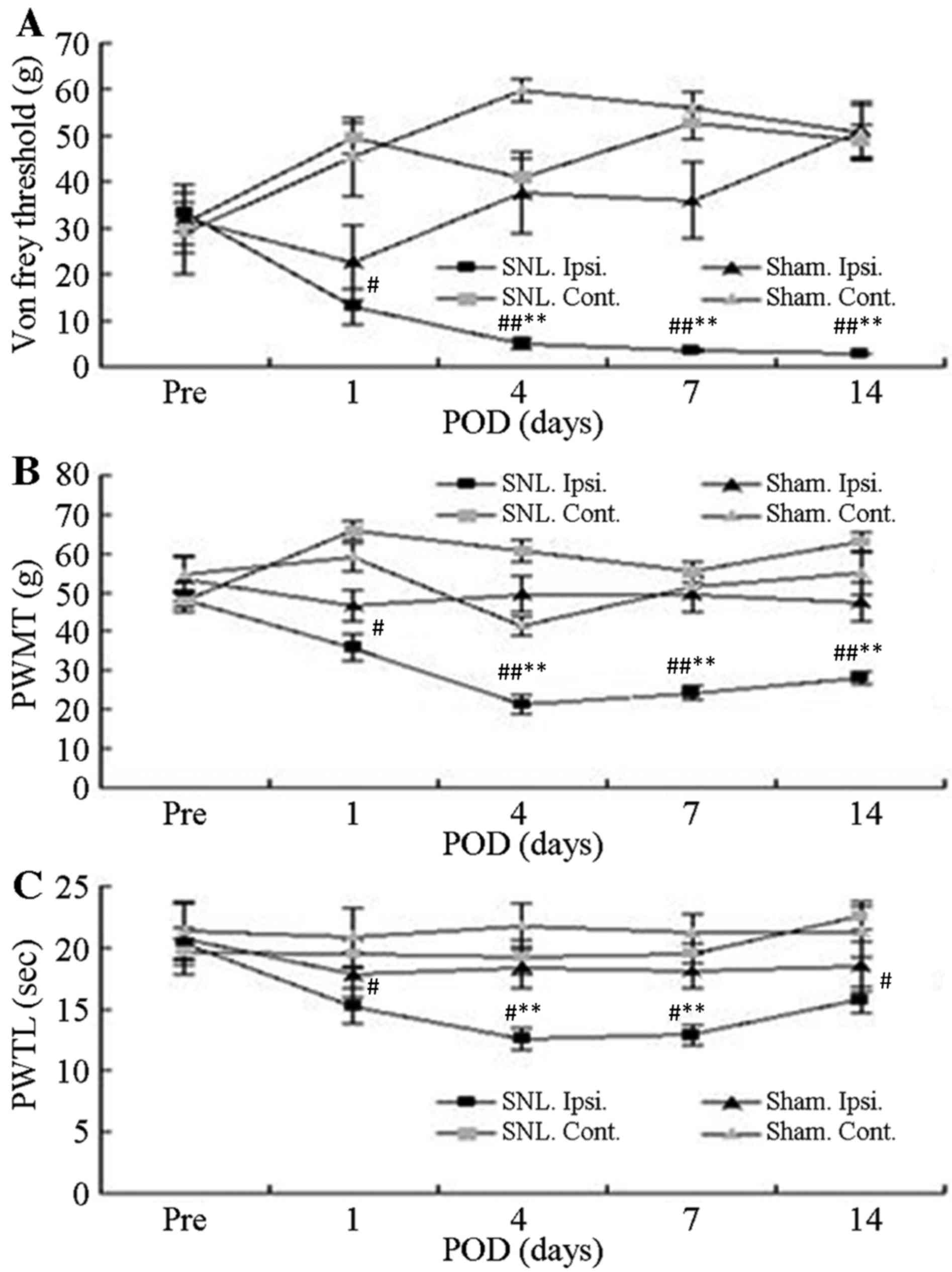 | Figure 1.Withdrawal responses of Ipsi. and
Cont. feet following mechanical and thermal stimulation. (A) Mean
threshold for withdrawal following Von Frey stimulation with
different bending forces. (B) Thresholds of paw withdrawal in
response to mechanical stimuli. (C) Mean latencies of thermal
withdrawal responses. Values are expressed as the mean ± standard
deviation. #P<0.05 and ##P<0.01, Pre
vs. Pod (paired t-test); *P<0.05 and **P<0.01, Ipsi.
foot responses in SNL (n=16) vs. sham group (n=7) (analysis of
variance). Pre, pre-operative period; Pod, post-operative days;
Ipsi., paw ipsilateral to surgery; Cont., paw contralateral to
surgery; PWMT, paw withdrawal mechanical threshold; PAWTL, paw
withdrawal time latency; SNL, spinal nerve ligation; sham, sham
operation. |
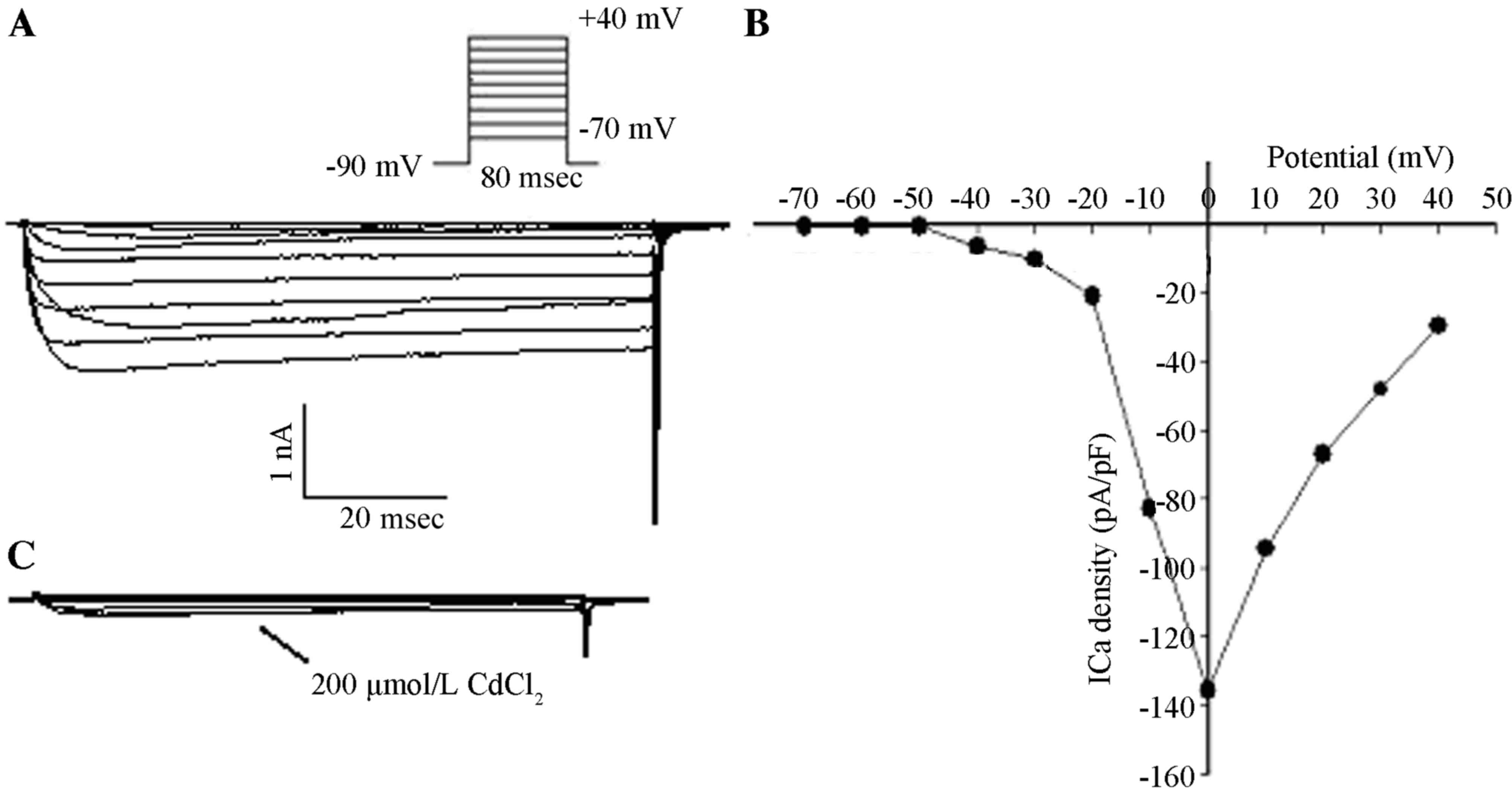
Dose-dependent inhibition of the
HVA-Ca2+ peak current by GBP
After entering the whole-cell recording
configuration, inward currents were activated under the command
voltage from −70 to +40 mV at 80-msec intervals in 10 mV increments
and with a holding potential (VH) of −90 mV. There were
no low-voltage-activated (LVA) components and no fast component of
inactivation in the inward currents. Activation of inward currents
