Introduction
Peripheral artery disease (PAD) is an occlusive
disease of the peripheral circulation system. PAD is one of the
major syndromes of atherothrombosis and is found in 15–20% of
individuals aged >70 years (1).
Early diagnosis is important to improve patient quality of life and
prevent secondary vascular events, such as stroke or acute
myocardial infarction (AMI) (2). The
presence of the occlusion is effectively measured by the
ankle-brachial index (ABI), a non-invasive technique (2). Intermittent claudication in the lower
limbs is a common clinical symptom of PAD and at present, the most
effective treatment for occluded arteries is conventional surgery
(2). There are a number of common
risk factors for PAD, including age, diabetes mellitus,
hypertension, smoking and hyperlipidemia (3). However, the development of PAD may also
be independently influenced by genetic factors (4). Atherosclerosis accounts for >90% of
PAD cases in the United States (3).
Central lipid core, connective tissue, inflammatory
cells and smooth muscle cells (SMCs) form plaques covered by a
fibrous cap, which are characteristic of atherosclerotic lesions.
Atherosclerotic plaques are typically located at the bifurcations
or proximal segments of large- and medium-sized arteries (3). The popliteal and femoral arteries are
affected in 80–90% of symptomatic PAD patients, the tibial and
peroneal arteries are affected in 40–50% of patients and the
aorto-iliac arteries are affected in 30% of patients (3).
Oxidized low-density lipoprotein receptor 1 (OLR1),
also known as LOX1, has a pro-inflammatory role in atherogenesis.
Lipoprotein modification in the arteries causes the peroxidation of
lipids and generates aldehyde products, such as malondialdehyde.
Lipid peroxidation induces inflammatory processes in vascular cells
and inflammatory mediators accelerate the uptake of
lipoprotein-derived lipids. Modified lipoproteins, including
acetylated low-density lipoprotein (LDL) and oxidized LDL (oxLDL),
cannot be detected by native LDL receptors. Macrophage receptor
families recognize these modified lipoproteins and facilitate the
formation of lipid-filled macrophages. oxLDL is self-promoting and
pro-inflammatory cytokines are produced alongside the formation of
more oxLDL through an OLR1-mediated cycle. The binding of oxLDL to
OLR1 leads to an increase in intracellular reactive oxygen species
(ROS). The elevated ROS level may cause superoxide anions to react
with intracellular nitric oxide, resulting in endothelial
dysfunction (5,6).
Interleukin 17 (IL17) is a unique cytokine that has
six isoforms (A-F) produced by a novel T helper (Th) subset, Th17
cells, and other cells in the immune system. IL17A has been studied
as part of numerous inflammatory diseases (7,8) and has
been demonstrated to serve an essential role in the maintenance of
angiotensin II-induced hypertension and vascular dysfunction
(9). Another study has indicated
that IL17A gene variants are significantly associated with an
increased risk of developing coronary artery disease (CAD) and that
IL17A is overexpressed in patients with AMI (10). In a recent study, oxLDL, a
hyperlipidemia stimulus, was found to upregulate IL17 receptors in
human primary aortic cells (11).
The OLR1 gene, located on chromosome 12p13.1-p12.3,
spans over 7 kb and consists of six exons. A single nucleotide
polymorphism (SNP) at rs11053646 (c.501 G>C) on exon 4 resulting
in a lysine (K) to asparagine (N) amino acid substitution at
position 167 (p.K167N) has been identified to be associated with
the decreased binding and internalization of oxLDL (12). Another SNP at rs11053646 was found to
be associated with hypertension, MI and carotid atherosclerosis
(13,14) as well as ischemic stroke (15–17).
The IL17A SNP rs8193037 (−121 G>A) and IL17A SNP
rs3819025 (+45 G>A) are located in the 5′ region of intron 1 of
the IL17A gene. It has been demonstrated that IL17A gene variants
are associated with CAD (10).
The OLR1 and IL17A genes may jointly impact the
phenotype during the development of PAD. Investigating the possible
association between OLR1 and IL17A gene expression and OLR1
rs11053646 and IL17A rs8193037 and rs3819025 variants may therefore
help to determine the pathogenesis of PAD. To the best of our
knowledge, no studies have yet examined the association of OLR1 and
IL17A with femoropopliteal (FP) artery disease, a sub-type of PAD.
Due to OLR1 and IL17A exhibiting a functional significance in
atherogenesis, the present study assessed the mRNA expression and
frequency of OLR1 rs11053646 and IL17A rs8193037 and rs3819025
polymorphisms, as well as the levels of Th17-associated cytokines
in a sample of Turkish patients with FP artery disease. It was then
evaluated whether mRNA expression of OLR1 and IL17A was associated
with peripheral circulation and its pathology.
Materials and methods
Patients
OLR1 and IL17A mRNA levels, OLR1 rs11053646 and
IL17A rs8193037 and rs3819025 genotypes as well as plasma cytokine
levels were compared between patients diagnosed with FP artery
disease and healthy controls. The present study included 150
Turkish patients, consisting of 70 patients with FP artery disease
(50 male, 20 female; mean age, 61.76±10.95 years; range, 42–82
years) and 80 healthy controls (56 male, 24 female; mean age,
60.61±6.34 years; range, 50–78 years) recruited at the
Cardiovascular Surgery Department at the Istanbul University
Cerrahpasa Medical Faculty between January 2015 and January 2016.
The present study was conducted according to the principles of the
Declaration of Helsinki and was approved by the Local Ethics
Committee of the Cerrahpasa Medical Faculty, Istanbul University
(Istanbul, Turkey). Prior to participation, all participants
provided written informed consent.
Eligible patients were recruited on the basis of
atherosclerotic occlusions of the FP peripheral arteries (≥50%)
detected by physical examination, duplex Doppler ultrasound, ankle
brachial index (ABI), magnetic resonance angiography, computed
tomography-angiography and digital subtraction angiography.
Exclusion criteria for the patient group were presence of acute or
chronic inflammatory disease, immunological disease, cancer and
pregnancy. All subjects with FP artery disease underwent surgery at
the Cardiovascular Surgery department of the Cerrahpasa Medical
Faculty, Istanbul University, which was scheduled following blood
tests; all of the patients received statin treatment.
