Introduction
Spinal cord injury (SCI), which is attributed to
various factors that include mechanical factors as well as other
mechanisms caused by the trauma, often leads to the severe
dysfunction of limbs and trunk below the damaged section, and is a
major cause of permanent disability in children and adults
(1,2). In addition to direct injury caused by
primary trauma following SCI, a variety of extended
neuropathophysiological alterations occur. Secondary damage plays a
part in increasing the extent of the pathological effects of SCI
where excitotoxicity, inflammation, autophagy as well as apoptosis
play vital roles (3–6). Over the past decades various strategies
have been used to elucidate the molecular basis of SCI; however, no
fully effective treatments for SCI are available to date.
Therefore, the development of a safe and efficient treatment for
SCI is greatly complicated by the existence of a highly complex
injury environment.
Oxidative stress has been implicated to play a
significant role in the pathology of SCI (7,8).
Nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase is a
membrane enzyme composed of several subunits that include NOX and
phox subunits, and is responsible for the production of reactive
oxygen species (ROS). A previous study has suggested that NADPH
oxidase is expressed in neurons, astrocytes and microglia (9). Another study has indicated that NADPH
oxidase-derived ROS is involved in the modulation of neurological
function under physiological and pathological conditions (10), and inhibition of this enzyme
represents an attractive therapeutic target for the treatment of a
number of nervous system diseases.
Apocynin, an inhibitor of NADPH oxidase, is a
natural organic compound isolated from the roots of Apocynum
cannabinum (Canadian hemp) (11). Studies have been conducted to
determine its disease-fighting capabilities and application in
several types of brain damage, such as traumatic brain injury and
stroke (12,13). Notably, the beneficial effects of
apocynin following SCI in rats have been demonstrated to be
associated with its antioxidant and anti-inflammatory properties
(14). Therefore, the protective
effect and mechanisms of apocynin after SCI require further
exploration.
In the present study, the effect of apocynin on
oxidative stress, apoptosis, inflammation and function following
SCI were examined in rat model. It was found that SCI-induced
oxidative damage, neuronal injury, microglial activation and motor
deficits were prevented through the anti-apoptotic and
anti-inflammatory effects of apocynin. Therefore, the present
results suggest that treatment with apocynin following SCI may have
the potential to reduce SCI-induced neuronal death.
Materials and methods
Experiment animals and SCI model
Adult male Sprague-Dawley rats (250–300 g) were
obtained from Xi'an Medical University Experimental Animal Center
(Xi'an, China). The animals were housed in a temperature- and
humidity-controlled environment (22–24°C, 55+5% humidity and a
standard 12 h light/dark cycle), and supplied with food and water
ad libitum. A total of 60 rats were utilized in this study.
All experimental protocols were approved by the Xi'an Medical
University Animal Care and Use Committee. SCI models were created
using standardized mid-thoracic spinal cord compression injury as
described by Rivlin and Tator (15),
which has been well established in our laboratory for several
years. Rats were anesthetized with 10% chloral hydrate (3 ml/kg;
Bio-Rad Biotechnology, Inc., Shanghai, China), and then positioned
on a thermostat-controlled heating pad at 37°C in a prone position.
Under sterile conditions, a longitudinal incision was made on the
midline of the back, exposing the paravertebral muscles and then
dissection was conducted to expose the T6/T7 vertebrae. Laminectomy
was performed, and the spinal cord was exposed without opening the
dura mater. In this model, an aneurysm clip with a calibrated
closing force of 24 g was closed around the mid-thoracic spinal
cord for 1 min, which rendered the animals completely paraplegic.
The incision was sutured and the rats were placed on heat pads
(37°C) for 2–4 h to maintain normal body temperature until they
were completely awake.
Groups and drug administration
Rats were randomly divided into three groups with 20
rats in each: The sham group, SCI group and SCI + apocynin groups.
Animals in the sham group were subjected to the surgical procedure
described above, with the exception that the aneurysm clip was not
applied. Apocynin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was dissolved in dimethyl sulfoxide (DMSO) and
physiological saline (1:1; 1 mg/ml), and then injected (50 mg/kg)
intraperitoneally 30 min after SCI and then every 12 h for 3 days.
The sham and SCI groups received equal volumes of DMSO and saline
intraperitoneally at the same times daily. A sub-group of 5 rats
from each group were sacrificed 3 days following SCI, and the
remaining rats were subjected to behavioral testing. Rats were
anesthetized with 10% chloral hydrate (3 ml/kg; Bio-Rad
Biotechnology, Inc.). All rats were sacrificed by exsanguination
following anesthesia.
Oxidative stress and antioxidant
assays
Measurement of tissue myeloperoxidase (MPO)
activity
MPO activity in the spinal cord tissues was
determined 3 days after SCI as previously described (16). Tissue samples were obtained and
homogenized in 50 mM potassium phosphate buffer (PB) with a pH of
7.0, and centrifuged for 10 min at 4,000 × g at 4°C. The
pellets were suspended in 50 mM PB containing 0.5%
hexadecyltrimethylammonium bromide, then subjected to three freeze
and thaw cycles with sonication and centrifuged again (10 min at
4,000 × g at 4°C). An aliquot of the supernatant (0.3 ml)
was added to 2.3 ml reaction mixture containing
o-dianisidine, 50 mM PB and 20 mM H2O2
solution. The rate of change in absorbance was measured
spectrophotometrically at 460 nm. MPO activity was expressed as U/g
tissue.
Measurement of tissue malondialdehyde (MDA) and
glutathione (GSH) levels
Spinal cord tissue samples were homogenized with
ice-cold 150 mM KCl for the determination of MDA and GSH levels.