occurred at −40 mV and peak inward currents occurred at 0 mV
according to the current density-potential (I–V) curve. Inward
currents were completely suppressed following the addition of 200
µmol/l CdCl2 with the same stimuli, which was in
accordance with the properties of HVA-Ca2+ currents
(Fig. 2).
Voltage steps of 80 msec were applied to various
test potentials between −70 and +40 mV in 10-mV steps with 5-sec
intervals from a VH of −90 mV. This was performed to
examine current-voltage associations in axotomized, adjacent
neurons and control neurons from the L4 and
L5 DRGs and attached dorsal roots of sham-operated rats.
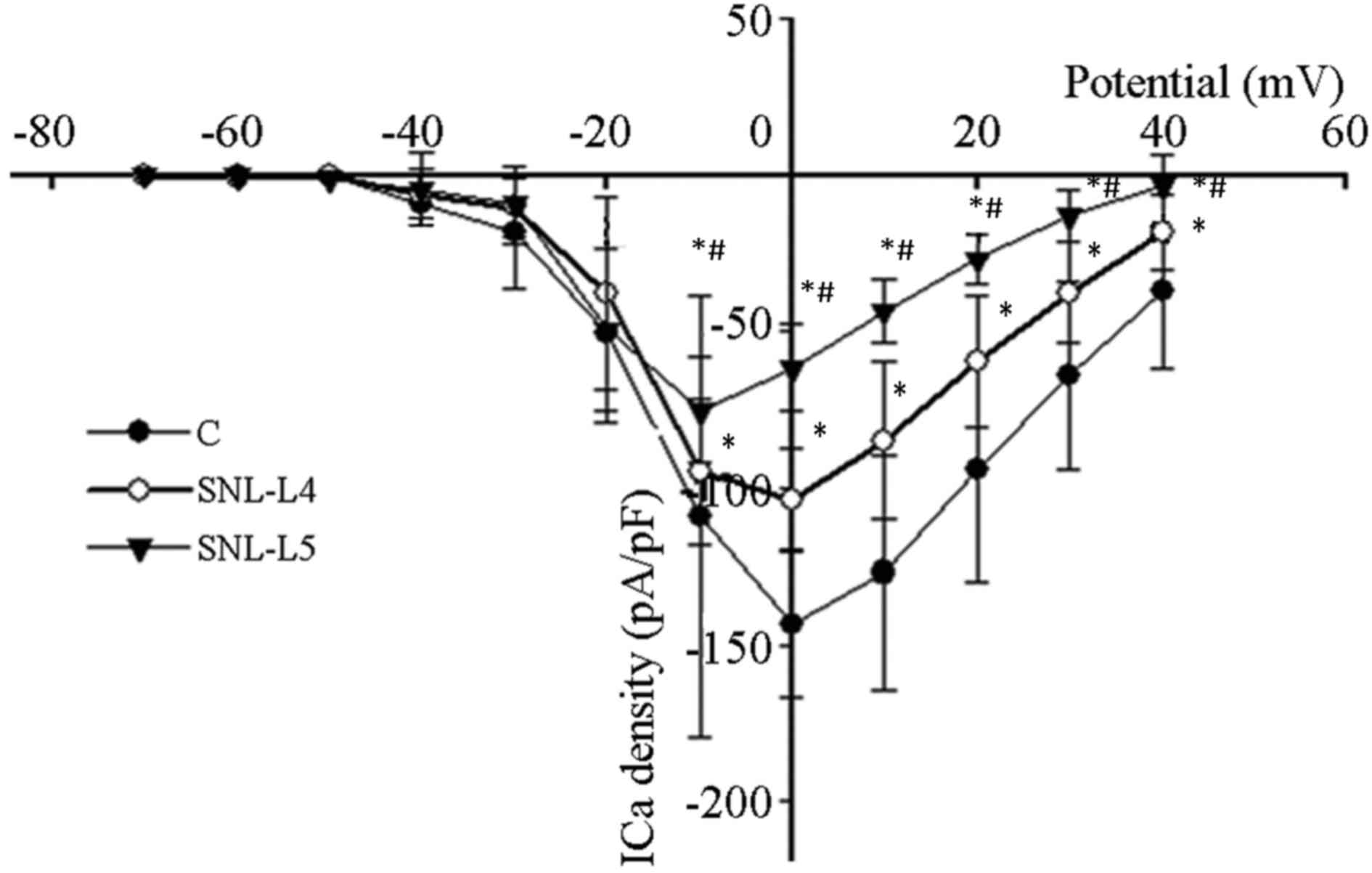
Peak inward calcium current levels were measured, corrected for
cell size and subsequently expressed as the peak current density
(pA/pF). Peak current densities of control, adjacent and axotomized
neurons were 144±24, 104±17 and 75±17 pA/pF, respectively. Compared
with that of control neurons, the peak current density of adjacent
and axotomized neurons decreased significantly (P<0.05) and the
peak current density of axotomized neurons was significantly lower
than that of adjacent neurons (P<0.05). The activation voltage
of the peak HVA-Ca2+ current density in control and
adjacent neurons was 0 mV, while that of axotomized neurons was −10
mV (Fig. 3).