The control group consisted of 80 healthy
individuals who visited the Cerrahpasa Medical Faculty Hospital
(Istanbul, Turkey) for regular health screening without any
clinical findings of PAD and were randomly selected. Inclusion
criteria were no use of statins and a normal lipid profile. The
exclusion criteria included the presence of cardiovascular disease,
severe kidney and hepatic diseases, diabetes, cancer, hypertension,
autoimmune diseases, pregnancy and any atherosclerosis risk factor
such as obesity, smoking or a family history of cardiovascular
disease. Furthermore, healthy controls were excluded from the study
if intermittent claudication with palpable pulses on their lower
extremity arteries was identified upon examination.
Blood samples and DNA extraction
In order to isolate DNA, venous blood samples from
all participants were collected into EDTA tubes and stored at −20°C
in aliquots until use. Genomic DNA was extracted from whole blood
using a high pure PCR template preparation kit (Roche Diagnostics
GmbH, Mannheim, Germany) according to the manufacturer's
protocol.
Blood samples and isolation of
peripheral blood mononuclear cells (PBMCs) and RNA
Venous blood samples obtained from all participants
were collected into heparin tubes and immediately underwent RNA
extraction and lymphocyte separation. Using the
PureLink® RNA Mini kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) total RNA was extracted from freshly isolated
PBMCs according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following RNA extraction, 400 ng of total RNA was
reverse-transcribed into complementary (c)DNA with random hexamers
as primers using the Transcriptor High Fidelity cDNA Synthesis kit
(Roche Diagnostics), according to the manufacturer's protocol. OLR1
and IL17A expression in PBMCs was determined by qPCR using a
LightCycler® 1.5 detection system (Roche Applied
Science, Pleasanton, CA, USA) with TaqMan probe technology (TIB
Molbiol GmbH, Berlin, Germany). cDNA samples were amplified with
hydrolysis probes in qPCR reactions for pre-incubation at 95°C for
10 min, 45 cycles of denaturation at 95°C for 10 sec, annealing at
60°C for 30 sec and extension at 72°C for 1 sec, followed by
cooling at 40°C for 30 sec. OLR1 (catalogue no.
05532957001–90015528) and IL17A (catalogue no.
05532957001-90015530) mRNA levels were normalized to endogenous
reference genes: ACTB (catalogue no. 05532957001-90018066), B2M
(catalogue no. 05532957001-90010199) and GAPDH (catalogue no.
05532957001-90015529) using the 2−ΔΔCq method (18). The primers and probes were chosen
from Roche UPL system (Roche Diagnostics GmbH, Mannheim, Germany).
Three endogenous stably expressed reference genes were used to
prevent erroneous normalization. Primers were designed as follows:
ACTB, forward 5′-AGAGCTACGAGCTGCCTG AC-3′ and reverse
5′-CGTGGATGCCACAGGACT- 3′; and B2M, forward
5′-ATCTGAGCAGGTTGCTCCAC-3′ and reverse
5′-GACCAAGATGTTGATGTTGGATAA-3′. The amplicon lengths of OLR1,
IL17A, ACTB, B2M and GAPDH were 62, 69, 114, 95, and 66 nt,
respectively. Cq values of 40 were excluded from the study. The
experiments were performed twice.
Genotyping of OLR1 rs11053646 and
IL17A rs8193037 and rs3819025 SNPs
OLR1 rs11053646 (catalogue no. 29931401) and IL17A
rs8193037 (catalogue no. 31931501) and rs3819025 (catalogue no.
32321401) SNPs (TIB Molbiol GmbH) were determined using the
LightCycler® 1.5 detection system with hybridization
probes consisting of 3′-fluorescein and a
5′-LightCycler® Red-labeled pair of oligonucleotide
probes (TIB Molbiol GmbH). Genotyping was performed using a total
reaction volume of 20 µl containing 1.0 µl primer-probe mix 2.0 µl
LightCycler® FastStart DNA Master HybProbe (Roche
Diagnostics GmbH), 3.0 mM magnesium chloride and 50 ng genomic DNA.
The melting temperature profiles and the results of melting curve
analysis were used to identify the genotype of PCR products. The
quality of SNP genotyping was verified by independent replications
of the genotyping, using randomly selected samples; quality control
results agreed with the initial genotyping results.
Multiplex immunoassay
The levels of the plasma cytokines IL1B, −4, −6,
−10, −17A, −17F, −21, −22, −25, −31 and −33 as well as interferon-γ
(IFNG), soluble cluster of differentiation 40 ligand (SCD40L) and
tumor necrosis factor-α (TNF-α), were measured using the
Bio-Plex® system (catalogue no. 171-AA001M; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol. The 96-well plates were prepared with
assay buffer (Bio-Rad Laboratories, Inc.). Controls, standards and
samples to be analysed were added (total volume, 50 µl) to the
wells and incubated with antibody-immobilized microbeads (capture
antibody; 1:20) for 1 h at room temperature (catalogue no.
171-AA001M, Bio-Rad Laboratories, Inc.). Following washing,
biotinylated detection antibodies at a dilution of 1:20 (anti-human
IL1B, −4, −6, −10, −17A, −17F, −21, −22, −25, −31, −33, IFNG, SCD40
L, and TNF-α antibodies; catalogue no. 171-AA001 M, Bio-Rad
Laboratories, Inc.) were incubated in the dark for 30 min at RT
with the bound cytokines. Fluorescent (phycoerythrin-labeled)
streptavidin (1:100; Bio-Rad Laboratories, Inc.) was added. A final
wash was completed prior to resuspension in sheath fluid for
analysis in the Bio-Plex® array reader using the
Bio-Plex Manager 4.1 software (Bio-Rad Laboratories, Inc.). The
concentration of each cytokine (pg/ml) was calculated against a
standard curve plotted using a five-parameter logistic
regression.