The levels of MDA were assayed for products of lipid peroxidation
in the spinal cord tissues homogenates using the thiobarbituric
acid (TBA) reaction method, as described previously (17). MDA levels which were determined by
measurement of absorption at a wavelength of 532 nm following
reaction with TBA to form a pink chromogen, were expressed as nmol
MDA/g tissue. GSH measurements were performed using a kit supplied
by Cayman (Cayman Chemical Company, Ann Arbor, MI, USA) according
to the manufacturer's instructions. GSH levels were expressed as
mmol GSH/g tissue.
Measurement of superoxide dismutase (SOD)
activity
SOD activity in the spinal cord tissues was measured
with a Shimadzu UV-2100 spectrophotometer (Shimadzu International
Trading (Shanghai) Co., Ltd., Shanghai, China). The assay for SOD
was based on the activity of this enzyme in the xanthine-xanthine
oxidase system (18). The changes in
absorbance were measured at 550 nm with a spectrophotometer. The
SOD levels were expressed as activity units per g protein.
Histological analysis
Histological examination of the SCI was performed by
NeuN and terminal deoxynucleotidyl-transferase-mediated dUTP nick
end labeling (TUNEL) staining as described previously (19). At day 3 after SCI, animals were
anesthetized and transcardially perfused with 4% paraformaldehyde
(PB, pH 7.4). A 5-mm spinal cord segment, 2.5 mm caudal and 2.5 mm
rostral to the injury site, was extracted. Spinal cords were kept
in 4% paraformaldehyde for 24 h and then transferred to a 30%
sucrose solution (0.1 M PBS, pH 7.4). Spinal cords were then cut
into 30-µm axial sections. The sections were permeabilized using
0.4% Triton X-100 for 30 min, then incubated with 5% normal donkey
serum (Bio-Rad Biotechnology, Inc.) for 1 h at room temperature,
followed by incubation with mouse anti-NeuN monoclonal antibodies
(diluted 1:100; ab104224; Abcam, Cambridge, UK) overnight at 4°C.
The following day, the sections were incubated with secondary
antibodies (Alexa Fluor 488 donkey anti-mouse IgG; diluted 1:100;
ab150105; Abcam) at room temperature for 2 h. TUNEL staining was
performed using an In Situ Cell Death Detection kit (Roche
Diagnostics GmbH, Mannheim, Germany). Sections were incubated with
TUNEL reaction mixture including TdT enzyme and TMR red labeled
dUTP in a dark humidified chamber for 1 h at 37°C, followed by
three final washes, each for 10 min, and visualized using
converter-POD (a horseradish peroxidase-conjugated anti-fluorescein
antibody) with 0.03% 3,3′-diaminobenzidine (DAB).
Immunofluorescence was detected and photographed in the dorsal
column region within the 5-mm region of interest using a laser
scanning confocal microscope (Olympus FluoView™ FV1000; Olympus
Corporation, Tokyo, Japan). For quantitative analysis, the numbers
of surviving neurons and TUNEL-positive apoptotic cells were
quantified as previously described using pixel density measurements
in Scion Image 4.02 (Scion Corporation, Boston, MA, USA) (20). For all stains, at least five sections
taken from regular intervals within the 5-mm region of interest
were evaluated.
Immunohistochemical analysis
Immunohistochemical staining for spinal cord tissue
was performed on ice-cold sections (30 µm). Briefly, sections were
blocked with 0.01 mol/l phosphate-buffered saline (PBS) containing
5% goat serum for 1 h at room temperature. Sections were then
incubated overnight at 4°C with rabbit anti-caspase 3 polyclonal
antibodies (ab4051; diluted 1:100), mouse anti-CD11b monoclonal
antibodies (ab1211; diluted 1:100) and mouse anti-glial fibrillary
acidic protein (GFAP) monoclonal antibodies (ab10062; diluted
1:100), all from Abcam, and then with horseradish
peroxidase-conjugated anti-rabbit (ab150077) or mouse (ab6785) IgG
antibodies (diluted 1:500), all from Abcam, for 30 min. DAB was
used to reveal the immunohistochemical reaction. Primary antibodies
were replaced with PBS in the negative control. Cell images were
captured with a microscope (Nikon Eclipse E800; Berlin, Germany)
equipped with a Spot RT digital camera (Diagnostic Instruments Inc,
Sterling Heights, CA, USA). The number of caspase 3-positive cells
was counted manually under a high-power field (×400) and the mean
percentage of positive cells in six different non-overlapping
fields of view (three gray matter and three white matter fields)
from each slide was calculated.
Western blot analysis
Rats were euthanized as described above, and the
spinal cord was rapidly isolated. Proteins were extracted from the
spinal cord (4°C) using protein extraction reagent (Bio-Rad
Biotechnology) surrounding the injured area and the protein
concentration was quantified using a BCA protein assay kit (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China)
according to the manufacturer's protocol. Samples were loaded (3 µl
per lane) onto SDS-polyacrylamide gel [0.8% w/v acrylamide
bisacrylamide (2.5 ml), 1.0 M Tris-Cl pH 8.8 (3 ml), 20% SDS (38
µl) and H2O (1.9 ml)] for electrophoresis. Separated
proteins on the gel were transferred onto PVDF membranes (Roche
Diagnostics). The blots were blocked with 5% non-fat milk for 2 h
at room temperature, followed by incubation with primary antibodies
at 4°C overnight. The antibodies comprised rabbit anti-bax
polyclonal antibodies (ab32503; diluted 1:1,000), rabbit anti-bcl-2
polyclonal antibodies (ab32124; diluted 1:500), rabbit anti-tumor
necrosis factor (TNF)-α polyclonal antibodies (ab6671; diluted
1:500), rabbit anti-interleukin (IL)-1β polyclonal antibodies
(ab2105; diluted 1:500), rabbit anti-IL-6 polyclonal antibodies
(ab6672; diluted 1:500) and rabbit anti-β-actin polyclonal
antibodies (ab8227; diluted 1:1,000) all from Abcam. The membranes
were then incubated with secondary antibodies (goat anti-rabbit
IgG; ab6721; Abcam) at 37°C for 1 h. The bands were illuminated
using an ECL system (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Immunoreactive bands of all proteins were normalized to the
intensity of the corresponding bands for β-actin. Densitometric
analysis of the blots was performed using National Institutes of
Health image 1.41 software (Bethesda, MD, USA).