Steady maximal activation of HVA-Ca2+
currents was achieved with a voltage of V= 10 or 0 mV at 80-msec
intervals and the dose-dependent inhibition effect of GBP was
subsequently determined using 0.1, 1, 10, 100 and 300 µmol/l GBP.
Changes in peak inward Ca2+ flux (ICa) were recorded and
the inhibition of currents with GBP at various doses was
calculated. The peak ICa was significantly enhanced in cells
exposed to higher concentrations of GBP (10, 100 and 300 µmol/l;
P<0.05), in comparison with cells exposed to lower
concentrations of GBP (0.1 and 1 µmol/l). GBP significantly
inhibited the peak inward ICa in a dose-dependent manner.
Furthermore, the inhibitory effects of GBP on the peak current of
axotomized neurons at concentrations of 10, 100 and 300 µmol/l were
significantly higher than those on the current of control and
adjacent neurons (P<0.05). There were no significant differences
in the inhibitory effects on the peak current between control and
adjacent neurons at various concentrations of GBP (P>0.05;
Table I).
 | Table I.Comparison of peak current inhibition
rates (%) among the three groups at various gabapentin
concentrations. |
Table I.
Comparison of peak current inhibition
rates (%) among the three groups at various gabapentin
concentrations.
|
| Gabapentin
concentration (µmol/l) |
|---|
|
|
|
|---|
| Group | 0.1 | 1 | 10 | 100 | 300 |
|---|
| C (n=7) | 10.8±1.5 | 12.8±1.8 |
15.6±2.1a |
27.3±2.9a,b |
28.1±3.3a,b |
| SNL-L4
(n=5) | 10.5±1.6 | 12.2±2.1 |
16.0±1.9a |
26.9±2.0a,b |
27.4±2.3a,b |
| SNL-L5
(n=8) | 11.4±1.5 | 12.9±1.0 |
18.5±1.7a,c,d |
32.0±2.6a–d |
32.7±2.8a–d |
Effects of GBP on the kinetics of
HVA-Ca2+ channels
The membrane VH was set at −90 mV and
calcium currents were elicited by a series of command voltages
(range, −70 to +40 mV) with successive increments of 10 mV at
80-msec intervals. As shown in Fig.
4, the midpoint potentials for the activation curves
(Va1/2) of control, adjacent and axotomized neurons were
−16.23±1.92, −18.03±0.37 and −20.73±0.33 mV, respectively, with the
Va1/2 of axotomized neurons being significantly lower
than that of the control and adjacent neurons (P<0.05; Fig. 4C); however, no difference was
observed between those of the control and adjacent neurons
(P>0.05; Fig. 4C). To study the
kinetics of channel inactivation, calcium currents were generated
by applying a membrane VH at −90 mV for 10 msec,
followed by series of command voltages from −70 to +20 mV with
successive increments of 10 mV at 500-msec intervals. Subsequently,
command voltages of −10 mV at 80-msec intervals were applied. The
midpoint potentials for the inactivation curves (Vi1/2)
of control, adjacent and axotomized neurons were −31.93±1.03,
−31.45±1.87 and −32.73±1.37 mV, respectively. There were no
significant differences in the midpoint potentials among the three
types of neurons (P>0.05; Fig.
4D).
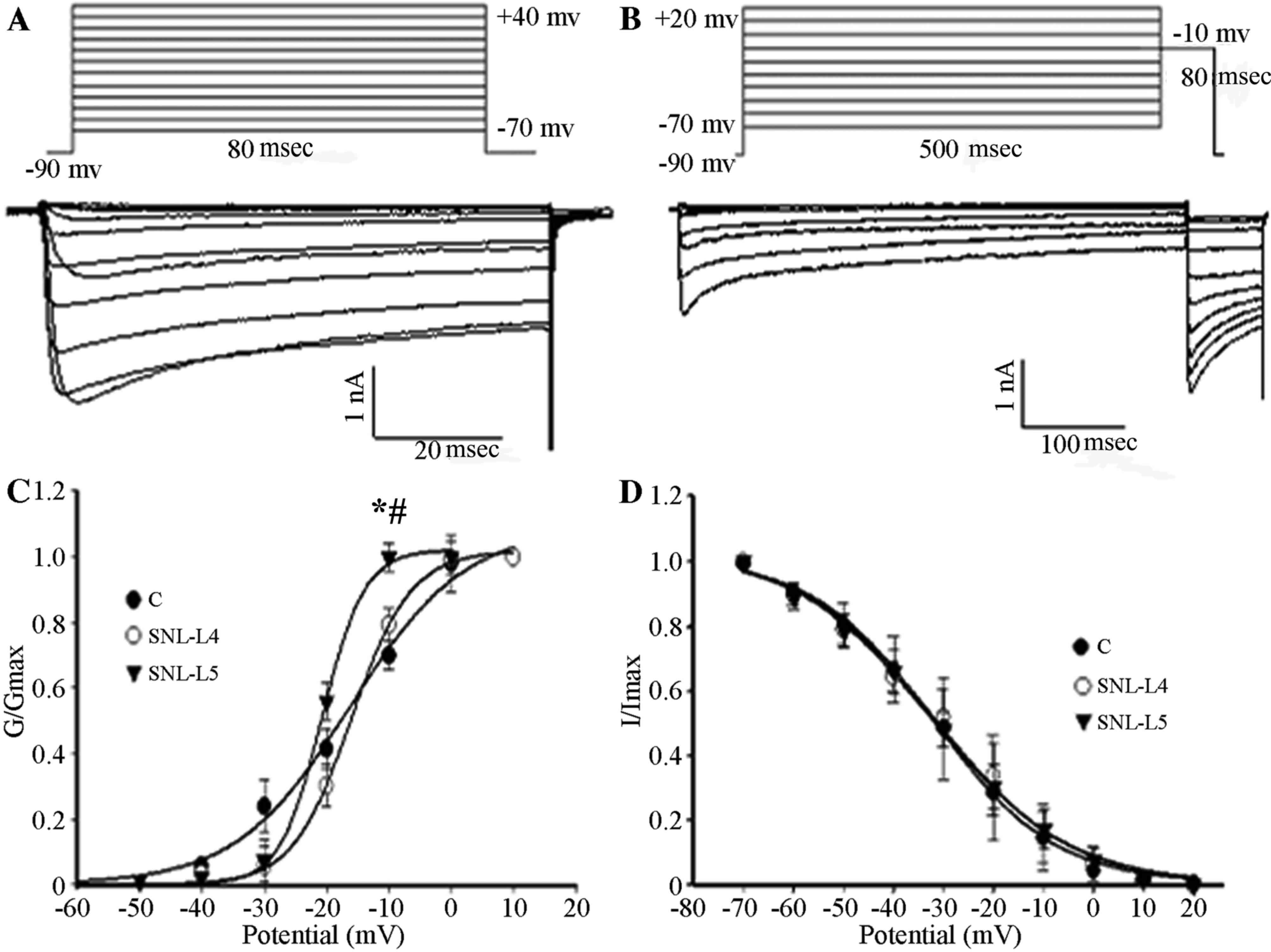 | Figure 4.(A) Activation. The membrane
VH was set at −90 mV, calcium currents were elicited by
a series of command voltages ranging from −70 to +40 mV with
successive increments of 10 mV with 80-msec intervals. (B)
Steady-state inactivation. Ca2+ currents were generated
by the membrane VH at −90 mV for 10 msec, immediately followed by a
series of command voltages from −70 to +20 mV with successive
increments of 10 mV at 500-msec intervals. Subsequently, command
voltages of −10 mV were applied at 80-msec intervals. (C)
Activation curves were fitted using the Boltzmann equation (control
neurons, n=10; adjacent neurons, n=5; axotomized neurons, n=8). A
depolarized shift was observed in adjacent and axotomized neurons.