Statistical analysis
Continuous variables were compared between healthy
controls and patients with FP artery disease using the Student's
t-test or Mann-Whitney U Test; data were expressed as the mean ±
standard deviation. For categorical variables, the χ2
test or two-sided Fisher's exact test were used. These included
genotype and allele frequencies to compare the association between
genotypes and alleles among cases and controls and to test the
deviation of genotype distribution from the Hardy-Weinberg
equilibrium (HWE). P≤0.05 was considered to indicate a
statistically significant difference. To determine the strength of
the association between genotypes, alleles and case/control status,
odds ratios (OR) and their 95% confidence intervals (CIs) were
calculated. Furthermore, ORs and 95% CI of SNP and gene expression
levels were estimated by multiple logistic regression analysis with
adjustments for age, gender, serum CRP levels, LDL cholesterol and
hypertension status. Relative gene expression levels and
biochemical parameters were compared using the Spearman's
non-parametric correlation test. All statistical analyses were
performed using SPSS software for Windows, version 21.0 (IBM SPSS
Inc., Armonk, NY, USA).
Results
Demographic data
The demographic and clinical characteristics of
healthy controls and patients with FP artery disease are presented
in Table I. There were no
statistically significant differences observed between the groups
with regard to gender (P=0.86) or age (P=0.44). Patients with FP
artery disease and controls exhibited significant differences in
hematocrit (Z=−4.508, P<0.001), fasting glucose (Z=−5.563,
P<0.001), urea (Z=−2.934, P=0.003), creatinine (Z=−3.477,
P=0.001) and serum C-reactive protein (CRP; Z=−6.133, P<0.001)
levels but not in aspartate transaminase, alanine transaminase,
total high-density lipoprotein (HDL) and LDL cholesterol or
triglyceride levels (P>0.05; Table
I).
 | Table I.Demographic and clinical
characteristics of patients with FP artery disease and
controls. |
Table I.
Demographic and clinical
characteristics of patients with FP artery disease and
controls.
| Characteristic | FP artery disease
(n=70) | Control (n=80) | P-value |
|---|
| Age (years) | 61.76±10.95 | 60.61±6.34 | 0.44 |
| Gender, M/F
(%) | 50/20
(71.4/28.6) | 56/24
(70.0/30.0) | 0.86 |
| Hematocrit, % | 35.93±6.37 | 40.73±4.37 |
<0.001a |
| Fasting glucose,
mg/dl | 141.25±67.20 | 94.77±31.40 |
<0.001a |
| AST, U/L | 22.59±14.83 | 19.55±5.96 | 0.123 |
| ALT, U/L | 20.82±15.35 | 21.75±8.82 | 0.669 |
| T cholesterol,
mg/dl | 186.47±39.27 | 192.37±35.25 | 0.430 |
| HDL cholesterol,
mg/dl | 41.64±16.48 | 46.60±17.65 | 0.141 |
| LDL cholesterol,
mg/dl | 131.59±95.96 | 132.54±32.75 | 0.949 |
| Triglycerides,
mg/dl | 180.21±219.02 | 171.85±68.07 | 0.81 |
| Urea, mg/dl | 45.34±27.57 | 32.89±10.28 | 0.003a |
| Creatinine,
mg/dl | 1.19±1.17 | 0.82±0.23 | 0.001a |
| C-Reactive protein,
nmol/l | 46.92±70.21 | 3.99±4.28 |
<0.001a |
OLR1 and IL17A mRNA levels
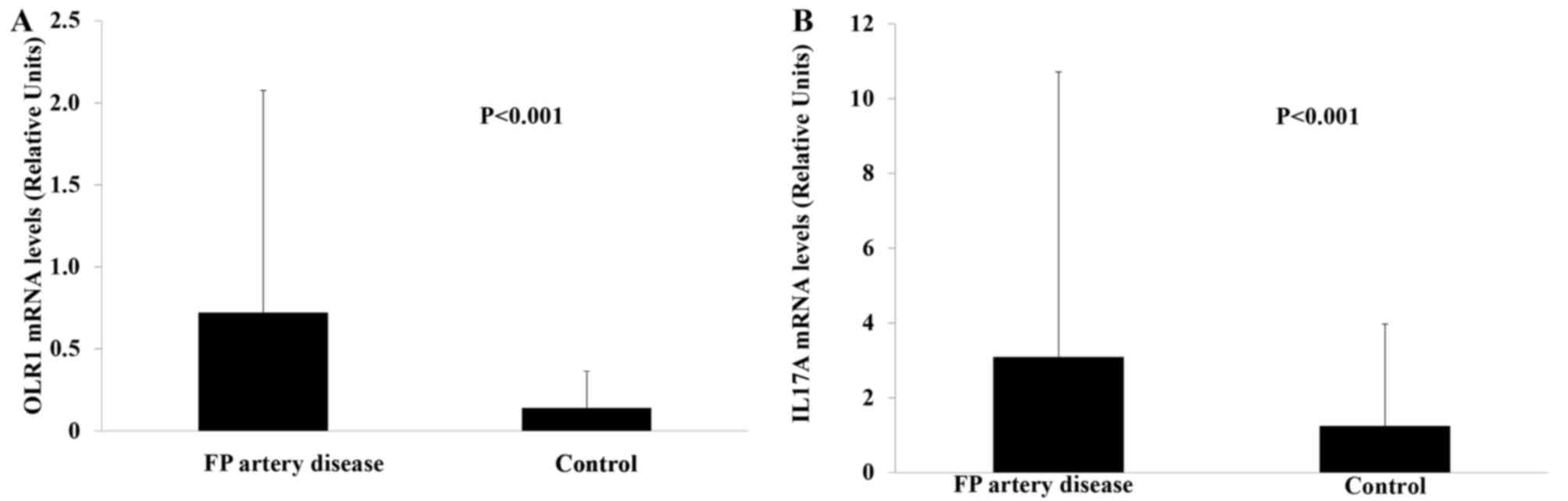
OLR1 and IL17A mRNA levels were significantly higher
in patients with FP artery disease (Z=−4.114) compared with those
in the healthy controls (Z=−5.679, P<0.001; Fig. 1).
OLR1 and IL17A mRNA levels were compared in male and
female patients. Male patients exhibited higher IL17A mRNA levels
compared with those in female subjects; however, this difference
was not statistically significant (P>0.05; results not
shown).