Behavioral assessment
Inclined plane test
Behavior was evaluated days 1, 3, 5, 7 and 14
postoperatively using the modified Rivlin's method (21), which tested the ability of animals to
balance on elevated wooden beams. A simple device was constructed
containing a moveable plate with an adjustable angle of 0–90°. The
rat's head was placed faced forward, and the angle of inclination
between the inclined plane and the horizontal plane was increased
gradually, until the rats were unable to maintain a constant
position for 5 sec. The angle was considered to be the critical
value and then recorded.
Basso, Beattie and Bresnahan (BBB) scores
Assessment of motor function recovery of the hind
limbs was conducted using the BBB scale, which is based on a
21-point scale originally developed for the SCI rat model (22). Perfectly healthy animals are assigned
a locomotor score of 21, and animals with complete hind limb
paralysis are scored as 0. The BBB scores were determined by at
least two observers blinded to the treatment. Rats were forced to
walk in an open field for 20 min at days 1, 3, 5, 7 and 14
postoperatively and their hind limb movement was observed. The
average rat hind limb motor function scores on the BBB scale were
recorded.
Statistical analysis
All experiments were repeated three times and
similar results were obtained. All data are presented as the mean ±
standard deviation and analyzed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). The significance of differences in the
experimental results was determined using one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Regulatory effect of apocynin on
secondary oxidative stress following SCI
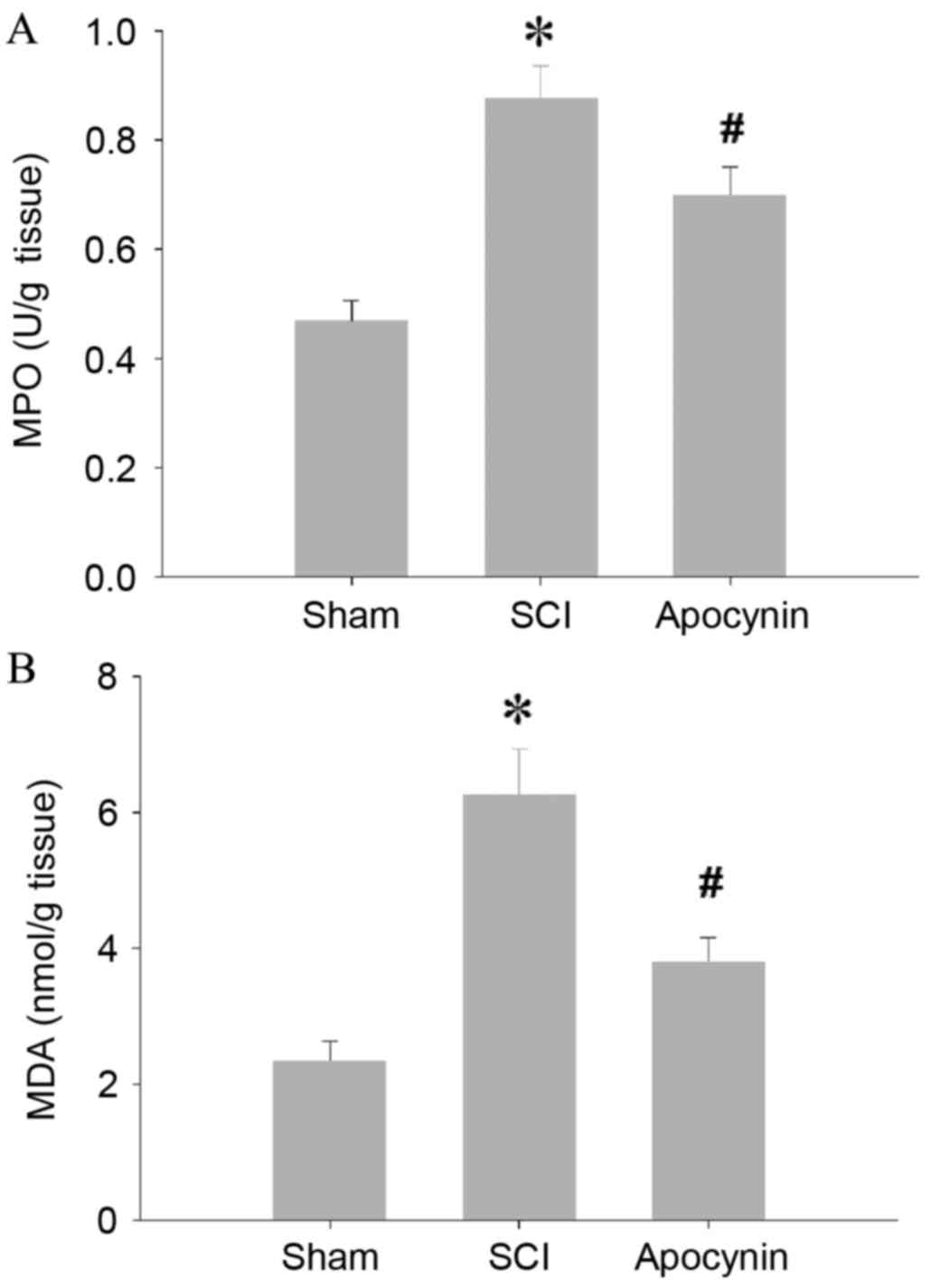
SCI caused a significant increase in the MPO
activity of spinal cord tissues when compared with the MPO activity
of the tissues from sham rats (Fig.