Va1/2 of three groups was compared. *P<0.05 vs. control neurons.
#P<0.05 regarding axotomized vs. adjacent neurons.
(D) Inactivation curves were fitted using the Boltzmann equation
(control neurons; n=6; adjacent neurons, n=6; axotomized neurons,
n=7). Vi1/2 of three groups was compared. Values are expressed as
the mean ± standard deviation. C, control; SNL, spinal nerve
ligation; VH, holding potential; G, conductance; I,
current; L4, adjacent neurons; L5, axotomized neurons. |
To study the effects of GBP on the kinetics of
HVA-Ca2+ channel activation, I–V associations for the
transient calcium channel currents were plotted using 10-mV
incremental step pulses from −70 to +40 mV at 80-msec intervals
with a membrane VH at −90 mV in the presence of 100
µmol/l GBP. The Va1/2 decreased from −16.23±1.92 to
−19.69±1.07 mV (P<0.05), −18.03±0.37 to −21.73±0.64 mV
(P<0.05) and −20.73±0.33 to −23.94±0.15 mV (P<0.05) in the
control, adjacent and axotomized neurons, respectively (Fig. 5). To investigate the effects of GBP
on the kinetics of HVA-Ca2+ channel inactivation,
steady-state inactivation was measured in a conventional way using
a 500-msec pre-pulse from −70 to +20 mV in 10-mV increments with a
−90-mV membrane VH for 10 msec followed by a second test
pulse at −10 mV for 80 msec in the presence of 100 µmol/l GBP. By
addition of 100 µmol/l GBP, the current required to inactivate
Va1/2 was decreased from −38.78±2.05 to −31.93±1.03 mV in control
neurons, from −36.22±2.80 to −31.45±1.87) mV in adjacent neurons
and from −44.23±2.30 to −32.73±1.37 mV in axotomized neurons
(Fig. 5).
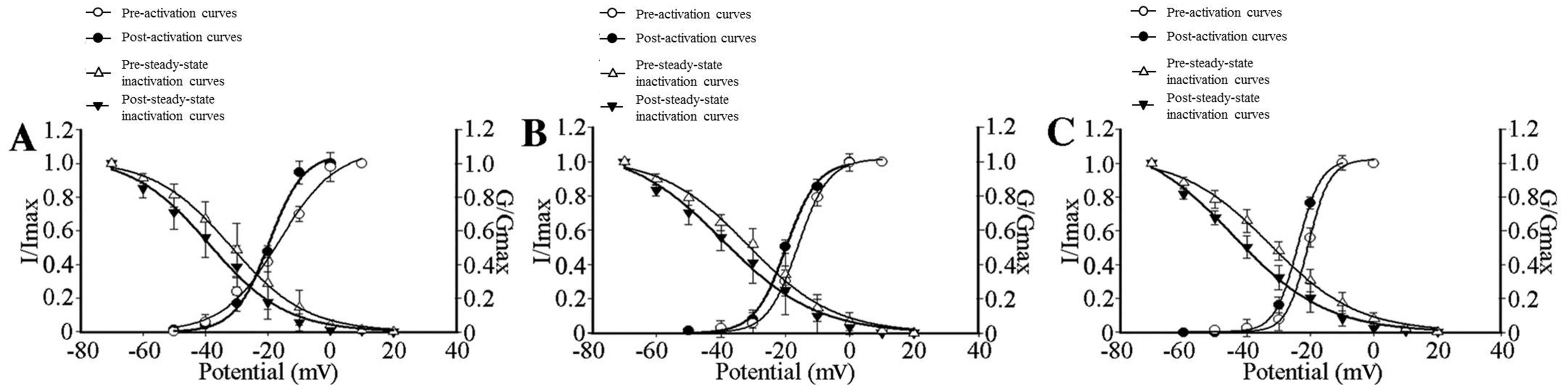 | Figure 5.HVA-Ca2+ current
activation and steady-state inactivation curves of control,
adjacent and axotomized neurons in the absence or presence of 100
µmol/l GBP. (A) Activation (n=10) and steady-state inactivation
(n=6) curves shifted towards a hyperpolarization direction in
control neurons, which reduced the ‘window currents’, in the
presence of 100 µmol/l GBP. Va1/2 decreased from
−16.23±1.92 to −19.69±1.07 mV with GBP added (P<0.05);
Vi1/2 was inactivated at −31.93±1.03 mV vs. −38.78±2.05
(P<0.05). (B) A hyperpolarized shift was observed in activation
(n=5) and steady-state inactivation (n=6) curves of adjacent
neurons; however, ‘window currents’ remained unchanged in the
presence of 100 µmol/l GBP. Va1/2 decreased from
−18.03±0.37 to −21.73±0.64 mV (P<0.05) with the addition of GBP;
Vi1/2 was inactivated at −31.45±1.87 mV vs. −36.22±2.80
mV (P<0.05). (C) Activation (n=8) and steady-state inactivation
(n=7) curves in axotomized neurons, showing reduced ‘window
currents’ in the presence of 100 µmol/l GBP. Compared without using
GBP, after adding GBP, Va1/2 and Vi1/2
decreased from −20.73±0.33 and −32.73±1.37 mV towards −23.94±0.15
mV (P<0.05) and −44.23±2.30 mV (P<0.05). Values are expressed
as the mean ± standard deviation. GBP, gabapentin; HVA,
high-voltage activation; Va1/2, voltage at the half
maximal activation current; Vi1/2, voltage at the half
maximal inactivation current; I, current; G, conductance. |
The voltage dependence of activation shifted in a
depolarized direction, whereas the Vi1/2 remained
unchanged in axotomized as well as adjacent uninjured DRG neurons,
compared with those in control neurons. The shift in activation
reduced the ‘window current’ between inactivation and activation,
thereby stopping the sustained inward ICa. Following the addition
of GBP, the activation and steady-state Vi1/2 of three
groups all shifted in a hyperpolarized direction. The shift in
activation and inactivation reduced the overlap (‘window currents’)
between inactivation and activation in control and axotomized
neurons, while the ‘window currents’ remained unchanged in adjacent
neurons (Fig. 5).