Plasma cytokine levels
Plasma levels of IL4, −6, −10, 17A, −22, −31, −33 as
well as SCD40 L and TNF-α were significantly higher in patients
with FP artery disease, compared with the controls (P<0.05;
Fig. 2). In addition, plasma IL1B
and IFNG levels were higher in patients with FP artery disease, but
the difference was not significant (P>0.05; Fig. 2).
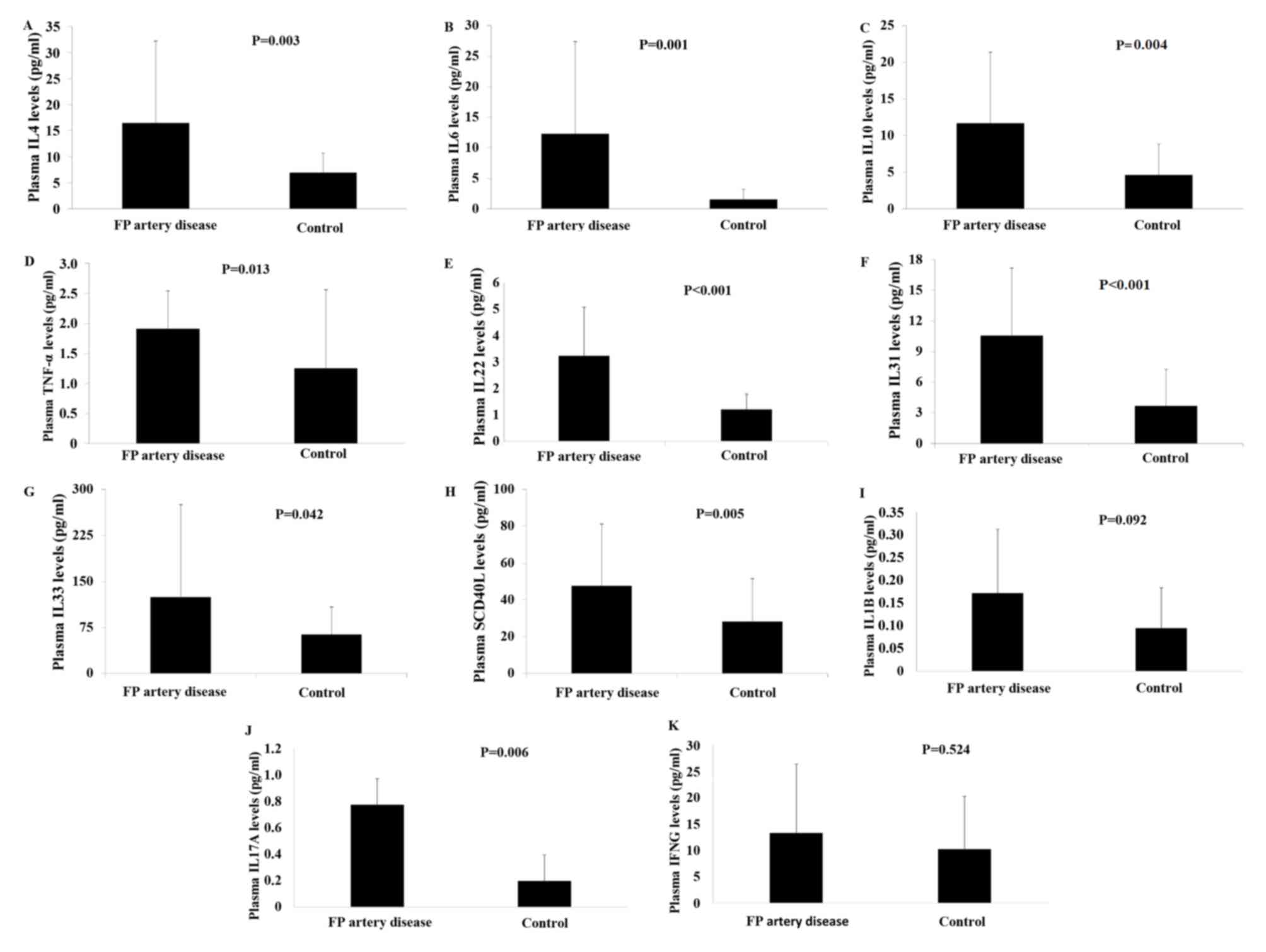 | Figure 2.Levels of plasma cytokines in patients
with FP artery disease and healthy controls. (A) Plasma IL4, (B)
plasma IL6, (C) plasma IL10, (D) plasma TNF-α levels, (E) plasma
IL22, (F) plasma IL31, (G) plasma IL33, (H) plasma SCD40 L, (I)
plasma IL1B, (J) plasma IL17A and (K) plasma IFNG. FP,
femoropopliteal; IL, interleukin; TNF, tumor necrosis factor; SCD40
L, soluble cluster of differentiation 40 ligand; IFNG,
interferon-γ. |
Correlation of OLR1 and IL17A gene
expression with blood lipid parameters
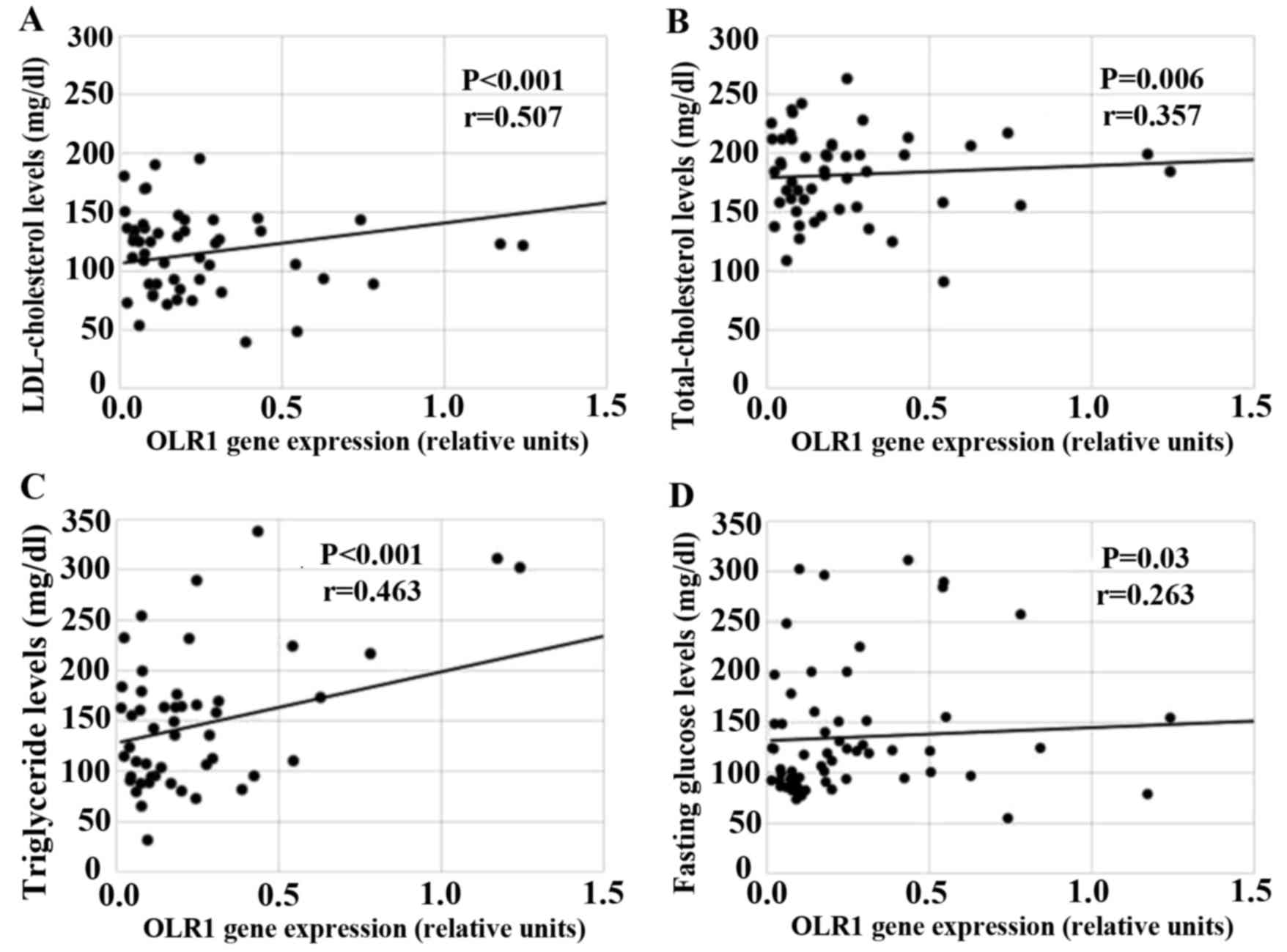
Potential associations between the expression of
OLR1 and IL17A and blood lipid parameters were investigated using
Pearson's correlation analysis. LDL and total cholesterol levels
were significantly correlated with OLR1 expression (r=0.507,
P<0.001; r=0.357, P=0.006, respectively; Fig. 3A and B) in patients with FP artery
disease. Furthermore, OLR1 expression was positively correlated
with the levels of triglyceride (r=0.463, P<0.001) and fasting
glucose (r=0.263, P=0.03; Fig. 3C and
D). However, no significant association was observed between
IL17A expression and blood lipid parameters in patients with FP
artery disease (P>0.05; results not shown).
Comparison between genotype
frequencies of OLR1 SNP rs11053646 and IL17A SNPs rs8193037 and
rs3819025
The genotype distribution for the SNPs OLR1
rs11053646 as well as IL17A rs8193037 and rs3819025, between