1A; P=0.007). In the SCI model rats that received apocynin
treatment, the elevations in MPO activity were decreased compared
with those in the SCI group (P=0.028). In the spinal cord tissues
of the SCI group, levels of MDA, an index of lipid peroxidation,
were found to be significantly higher than those of the sham group
(Fig. 1B; P=0.005), whereas
treatment with apocynin attenuated the SCI-induced elevations in
MDA levels (P=0.012). In accordance with the increased MPO activity
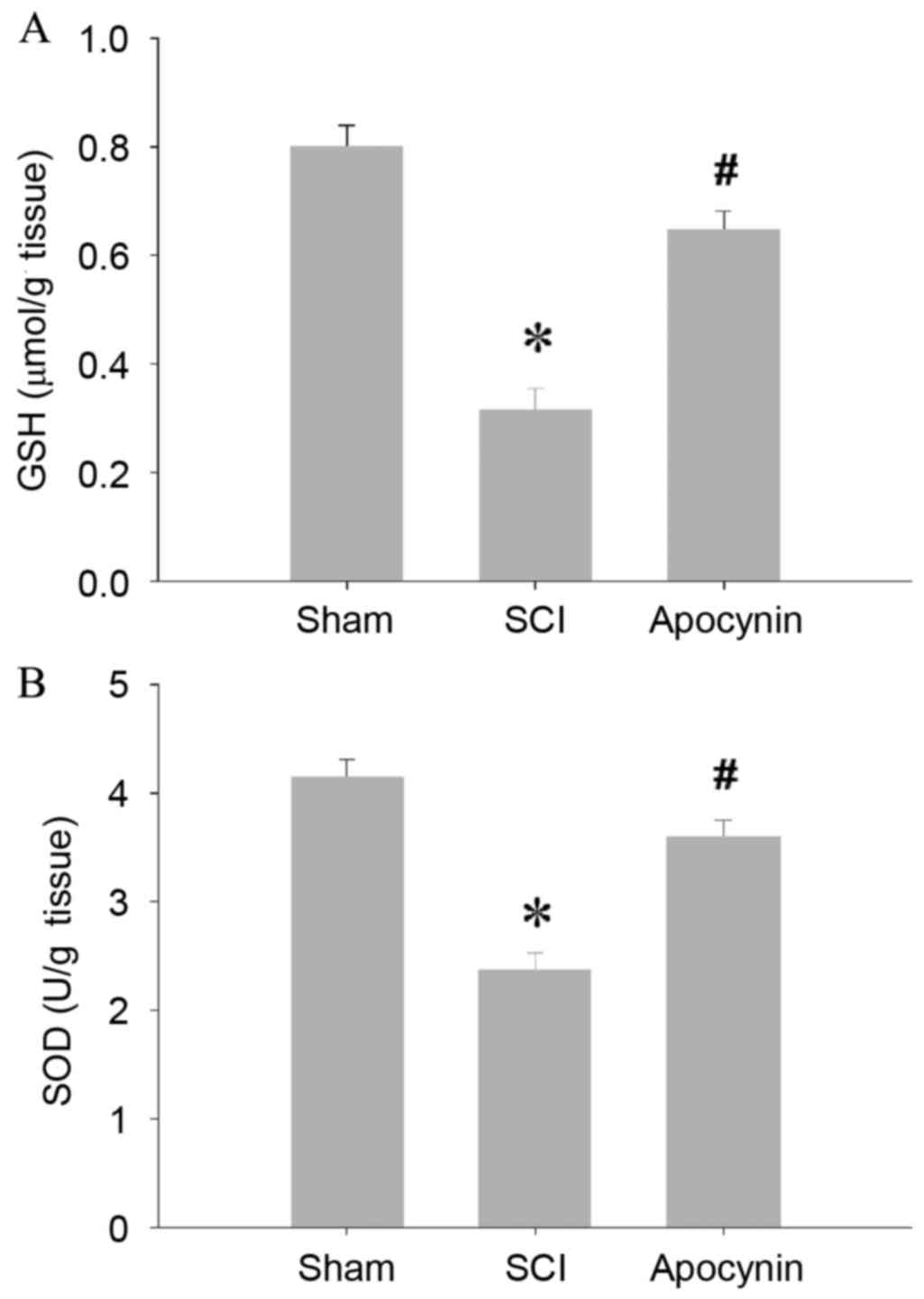
and MDA levels, the key antioxidant enzymes GSH and SOD, were found
to be significantly depleted in the spinal cord tissues of the SCI
group (Fig. 2; P=0.003), whereas the
antioxidant enzyme activities of the tissues were preserved in the
apocynin-treated SCI rats (Fig. 2;
P=0.026).