Effects of SNL injury and GBP on
HVA-Ca2+ channel currents
Maximal activation of the HVA-Ca2+
currents occurred with V=10 or 0 mV at 80-msec intervals, with a
membrane VH of −90 mV. To individually assess different
sub-types of HVA-Ca2+ channels present in the same cell,
20 µmol/l nifedipine (L-type calcium channel blocker), 2 µmol/l
ω-conotoxin MVIIC (P/Q-type calcium channel blocker) and 2 µmol/l
ω-conotoxin MVIIA (N-type calcium channel blocker) were applied to
the bath in sequence when stable currents were reached. Similar
activation voltages were applied to the same cells to evoke
Ca2+ currents. Currents subtracted prior to and
following application of respective blocker yielded L-, N-,
P/Q-type currents. Subsequently, 100 µmol/l GBP was applied to
determine its inhibitory efficacy on various sub-types of HVA
calcium channel current.
As shown in Fig. 6,
the proportion of N-type Ca2+ currents in axotomized
neurons was significantly higher (50.0±2.7%) than that in control
neurons (35.9±1.4%) and adjacent neurons (37.1±2.0%; P<0.05;
Fig. 6B). The contribution of L-type
channels to the HVA-Ca2+ influx in axotomized neurons
was markedly reduced, as 15.4±2.3% of the HVA-Ca2+
current was blocked by 20 µmol/l nifedipine compared to 27.9±2.5%
in control neurons and 26.1±1.8% in adjacent neurons (P<0.05).
There was no significant difference in the proportion of each
sub-type of HVA-Ca2+ channel current between adjacent
and control neurons (P>0.05; Fig.
6B). However, the P/Q-type current was similar between the
three groups.
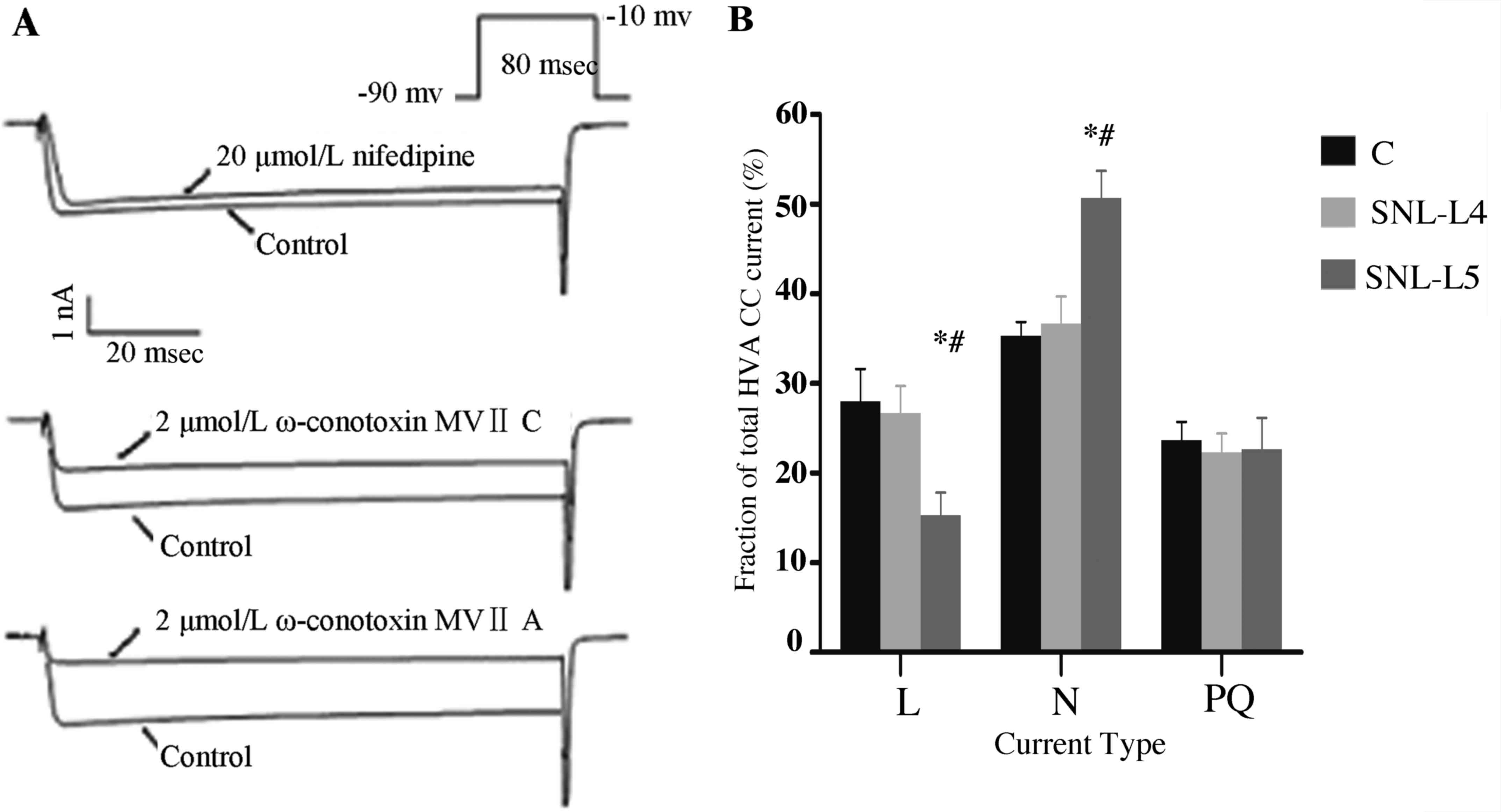 | Figure 6.Sub-types of HVA-Ca2+
currents identified by application of specific channel blockers.