patients with FP artery disease and healthy controls is presented
in Table II. The distribution of
OLR1 rs11053646 as well as IL17A rs8193037 and rs3819025 genotypes
was consistent with HWE expectations for patients and controls
(P>0.05).
 | Table II.Distribution of OLR1 SNP rs11053646
and IL17A SNPs rs8193037 and rs3819025 genotype and allele
frequencies among patients with FP artery disease and healthy
controls. |
Table II.
Distribution of OLR1 SNP rs11053646
and IL17A SNPs rs8193037 and rs3819025 genotype and allele
frequencies among patients with FP artery disease and healthy
controls.
|
Genotype/allele | FP artery disease
(n=70) | Control (n=80) | P-value | OR | 95% CI |
|---|
| OLR1
rs11053646 |
|
|
|
|
|
| CC; n
(%) | 61 (87.1) | 69 (86.3) |
| 0.99 | 0.873–1.122 |
| GC +
GG; n (%) | 9
(12.9) | 11 (13.8) | 0.87 | 1.069 | 0.471–2.429 |
| C
allele frequency | 0.93 | 0.92 |
|
|
|
| G
allele frequency | 0.07 | 0.08 | 0.79 |
|
|
| IL17A
rs8193037 |
|
|
|
|
|
| GG; n
(%) | 61 (87.1) | 71 (88.8) |
| 1.018 | 0.904–1.147 |
| GA +
AA; n (%) | 9
(12.9) | 9
(11.3) | 0.80 | 0.875 | 0.368–2.081 |
| G
allele frequency | 0.94 | 0.94 |
|
|
|
| A
allele frequency | 0.06 | 0.06 | 0.86 |
|
|
| IL17A
rs3819025 |
|
|
|
|
|
| GG; n
(%) | 59 (84.3) | 67 (83.7) |
| 0.994 | 0.864–1.143 |
| GA +
AA; n (%) | 11 (15.7) | 13 (16.3) | 0.92 | 1.034 | 0.495–2.159 |
| G
allele frequency | 0.91 | 0.91 |
|
|
|
| A
allele frequency | 0.09 | 0.09 | 0.80 |
|
|
There was no significant difference in the genotype
frequencies of the OLR1 rs11053646 polymorphism between the
individuals with FP artery disease and controls (P=0.87). In
addition, no significant difference was observed between the
genotype frequencies of the IL17A rs8193037 and rs3819025
polymorphisms between the patients with FP artery disease and
healthy controls (P=0.80 and 0.92, respectively; Table II).