Apocynin treatment attenuates neuronal
injury in spinal cord tissues following SCI
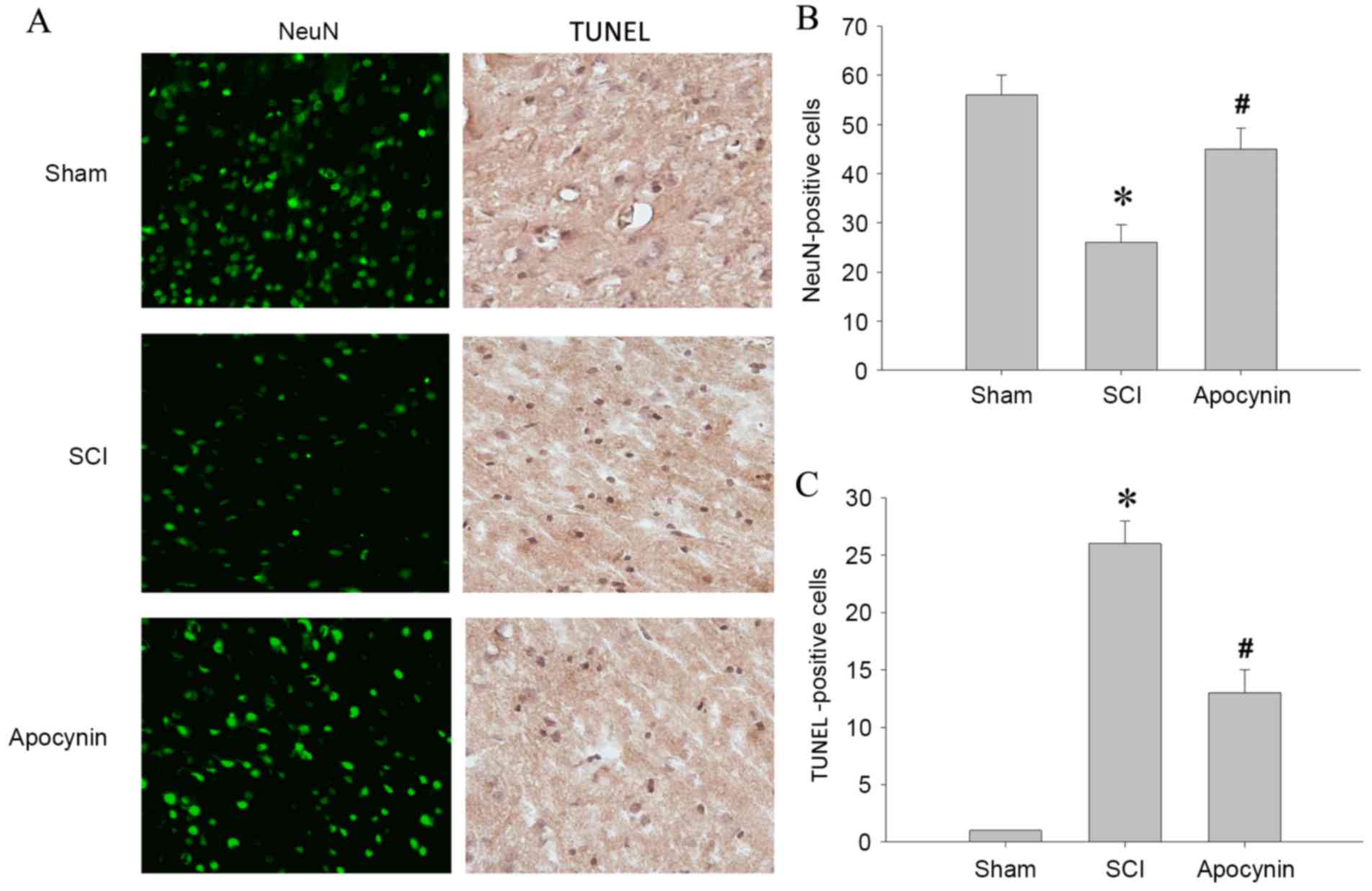
The effect of apocynin on neuronal injury around the
damaged area following SCI was investigated. As shown in Fig. 3, representative photomicrographs and
cell counting showed that SCI induced a significant loss of spinal
cord neurons, as indicated by a marked reduction of NeuN-positive
cells in the SCI group compared with the sham group (Fig. 3B; P=0.004). In addition, the number
of TUNEL-positive cell was markedly increased in the spinal cord of
SCI group, compared with sham (Fig.
3C; P=0.001). Notably, apocynin treatment strongly attenuated
neuronal cell death in the spinal cord, as indicated by a decreased
number of TUNEL-positive cells and increased number of
NeuN-positive cells compared with the SCI group (Fig. 3B, P=0.036; Fig. 3C, P=0.025). These observations
demonstrate that apocynin protects the spinal cord from SCI-induced
neuronal cell death.
Apocynin treatment attenuated
apoptosis
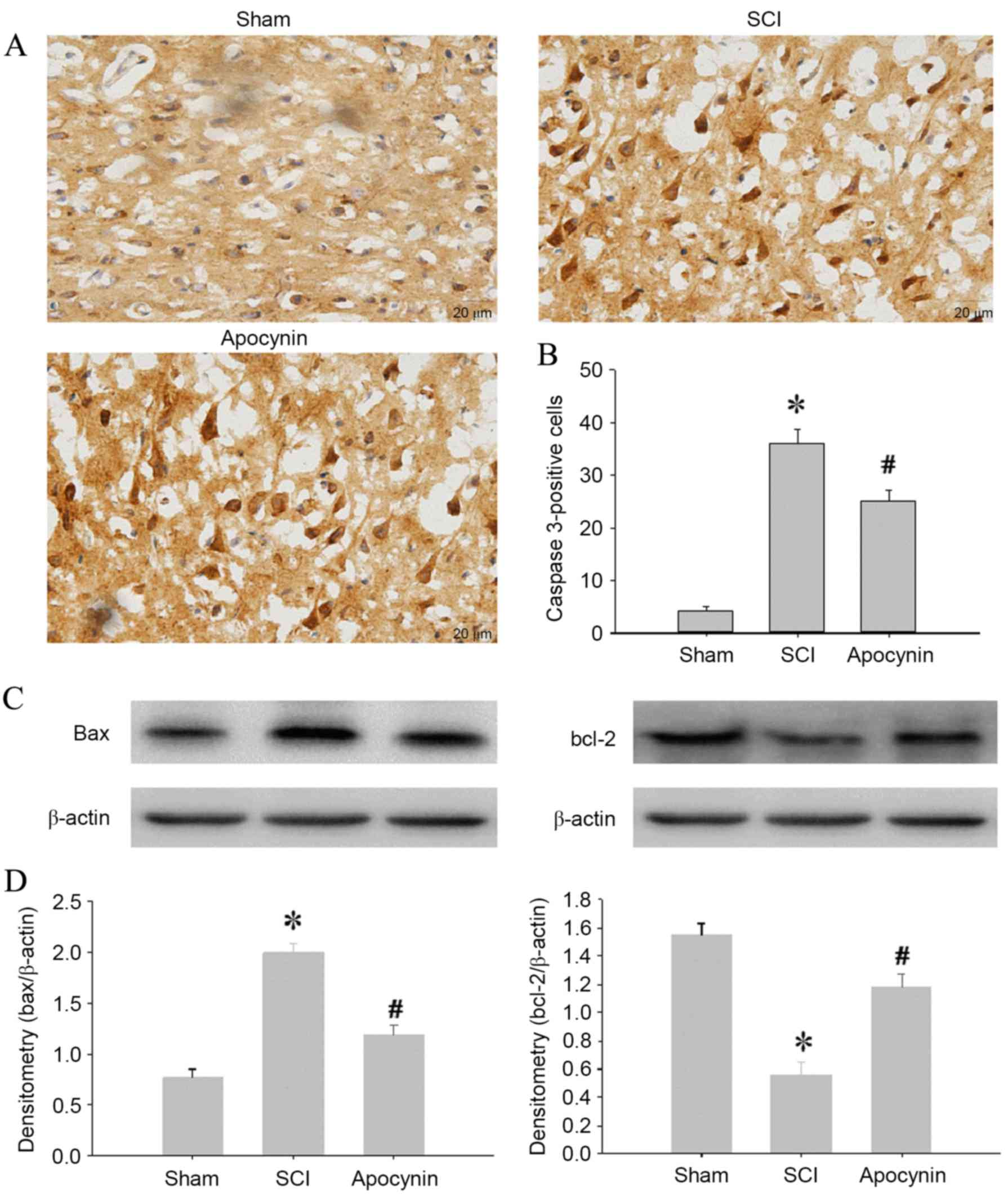
Neuronal apoptosis was also assessed by the analysis
of activated caspase 3 expression using immunohistochemical
staining. As shown in Fig. 4A,
representative photomicrographs revealed a high density of caspase
3-positive cells in the SCI group compared with sham group
(Fig. 4A, P=0.002). Following