(A) To individually assess various types of calcium channels
present in the same cell, 20 µmol/l nifedipine (L-type
Ca2+-specific channel blocker), 2 µmol/l ω-conotoxin
MVIIC (P/Q-type Ca2+ channel-specific blocker) and 2
µmol/l ω-conotoxin MVIIA (N-type Ca2+ channel-specific
blocker) were applied to the bath in sequence when stable currents
were reached. (B) Fraction changes of each sub-type (L, N, P/Q)
Ca2+ channel current over the total HVA-Ca2+
peak currents in control, adjacent and axotomized neurons. Values
are expressed as the mean ± standard deviation (n=10). *P<0.05
vs. control neurons (ANOVA); #P<0.05 regarding
axotomized vs. adjacent neurons (ANOVA). HVA, high-voltage
activation; ANOVA, analysis of variance; SNL, spinal nerve
ligation; L4, adjacent neurons; L5, axotomized neurons. |
The proportion of N-type Ca2+ currents in
axotomized neurons was significantly higher (81.0±2.8%) than that
in control and adjacent neurons (68.8±4.5 and 69.7±3.3%,
respectively; P<0.05), suggesting that the N-type
Ca2+ channel current is the GBP-sensitive sub-type of
HVA-Ca2+ channel currents (Table II). However, the N- and P/Q-type
currents were not affected by GBP.
 | Table II.Sensitive Ca2+ current
ratios (%) of each channel sub-type (L, P/Q and N) among the three
groups. |
Table II.
Sensitive Ca2+ current
ratios (%) of each channel sub-type (L, P/Q and N) among the three
groups.
|
| HVA-Ca2+
subtype |
|---|
|
|
|
|---|
| Group | L | P/Q | N |
|---|
| C | 35.2±4.3 | 43.4±3.5 | 68.8±4.5 |
| SNL-L4 | 34.9±3.7 | 43.6±4.7 | 69.7±3.3 |
| SNL-L5 | 32.5±3.4 | 45.6±4.6 |
81.0±2.8a,b |
Discussion
The present study demonstrated that SNL of the
peripheral nerve of rats as a model of neuropathic pain injury
significantly reduced HVA-Ca2+ currents. A decrease in
current density, changes in the proportion of HVA-Ca2+
channel current sub-types and a depolarizing shift in the voltage
dependence of activation appeared to cause this reduction in
HVA-Ca2+ currents.
According to the I–V curves, the peak current
density of axotomized neurons decreased and was lower than that of
control and adjacent uninjured DRG neurons. Similar results have
been observed in other rat models, including that of chronic
constriction injury (CCI). In this model, the somata of acutely
dissociated DRG neurons from hyperalgesic rats exhibited a
decreased ICa and injury of medium and large neurons decreased the
peak calcium channel current density (15). McCallum et al (15) examined the ICa specifically in
axotomized neurons generated by SNL and indicated that current loss
is present in all neuronal size groups. Furthermore, current loss
was evident in the adjacent L4 neurons. These results
indicated that various types of nerve injury, such as axotomy,
result in ICa loss in primary sensory neurons of all sizes and
includes HVA and LVA current types. This may be a common feature of
nerve injury (16).
One possible explanation for the reduction in
Ca2+ currents in DRG neurons observed in the present
study is that the calcium channel density in the membrane decreases
following axotomy. This may occur due to alterations in channel
synthesis or degradation, post-translational modifications
affecting the insertion of the channel into the membrane or the
transport rate of channels to more distal locations. Alternatively,
changes in the single-channel properties of somatic calcium
channels may occur (17). The
present study compared changes in the HVA-Ca2+ channel
kinetics in control, adjacent and axotomized DRG neurons in model
rats subjected to SNL. It was demonstrated that neuropathic injury
shifted the voltage dependence of activation in a depolarized
direction, whereas Vi1/2 remained unchanged in
axotomized and adjacent uninjured DRG neurons. This shift in
activation reduced the ‘window current’ between inactivation and
activation, thereby attenuating the sustained inward ICa. A similar
decrease in the overlap of the Ca2+ influx has
previously been observed in a rat model of CCI (18).
The importance of ICa in the functioning of neurons
means that altered ICa may contribute to functional abnormalities
that accompany neuropathic pain (16). It is possible that the elevated
excitability observed in neurons following axonal injury is the
result of diminished ICa. It has been demonstrated that the
tetradotoxin-resistant (TTX-R) sodium current provides the greatest
amount of total inward current during the downstroke and HVA
calcium channels carry a substantial inward current in the form of
an AP (19). These currents
decreased in the TTX-R sodium channel and HVA calcium channels
following axonal injury. The direct effect of ICa contributes to AP
formation and admits Ca2+ through voltage-dependent
calcium channels (VGCCs), which acts on Ca2+-sensitive
K+ channels thus generating inward Ca2+ flux
through Ca2+-sensitive K+ channels [IK(Ca)].
In surgery, they contribute to the repolarization of the AP and the
generation of AHP (16). AHP
determines spike frequency adaptation and the ability of sensory
neurons to maintain rapidly firing pulse trains, thus regulating a
critical aspect of neuronal excitability (20). There is a correlation between reduced
inward ICa and delayed AP depolarization, whereas the outward
current, presumably IK(Ca), is reduced at the onset of AHP and
during the repolarization phase of the AP (16). Increased burst firing from decreased
AHP results in greater nociceptive traffic on the secondary neurons
of the dorsal horn, where prolonged AP duration results in greater
excitatory neurotransmitter release (16). Increased repetitive firing during
sustained depolarization occurs in traumatized nociceptive neurons
following axotomy. Thus, axotomized neurons, particularly
pain-conducting ones, become unstable and exhibit increased
excitability (16).
In the present study, the reduction of peak current
density and ‘window currents’ was observed in adjacent neurons,
similar to that in axotomized neurons, due to the injury-induced
change of channel activation. L4 afferent fibers
commingled with degenerating L5 axons in the peripheral
nerve, therefore, Wallerian degeneration may result in enhanced
excitability of intact neurons with somata in L4 DRGs
adjacent to the axotomized L5 DRGs. For example, glial
cell line-derived neurotrophic factor or nerve growth factor;
cytokines, including tumor necrosis factor, interleukin-1 or other
inflammatory mediators released by immune cells; and Schwann cells
activated by L5 axonal degeneration, may activate
adjacent neurons and lead to hyperexcitability in adjacent neurons,
which may result in changes occurring in the distribution and
activation state of ion channels (6). Furthermore, intense bursts of activity
along the L5 pathway sensitize the spinal dorsal horn to
input along intact (i.e. SNL L4) pathways, so that
natural stimuli are perceived as more intense (16). The process of cross-excitation
spreads activity among adjacent neurons in the DRG. The injured
adjacent afferents remaining intact following SNL serve a critical
role in the development of neuropathic pain by interacting with
degenerating afferents and by peripherally-propagating injury
discharge, thus inducing peripheral sensitization (21).
The present study revealed that in injured neurons,
the proportion of L-type calcium currents decreased and N-type
calcium currents increased, whereas the proportion of P/Q-type
calcium currents remained unchanged compared with currents in
control and adjacent uninjured neurons. Yang et al (22) and Sun et al (23) observed an upregulation of the N-type
Ca2+ current in injured DRG neurons in partial sciatic
nerve ligation and SNL models, respectively. Hogan et al
(24) indicated that injury affected
the HVA-Ca2+ currents of the N- and P/Q-type channels,
whereas no effects on the L-type channel current were noted.