Multivariate logistic regression
analysis of OLR1 and IL17A genes with regard to factors associated
with FP artery disease
The potential roles of OLR1 and IL17A mRNA levels in
FP artery disease and the possible risk regarding its development
associated with OLR1 SNP rs11053646 and IL17A SNPs rs8193037 and
rs3819025 were assessed using multiple logistic regression analysis
with adjustments for a number of factors associated with FP artery
disease. These factors, including serum CRP levels (P=0.038,
OR=1.587; 95% CI: 1.025–2.456) together with OLR1 mRNA levels
(P=0.006) were significantly associated with FP artery disease.
Furthermore, hypertension (P=0.034) was significantly associated
with FP artery disease. However, neither the OLR1 rs11053646 nor
the IL17A rs8193037 and rs3819025 genotypes and gender were not
associated with a risk of FP artery disease (P>0.05; Table III).
 | Table III.Logistic regression analysis for FP
artery disease risk. |
Table III.
Logistic regression analysis for FP
artery disease risk.
|
Genotype/allele | P-value | Exp (B) | 95% CI |
|---|
| OLR1 mRNA
levels | 0.006 |
|
|
| Serum CRP
levels | 0.038 | 1.587 | 1.025–2.456 |
| LDL-cholesterol
levels | 0.081 | 0.945 | 0.887–1.007 |
| Hypertension
(+) | 0.034 |
|
|
| Constant | 0.393 |
|
|
Discussion
Atherosclerosis is a multifactorial and multistep
disease involving inflammation at all stages from initiation to
progression, as well as plaque rupture (19). OLR1 (also known as LOX1) is a
membrane protein previously identified in endothelial cells as an
oxLDL receptor (20). OLR1 may serve
various roles in endothelial dysfunction and proinflammatory
signaling (20). In addition, IL17
secreted from Th17 cells, may have diverse roles in various
inflammatory diseases (11). IL17
has proatherogenic effects, inducing chemokine, cytokine and matrix
metalloproteinase production (21).
A number of peripheral arterial diseases, including FP artery
disease, are primarily caused by atherosclerosis and it has been
determined that OLR1 and IL17 are associated with plaque formation
and atherosclerosis (3,20,21). Lim
et al (22) demonstrated that
atherogenic mice presented with increased serum levels of IL17,
which was in turn associated with an increased level of Th17 cells
in the secondary lymphoid organs. Furthermore, it was determined
that dendritic cell-mediated Th17 polarization by triggering IL6
production was induced by oxLDL uptake (22).
LDL is passed into the subendothelial layer of the
artery and oxidized by various biochemical mediators and enzymes,
resulting in the production of oxLDL. LDL is recognized by the
specific LDL receptor (LDLr); however, oxLDL is recognized by
various receptors, including oxLDL receptor-1 (OLR1), cluster of
differentiation (CD) 36, Toll-like receptors, scavenger receptors
and CD205 (23). OxLDL induces
inflammatory mediators and cell adhesion molecules that recruit
inflammatory cells and macrophages into the subendothelial layer
(24). Arjuman and Chandra (6) evaluated the modulation of OLR1 in the
presence of IL10 and determined that oxLDL and IL10 stimulated cell
surface expression of OLR1 in the THP-1 macrophage cell line.
Ox-LDL-induced OLR1 subsequently promoted intracellular nitric
oxide, which acts as a pro-inflammatory substance (6). In the present study, a significant
increase in hematocrit, fasting glucose, urea, creatinine and serum
CRP levels was detected in patients with FP artery disease compared
with controls. This suggested the emergence of induced inflammatory
pathways in the patient group. However, no significant difference
was observed in the total, HDL and LDL cholesterol levels between
the control and FP artery groups. This may be attributed to the use
of statins by the FP artery disease group of the present study.
OLR1 is a scavenger receptor that mediates the
uptake and binding of oxLDL by vascular cells during the
progression of atherosclerosis (25). Exposure to oxLDL induces OLR1
expression and further activates the signaling pathways associated
with the biological activity of OLR1, such as nuclear factor-κB (a
nuclear factor involved in the signal transduction of inflammation)
(25). Previous studies have
investigated the changes in soluble OLR1 levels (sOLR1) in
atherosclerotic diseases other than CAD. In one previous study,
increased serum sOLR1 levels were noted in diabetic patients with
PAD compared to those without PAD; these levels were inversely
correlated with ABI (26). These
findings suggested that the serum OLR1 concentration is associated
with ABI and PAD in patients with type 2 diabetes. In addition, the
differences in macrophage trafficking among wild-type OLR1
knock-out (KO), LDLr KO and LDLr/OLR1 double KO mice were
determined. OLR1 deletion evoked a reduction in macrophage
trafficking in the aorta of LDLr KO mice (27). The results of the present study
indicated that OLR1 mRNA expression was significantly higher in the
group with FP artery disease compared with that in healthy
controls. These results are consistent with the findings of Fukui
et al (26); however, in the
present study, the incidence of type 2 diabetes in the patient
group was only 26.5%. It was also observed that factors, including
serum CRP levels along with OLR1 mRNA expression and hypertension,
were significantly associated with FP artery disease.
To the best of our knowledge, the association
between OLR1 SNP rs11053646 and peripheral arterial diseases has
not yet been studied. In a recent meta-analysis of seven
case-control studies, it was observed that OLR1 SNP rs11053646
dominant and co-dominant models were significantly associated with
ischemic stroke (28). In the
present study, OLR1 SNP rs11053646 recessive and co-dominant models
were not significantly associated with the risk of FP artery
disease. This result may be due to the limited sample size of the
study.
Potekhina et al (29) reported that the anti-atherogenic
regulatory T cell/proatherogenic Th17 cell ratio declined in
patients with severe coronary atherosclerosis compared to those
with intact coronary artery and coronary artery without
atherosclerosis progression (29).
It was determined that the imbalance in the pro- and
anti-inflammatory/atherogenic lymphocyte subpopulations was
associated with the progression of atherosclerosis (29). In addition, it was demonstrated that
IL17A deficiency did not affect the aortic plaque burden in mice
fed a high-fat diet or subjected to angiotensin II infusion
(30).