treatment with apocynin for 3 days, the expression of activated
caspase 3 in the cells was significantly decreased compared with
that in the SCI group (Fig. 4B,
P=0.017). To investigate further the mechanisms of apocynin
apoptosis blockade, the changes in bcl-2 family members, including
the proapoptotic protein bax and antiapoptotic protein bcl-2, in
the area of injury were analyzed using western blot analysis. The
results showed that injury significantly upregulated bax expression
(P=0.003) and downregulated bcl-2 expression (P=0.004) in the
injured spinal cord tissue. Notably, treatment with apocynin
significantly decreased the bax protein level (P=0.024) and
increased the bcl-2 level (P=0.018) compared with that in the SCI
group (Fig. 4C and D).
Apocynin treatment reduced
inflammatory cytokine levels
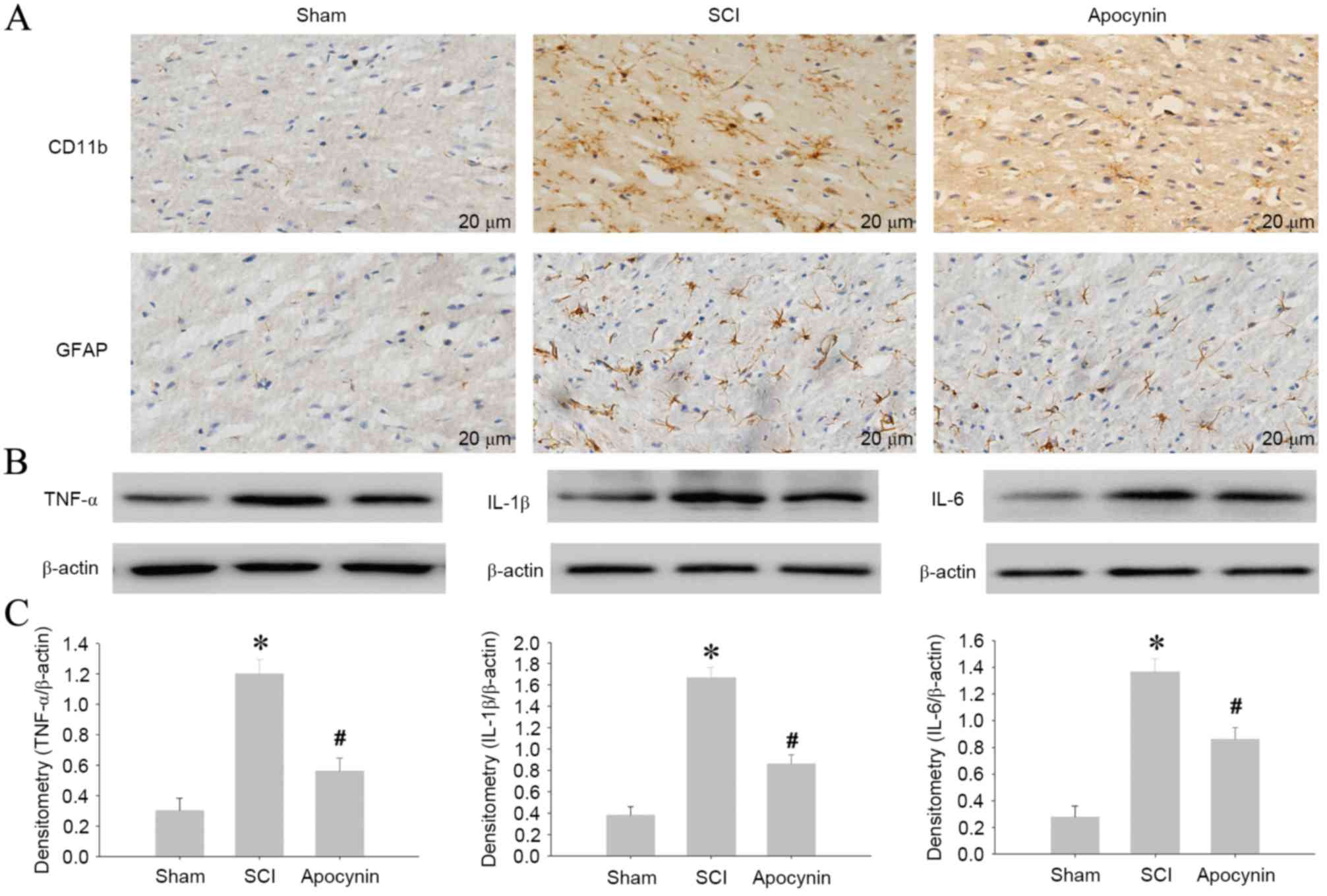
To further explore the effect of apocynin, changes
in expression levels of CD11b (as a marker for microglia) and GFAP
(as a marker for astrocytes) in the spinal cord tissue were tested
using immunohistochemical staining. As shown in Fig. 5A, the tissue from SCI model rats
clearly had stronger staining for CD11b- and GFAP-positive stains
than that from sham rats. However, in the groups treated with
apocynin, the expression levels of CD11b and GFAP were markedly
less than those in SCI group. In addition, the protein expression