Another study demonstrated that L-type calcium channel-associated
gene expression decreased in rat DRGs following CCI and sciatic
nerve axotomy (25). The proportion
of N-type calcium currents in adjacent uninjured neurons remained
unchanged, which may be due to the inflammatory environment caused
by peripheral nerve injury. It has been determined that N-type
calcium channel function changes in the rat dorsal horn during
inflammation (26). Selective
downregulation in the contribution of this calcium channel to
excitatory synaptic transmission recorded from neurokinin 1
receptor-positive neurons was observed in the laminae I (26).
Results from animal experiments (27,28) and
clinical applications (29) suggest
that GBP exhibits significant efficacy in treating chronic pains,
particularly neuropathological pain; however, distinct
neuroplasticities in various neuropathies may underlie the
complexity of the antiallodynic actions of GBP. This indicates that
the different effects in treating neuropathological pain may be due
to the specific mechanism of nerve injury. The results of the
present study demonstrated the dose-dependent inhibition of GBP
towards the HVA-Ca2+ currents of control, adjacent and
axotomized DRG neurons. Following application of 10, 100 and 300
µmol/l GBP, peak current densities decreased in control, adjacent
and axotomized neurons, among which the inhibitory effects on the
neuronal HVA-Ca2+ current of axotomized DRG neurons were
more significant. Sarantopoulos et al (30) demonstrated that GBP rapidly and
reversibly decreased the neuronal peak ICa of sham and neuropathic
rats in a concentration-dependent manner; however, with a lack of
significant differences in decreases among various groups.
The Va1/2 and Vi1/2, which
were fitted using the Boltzmann equation, indicated a shift towards
the hyperpolarized direction, and the ‘window current’ between the
activation curve and the homeostatic inactivation curve changed. An
in vivo experiment performed by Sutton et al
(31) revealed that the inhibitory
effect of GBP was dose- and voltage-dependent, producing a
hyperpolarizing shift in current-voltage curves and reducing the
non-inactivating component of the whole-cell current activated at
relatively depolarized potentials.
Spinal nerve injury may increase the expression of
the VGCC alpha-2-delta-1 sub-unit in the spinal dorsal horn and
sensory neurons in the DRG that correlate with established
neuropathic pain states (8,32) and GBP-sensitive allodynia (27). The auxiliary α2δ sub-unit is a
membrane-anchored protein and interacts with the extracellular
domains of the α1 sub-unit. Each auxiliary sub-unit is involved in
the trafficking of the channel complex to the membrane and modifies
current properties, including the kinetics of activation and
inactivation. α2δ sub-units modify inward calcium currents by
regulating the α1 sub-unit (33). In
addition, co-expression of α2δ with various α and β sub-units
results in the acceleration of current activation and inactivation,
an increase in current density and dihydropyridine binding sites,
and a hyperpolarising shift of the current-voltage curves (9). The a2δ-1 sub-unit represents the target
for gabapentin in the alleviation of hyperalgesia in experimental
models of neuropathic pain (34).
The findings of the present study revealed that the
mechanism of GBP blockage of HVA calcium channel currents
correlates with the injury-induced changes of activation and
inactivation kinetics of the HVA-Ca2+ channel.
In the SNL model, alterations of neural signaling in
injured and uninjured neurons contributed to the development of
neuropathic pain following peripheral nerve injury. However, the
pathological mechanisms of the two types of neuronal damage with
injured or uninjured neurons differ; the former may mainly be
affected by the direct damage of SNL, while the latter primarily
arises due to the reaction products associated with Wallerian
degeneration following the SNL (35). In the present study, it was observed
that the ‘window current’ decreased in control and axotomized
neurons, whereas the ‘window current’ in adjacent neurons was
unchanged following the application of GBP, indicating that its
inhibitory effects may depend on the particular neuropathological
or inflammatory conditions.
Furthermore, in the present study, there was a
certain degree of overlap in the inhibitory effects of various
Ca2+ channel blockers on the action of GBP. The mixed
pharmacology of the GBP-sensitive current suggests that inhibition
may reflect the direct action of GBP on a number of different
sub-types of calcium channel, dominated by the N-type current in
this cell-type, similar to results from the study by Sutton et
al (31).
Among all VACCs, N-type Ca2+ channels,
which are potential molecular targets, are considered to be the
most important ones in mediating neuropathic pain and the
GBP-dependent modulation of pre-synaptic N-type channels in DRG
neurons may prove an important means of mediating neuronal
excitability and neurotransmitter release (31). In the present study, the proportion
of N-type Ca2+ currents among the GBP-sensitive
Ca2+ currents was increased in axotomized neurons,
resulting in an increase of the main action target channels of GBP,
which may be one of the causes of its enhanced inhibitory effect on
HVA-Ca2+ currents in injured DRG neurons.
In conclusion, the present study demonstrated that
the inhibitory effect of GBP on HVA-Ca2+ currents was
enhanced in the injured DRG neurons of neuropathic rats. This
action may be associated with changes in the activation and
inactivation kinetics of Ca2+ channels, as well as an
increase in the proportion of N-type Ca2+ currents. The
different effects of GBP on HVA-Ca2+ currents in
axotomized vs. adjacent neurons may depend on the particular
neuropathological condition or inflammatory circumstances induced
by peripheral nerve injury.
Acknowledgements
The present study was supported by the Scientific
Research Fund of China Post doctorates of the Chinese Government
(nos. 20080431417 and 200902694).
References
|
1
|
Costigan M, Scholz J and Woolf CJ:
Neuropathic Pain: A maladaptive response of the nervous system to
damage. Annu Rev Neurosci. 32:1–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sommer C: Neuropathic pain:
Pathophysiology, assessment and therapy. Schmerz. 27:619–632.
2013.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laedermann CJ, Pertin M, Suter MR and
Decosterd I: Voltage-gated sodium channel expression in mouse DRG
after SNI leads to re-evaluation of projections of injured fibers.
Mol Pain. 10:192014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsantoulas C, Zhu L, Shaifta Y, Grist J,
Ward JP, Raouf R, Michael GJ and McMahon SB: Sensory neuron
downregulation of the Kv9.1 potassium channel subunit mediates
neuropathic pain following nerve injury. J Neurosci.
32:17502–17513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sapunar D, Ljubkovic M, Lirk P, McCallum
JB and Hogan QH: Distinct membrane effects of spinal nerve ligation
on injured and adjacent dorsal root ganglion neurons in rats.