Mai et al (11) demonstrated that the hyperlipidemia
stimulus oxLDL upregulated IL17 receptors in human and mouse
primary aortic endothelial cells and that IL17A, in turn, activated
human and mouse primary aortic endothelial cells via the
upregulation of pro-inflammatory cytokines such as IL6 and
granulocyte-macrophage colony-stimulating factor. In another study,
atherogenic mice exhibited increased levels of serum IL17 and Th17
cells (22). Pro-atherogenic factors
promoted the polarization and inflammatory function of autoimmune T
cells and antibodies directed against oxLDL inhibited cell
polarization. OxLDL, but not native LDL, promoted dendritic
cell-mediated Th17-cell polarization in atherogenic mice (22). In the present study, IL17A mRNA
expression in the PBMCs of patients with FP artery disease was
increased compared with that in healthy controls. In addition, OLR1
expression was compared with stratification by gender, which
demonstrated that male patients had higher IL17A mRNA levels
compared with female patients, however this difference was not
statistically significant. Furthermore, plasma cytokine levels in
patients with FP artery disease and healthy controls were assessed.
It was demonstrated that plasma IL4, −6, −10, −22, −31 and −33 as
well as SCD40 L and TNF-α levels were significantly higher in
patients with FP artery disease compared with controls. IL1B, IL17A
and IFNG levels were also increased in the patients with FP artery
disease; however, this difference was not significant.
Chronic inflammation in the arterial wall due to the
invasion, proliferation and differentiation of leukocytes is an
important phenomenon occurring during the development of
atherosclerotic lesions. Ge et al (31) reported that impaired renal function
increased the atherosclerotic lesion size and aortic leukocyte
infiltration. It was also demonstrated that renal impairment and
IL17A during myeloid cell differentiation enhanced
antigen-presenting cell marker expression and decreased oxLDL
uptake. Another study indicated that hypercholesterolemia resulted
in increased aortic inflammation and immune response to modified
lipids with the increase in splenic Th17-cell population. In
addition, the increase in Th17 cells was positively correlated with
the progression of atherosclerosis and immunoglobulin M antibodies
specific to oxLDL and Th17 cells were associated with
atherosclerosis development (32).
In the present study, associations between OLR1 and IL17A
expression and blood lipid parameters were investigated and it was
demonstrated that LDL and total cholesterol levels were
significantly with OLR1 expression in patients with FP artery
disease. OLR1 expression was also positively correlated with
triglyceride and fasting glucose levels. However, no significant
association was observed between IL17A expression and blood lipid
parameters in patients with FP artery disease.
Zhang et al (10) reported that the incidence of IL17A
rs8193037 GG homozygote and G allele was significantly higher in
patients with CAD than in the general Chinese Han population. The G
allele was associated with an increased risk of CAD in male
patients. In addition, plasma IL17A levels were higher in patients
with AMI, and the G allele was associated with increased expression
of IL17A in AMI patients and was a predictive factor for CAD. The
same study investigated the IL17A rs3819025 polymorphism in
patients with CAD and reported no significant difference in the
genotype and allele frequencies between patients with CAD and
controls (10). In another study, no
association was reported between rs8193037 and premature CAD;
however, certain haplotypes were involved in determining the risk
of developing premature CAD (33).
In the present study, no significant difference was observed in the
genotype and allele frequencies of IL17A SNP rs8193037 between
patients with FP artery disease and controls, consistent with
results of a study by Vargas-Alarcón et al (33). However, the results of the present
study were not consistent with the findings of Zhang et al
(10), which may be attributed to
ethnic differences between the populations studied. The role of the
IL17A rs3819025 polymorphism in the development of FP artery
disease was also investigated and consistent with the finding of
Zhang et al (10), as no
association between this polymorphism and FP artery disease was
observed.
In conclusion, although the sample size of the
present study was limited it is, to the best of our knowledge, the
first to identify an association between OLR1 and IL17A mRNA
expression in PBMCs and FP artery disease. The present study also
reported some large standard deviation values, which is another
limitation. As FP artery disease has a multifactorial inheritance,
it has both genetic and environmental based complex development and
many factors may affect its pathogenesis, thus expanding the
standard deviation values. The results suggested that OLR1, IL17A
and various cytokines may serve a significant role in the
inflammatory mechanism involved in the development of FP artery
disease and are associated with blood lipid parameters. The
susceptibility genes involved in PAD development remain elusive.
Future studies should focus on the regulatory non-coding RNAs
involved in the formation of oxLDL and its association with
IL17.
Acknowledgements
The present study was supported by the Scientific
Research Projects Coordination Unit of Istanbul University (grant
nos. 39959 and 46022).
References
|
1
|
Norgren L, Hiatt WR, Dormandy JA, Nehler
MR, Harris KA, Fowkes FG; TASC II Working Group; Bell K, Caporusso
J, Durand-Zaleski I, et al: Inter-Society consensus for the
management of peripheral arterial disease (TASC II). Eur J Vasc
Endovasc Surg. 33:(Suppl 1). S1–S75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernando FJ Serrano and Martín Conejero A:
Peripheral artery disease: Pathophysiology, diagnosis and
treatment. Rev Esp Cardiol. 60:969–982. 2007.(In Spanish).
PubMed/NCBI
|
|
3
|
Mahameed AA: Peripheral arterial disease.
http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/cardiology/peripheral-arterial-disease/Accessed.
January 11–2009.
|
|
4
|
Kullo IJ and Leeper NJ: The genetic basis
of peripheral arterial disease: Current knowledge, challenges, and
future directions. Circ Res. 116:1551–1560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XP and Du GH: Lectin-like oxidized
low-density lipoprotein receptor-1: Protein, ligands, expression
and pathophysiological significance. Chin Med J (Engl).
120:421–426. 2007.PubMed/NCBI
|
|
6
|
Arjuman A and Chandra NC: Effect of IL-10
on LOX-1 expression, signaling and functional activity: An
atheroprotective response. Diab Vasc Dis Res. 10:442–451. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tesmer LA, Lundy SK, Sarkar S and Fox DA:
Th17 cells in human disease. Immunol Rev. 223:87–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taleb S, Tedgui A and Mallat Z: Adaptive T
cell immune responses and atherogenesis. Curr Opin Pharmacol.