levels of TNF-α, IL-1β and IL-6 were detected by western blot
analysis. In comparison with the sham group, the protein levels of
these markers were increased significantly in SCI group on day 3
post-SCI, and treatment with apocynin significantly downregulated
the levels of TNF-α (P=0.013), IL-1β (P=0.019) and IL-6 (P=0.026)
at the same time point following SCI (Fig. 5B and C). These results indicate that
apocynin significantly reduces the production of inflammatory
factors in the spinal cord tissue in a rat model of SCI.
Protective effects of apocynin on
spinal cord motor function in SCI rats
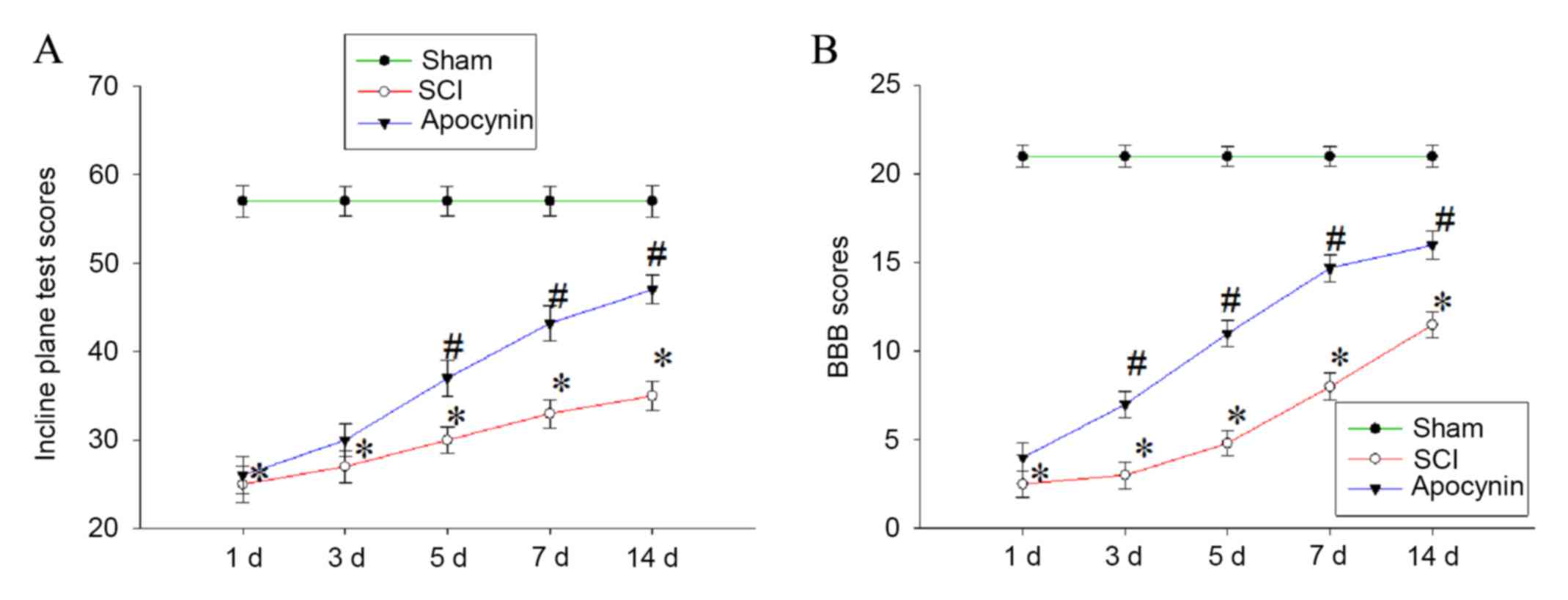
Finally, whether apocynin is able to improve the
recovery of locomotor function in SCI rats by regulating secondary
oxidative stress and suppressing apoptosis and inflammatory
response following SCI was evaluated. Locomotion was assessed using
the inclined plane test (the modified Rivlin's method) and the BBB
locomotor scale at 1, 3, 5, 7 and 14 days post-SCI. The results
showed that animals in the SCI group exhibited significant motor
deficits at 1, 3, 5, 7 and 14 days post-SCI compared with the sham
group (Fig. 6; P<0.01 vs. sham
group). Injured animals receiving apocynin, however, achieved
significantly greater plane inclinations and higher BBB locomotor
function scores compared with the SCI group (Fig. 6; P<0.05 vs. SCI group).