Anesthesiology. 103:360–376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma C, Shu Y, Zheng Z, Chen Y, Yao H,
Greenquist KW, White FA and LaMotte RH: Similar
electrophysiological changes in axotomized and neighboring intact
dorsal root ganglion neurons. J Neurophysiol. 89:1588–1602. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu YF, Wu Q and Henry JL: Changes in
functional properties of A-type but not C-type sensory neurons in
vivo in a rat model of peripheral neuropathy. J Pain Res.
5:175–192. 2012.PubMed/NCBI
|
|
8
|
Felix R, Calderón-Rivera A and Andrade A:
Regulation of high-voltage-activated Ca2+ channel function,
trafficking, and membrane stability by auxiliary subunits. Wiley
Interdiscip Rev Membr Transp Signal. 2:207–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou C and Luo ZD: Electrophysiological
characterization of spinal neuron sensitization by elevated calcium
channel alpha-2-delta-1 subunit protein. Eur J Pain. 18:649–658.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hooker BA, Tobon G, Baker SJ, Zhu C,
Hesterman J, Schmidt K, Rajagovindan R, Chandran P, Joshi SK,
Bannon AW, et al: Gabapentin-induced pharmacodynamic effects in the
spinal nerve ligation model of neuropathic pain. Eur J Pain.
18:223–237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kukkar A, Bali A, Singh N and Jaggi AS:
Implications and mechanism of action of gabapentin in neuropathic
pain. Arch Pharm Res. 36:237–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SH and Chung JM: An experimental model
for peripheral neuropathy produced by segmental spinal nerve
ligation in the rat. Pain. 50:355–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi Y, Yoon YW, Na HS, Kim SH and Chung
JM: Behavioral signs of ongoing pain and cold allodynia in a rat
model of neuropathic pain. Pain. 59:369–376. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harper AA and Lawson SN: Conduction
velocity is related to morphological cell type in rat dorsal root
ganglion neurons. J Physiol. 359:31–46. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCallum JB, Kwok WM, Sapunar D, Fuchs A
and Hogan QH: Painful peripheral nerve injury decreases calcium
current in axotomized sensory neurons. Anesthesiology. 105:160–168.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hogan QH: Role of decreased sensory neuron
membrane calcium currents in the genesis of neuropathic pain. Croat
Med J. 48:9–21. 2007.PubMed/NCBI
|
|
17
|
Baccei ML and Kocsis JD: Voltage-gated
calcium currents in axotomized adult rat cutaneous afferent
neurons. J Neurophysiol. 83:2227–2238. 2000.PubMed/NCBI
|
|
18
|
McCallum JB, Kwok WM, Mynlieff M, Bosnjak
ZJ and Hogan QH: Loss of T-type calcium current in sensory neurons
of rats with neuropathic pain. Anesthesiology. 98:209–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blair NT and Bean BP: Roles of
tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+
current, and Ca2+ current in the action potentials of nociceptive
sensory neurons. J Neurosci. 22:10277–10290. 2002.PubMed/NCBI
|
|
20
|
Lirk P, Poroli M, Rigaud M, Fuchs A,
Fillip P, Huang CY, Ljubkovic M, Sapunar D and Hogan Q: Modulators
of calcium influx regulate membrane excitability in rat dorsal root
ganglion neurons. Anesth Analg. 107:673–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang JH, Lee BH, Nam TS, Kim JW, Kim DW
and Leem JW: Peripheral contributions to the mechanical
hyperalgesia following a lumbar 5 spinal nerve lesion in rats.
Neuroscience. 165:221–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang L and Stephens GJ: Effects of
neuropathy on high-voltage-activated Ca (2+) current in sensory
neurones. Cell Calcium. 46:248–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun XD, Zhu MM, Chen XD, Li D, Wang Q,
Xiao H, Xu JG and Duan ML: Effects of gabapentin on
high-voltage-activated calcium current in dorsal root ganglion
neurons in rats. Zhonghua Yi Xue Za Zhi. 91:1713–1717. 2011.(In
Chinese). PubMed/NCBI
|
|
24
|
Hogan QH, McCallum JB, Sarantopoulos C,
Aason M, Mynlieff M, Kwok WM and Bosnjak ZJ: Painful neuropathy
decreases membrane calcium current in mammalian primary afferent
neurons. Pain. 86:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim DS, Yoon CH, Lee SJ, Park SY, Yoo HJ
and Cho HJ: Changes in voltage-gated calcium channel alpha(1) gene
expression in rat dorsal root ganglia following peripheral nerve
injury. Brain Res Mol Brain Res. 96:151–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rycroft BK, Vikman KS and Christie MJ:
Inflammation reduces the contribution of N-type calcium channels to
primary afferent synaptic transmission onto NK1 receptor-positive
lamina I neurons in the rat dorsal horn. J Physiol. 580:883–894.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo ZD, Calcutt NA, Higuera ES, Valder CR,
Song YH, Svensson CI and Myers RR: Injury type-specific calcium
channel alpha 2 delta-1 subunit up-regulation in rat neuropathic
pain models correlates with antiallodynic effects of gabapentin. J
Pharmacol Exp Ther. 303:1199–1205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gauchan P, Andoh T, Ikeda K, Fujita M,
Sasaki A, Kato A and Kuraishi Y: Mechanical allodynia induced by
paclitaxel, oxaliplatin and vincristine: Different effectiveness of
gabapentin and different expression of voltage-dependent calcium
channel alpha(2)delta-1 subunit. Biol Pharm Bull. 32:732–734. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gabapentin for Adults with Neuropathic
Pain, . A Review of the Clinical Evidence and Guidelines. Ottawa
(ON): Canadian Agency for Drugs and Technologies in Health;
2014
|
|
30
|
Sarantopoulos C, McCallum B, Kwok WM and
Hogan Q: Gabapentin decreases membrane calcium currents in injured
as well as in control mammalian primary afferent neurons. Reg
Anesth Pain Med. 27:47–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sutton KG, Martin DJ, Pinnock RD, Lee K
and Scott RH: Gabapentin inhibits high-threshold calcium channel
currents in cultured rat dorsal root ganglion neurones. Br J
Pharmacol. 135:257–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boroujerdi A, Zeng J, Sharp K, Kim D,
Steward O and Luo ZD: Calcium channel alpha-2-delta-1 protein
upregulation in dorsal spinal cord mediates spinal cord
injury-induced neuropathic pain states. Pain. 152:649–655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benedikt Nimmervoll: The function of the
Ca2+ channel α2δ subunits in synaptic release in hippocampal
neurons.
|
|
34
|
Lana B, Schlick B, Martin S, Pratt WS,
Page KM, Goncalves L, Rahman W, Dickenson AH, Bauer CS and Dolphin
AC: Differential upregulation in DRG neurons of an α2δ-1 splice
variant with a lower affinity for gabapentin after peripheral
sensory nerve injury. Pain. 155:522–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meyer RA and Ringkamp M: A role for
uninjured afferents in neuropathic pain. Sheng Li Xue Bao.
60:605–609. 2008.PubMed/NCBI
|