10:197–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madhur MS, Lob HE, McCann LA, Iwakura Y,
Blinder Y, Guzik TJ and Harrison DG: Interleukin 17 promotes
angiotensin II-induced hypertension and vascular dysfunction.
Hypertension. 55:500–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Pei F, Zhang M, Yan C, Huang M,
Wang T and Han Y: Interleukin-17A gene variants and risk of
coronary artery disease: A large angiography-based study. Clin Chim
Acta. 412:327–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mai J, Nanayakkara G, Lopez-Pastrana J, Li
X, Li YF, Wang X, Song A, Virtue A, Shao Y, Shan H, et al:
Interleukin-17A promotes aortic endothelial cell activation via
transcriptionally and post-translationally activating p38
mitogen-activated protein kinase (MAPK) pathway. J Biol Chem.
291:4939–4954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Biocca S, Falconi M, Filesi I, Baldini F,
Vecchione L, Mango R, Romeo F, Federici G, Desideri A and Novelli
G: Functional analysis and molecular dynamics simulation of LOX-1
K167N polymorphism reveal alteration of receptor activity. PLoS
One. 4:e46482009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tatsuguchi M, Furutani M, Hinagata J,
Tanaka T, Furutani Y, Imamura S, Kawana M, Masaki T, Kasanuki H,
Sawamura T and Matsuoka R: Oxidized LDL receptor gene (OLR1) is
associated with the risk of myocardial infarction. Biochem Biophys
Res Commun. 303:247–250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou XW, Wang LF, Wang N, Pang D, Hui B,
Zhou YL and He X: The G501C polymorphism of oxidized LDL receptor
gene [OLR-1] is associated with susceptibility and serum C-reactive
protein concentration in Chinese essential hypertensives. Clin Chim
Acta. 388:200–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hattori H, Sonoda A, Sato H, Ito D,
Tanahashi N, Murata M, Saito I, Watanabe K and Suzuki N: G501C
polymorphism of oxidized LDL receptor gene (OLR1) and ischemic
stroke. Brain Res. 1121:246–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Yin C, Zhang Y, Zhao L, Fu H and
Feng J: The role of OLR1 polymorphisms in determining the risk and
prognosis of ischemic stroke in a Chinese population.
NeuroRehabilitation. 32:391–396. 2013.PubMed/NCBI
|
|
17
|
Liu X, Zhu RX, Li L and He ZY: Association
of LOX-1 gene polymorphisms with cerebral infarction in northern
Chinese Han population. Lipids Health Dis. 13:552014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dunn S, Vohra RS, Murphy JE,
Homer-Vanniasinkam S, Walker JH and Ponnambalam S: The lectin-like
oxidized low-density-lipoprotein receptor: A pro-inflammatory
factor in vascular disease. Biochem J. 409:349–355. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liuzzo G, Trotta F and Pedicino D:
Interleukin-17 in atherosclerosis and cardiovascular disease: The
good, the bad, and the unknown. Eur Heart J. 34:556–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim H, Kim YU, Sun H, Lee JH, Reynolds JM,
Hanabuchi S, Wu H, Teng BB and Chung Y: Proatherogenic conditions
promote autoimmune T helper 17 cell responses in vivo. Immunity.
40:153–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goyal T, Mitra S, Khaidakov M, Wang X,
Singla S, Ding Z, Liu S and Mehta JL: Current concepts of the role
of oxidized LDL receptors in atherosclerosis. Curr Atheroscler Rep.
2012.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao S and Geng YJ: LOX-1: A male
hormone-regulated scavenger receptor for atherosclerosis. Vascul
Pharmacol. 59:138–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukui M, Tanaka M, Senmaru T, Nakanishi M,
Mukai J, Ohki M, Asano M, Yamazaki M, Hasegawa G and Nakamura N:
LOX-1 is a novel marker for peripheral artery disease in patients
with type 2 diabetes. Metabolism. 62:935–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding Z, Mizeracki AM, Hu C and Mehta JL:
LOX-1 deletion and macrophage trafficking in atherosclerosis.
Biochem Biophys Res Commun. 440:210–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Au A, Griffiths LR, Cheng KK, Wee Kooi C,
Irene L and Wei L Keat: The influence of OLR1 and PCSK9 gene
polymorphisms on ischemic stroke: Evidence from a meta-analysis.
Sci Rep. 5:182242015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Potekhina AV, Pylaeva E, Provatorov S,
Ruleva N, Masenko V, Noeva E, Krasnikova T and Arefieva T:
Treg/Th17 balance in stable CAD patients with different stages of
coronary atherosclerosis. Atherosclerosis. 238:17–21. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madhur MS, Funt SA, Li L, Vinh A, Chen W,
Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA and Harrison DG:
Role of interleukin 17 in inflammation, atherosclerosis, and
vascular function in apolipoprotein e-deficient mice. Arterioscler
Thromb Vasc Biol. 31:1565–1572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ge S, Hertel B, Koltsova EK,
Sörensen-Zender I, Kielstein JT, Ley K, Haller H and von
Vietinghoff S: Increased atherosclerotic lesion formation and
vascular leukocyte accumulation in renal impairment are mediated by
interleukin-17A. Circ Res. 113:965–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao LN, Ponnusamy T, Philip S,
Mukhopadhyay R, Kakkar VV and Mundkur L: Hypercholesterolemia
induced immune response and inflammation on progression of
atherosclerosis in Apob(tm2Sgy) Ldlr(tm1Her)/J mice. Lipids.
50:785–797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vargas-Alarcón G, Angeles-Martínez J,
Villarreal-Molina T, Alvarez-León E, Posadas-Sánchez R,
Cardoso-Saldaña G, Ramírez-Bello J, Pérez-Hernández N, Juárez-Rojas
JG, Rodríguez-Pérez JM, et al: Interleukin-17A gene haplotypes are
associated with risk of premature coronary artery disease in
Mexican patients from the Genetics of Atherosclerotic Disease (GEA)
study. PLoS One. 10:e01149432015. View Article : Google Scholar : PubMed/NCBI
|