Discussion
In this study, the neuroprotective role of the NADPH
oxidase inhibitor apocynin was evaluated and its ability to reduce
neuronal injury and improve motor deficits following SCI in rats
were investigated. The results show that apocynin led to
significant reductions in neuronal damage and improvements in
locomotor recovery following SCI. Recovery was observed at the
functional and cellular levels, including greater inclination in
the inclined plane test and higher BBB scores, reductions in
measures of oxidative stress, and reduced apoptosis and
inflammatory response through 3 days post-injury.
In addition to the direct injury caused by primary
trauma after SCI, secondary spinal cord damage occurs within min
and continues for hours or days, leading to further neurological
deterioration (23). Secondary SCI
involves numerous complex signal transduction systems, forming a
large gene regulation network. With greater understanding of SCI,
pathophysiological mechanisms and signaling pathways underlying
secondary injuries of the spinal cord have been proposed (24). In particular, oxidative stress has
been reported to be one of the earliest biochemical changes after
SCI, and an important factor contributing to deterioration of the
primary injury, which plays a key role in secondary damage
following SCI (25). Therefore, it
is important to effectively regulate secondary oxidative stress
following SCI. A number of studies have focused on the oxidative
properties of apocynin. Apocynin has been shown to reduce oxidative
stress in the central nervous system during conditions including
sepsis (26) and brain injury
(27,28), providing significant neuroprotection.
Myeloperoxidase (MPO) is a cationic protein present in primary
azurophilic granules of neutrophils and monocytes, MDA is the end
product of lipid peroxidation, and GSH and SOD are important
scavenger enzymes of free radicals. The present study determined
their activity in the spinal cord tissues to evaluate oxidative
stress injury secondary to SCI. Results from the present study
showed that from 3 days post-SCI, spinal cord MPO activity and MDA
levels significantly increased, whereas GSH and SOD activity
significantly decreased. However, apocynin significantly inhibited
the increases in MPO activity and MDA levels, and increased GSH and
SOD activity post-SCI. These results are similar to those of
previous studies reporting that apocynin treatment significantly
reduces oxidative stress damage after SCI (14,26).
During the secondary injury following SCI,
continuous ischemia and hypoxia induce the release of pro-apoptotic
factors and pro-inflammatory cytokines, which mainly mediate
activation of MAPK pathways (24).
Microglia, which are recruited to the injury site, magnify the
extent of inflammation through the secretion of proinflammatory
cytokines, including TNF-α, IL-1β and IL-6 (29). At the early stage of SCI, reactive
astrocytes start to synthesize abundant GFAP and release various
cytokines such as nerve growth factor and basic fibroblast growth
factor, which promote the recovery of neuron damage (30). However, the level of GFAP, as a
marker of astrocytes, reflects the degree of neurological damage.
Therefore, prevention of an inflammatory response may be important
in neurological recovery. In the present study, immunohistochemical
analysis revealed that CD11b and GFAP expression was markedly
reduced by apocynin, suggesting that apocynin may contribute to
neuronal recovery through attenuating the secretion of inflammatory
cytokines. The protein expression levels of TNF-α, IL-1β and IL-6
were detected by western blot analysis. The levels of these
proinflammatory cytokines were found to be increased after SCI,
demonstrating a systemic inflammatory response to spinal cord
trauma, whereas apocynin treatment attenuated these increases.
Previous studies have demonstrated a significant increase in the
production of these proinflammatory cytokines in an experimental
model of SCI in mice (31), which
are consistent with the finding in the present study that the
expression levels of TNF-α, IL-1β and IL-6 protein were markedly
downregulated in rats treated with 50 mg/kg apocynin for 3 days as
compared with those in rats in SCI group, thus limiting
neuroinflammation and promoting neurological recovery.
In addition to oxidative stress and inflammatory
response, apoptosis is an important mediator of secondary damage
after SCI (32). It initially occurs
6 h post-injury at the lesion center and continues for several
days, with a steadily increasing number of apoptotic cells in this
region. Caspase 3, which is an important component of caspase
cascades, has been associated with apoptosis in SCI (33). The results of the present study
demonstrate apocynin attenuates the degree of apoptosis, measured
by TUNEL detection and caspase-3 immunohistochemical staining, in
the spinal cord following SCI. The numbers of TUNEL and activated
caspase 3-positive cells were reduced in apocynin-treated SCI rats
compared with those in the SCI group, indicating that apocynin
promoted neural functional recovery in SCI rats by decreasing the
number of apoptotic cells in the injured spinal cord tissue.
Subsequently, the protein expression of bax and bcl-2 in the
injured area were detected by western blot analysis. Bax is a key
component in the induction of cellular apoptosis through
mitochondrial stress, whereas bcl-2 inhibits mitochondrial
permeabilization by binding membrane-inserted bax monomers, thus
preventing the functional oligomerization of bax (34). The results of the present study
indicate that apocynin significantly increased the expression of
the antiapoptotic protein bcl-2 and decreased the expression of the
proapoptotic protein bax in the injured spinal cord tissue compared
with the SCI control. These results are consistent with a previous
study, which reported that apocynin treatment could significantly
suppressed apoptosis after SCI (14).
Finally, whether apocynin was able to improve spinal
cord function in SCI rats by regulating secondary oxidative stress
and inhibiting apoptosis and inflammation following SCI was
investigated. The inclined plane test and 21-point BBB open field
locomotor score are widely accepted for assessing locomotor
recovery of animals. In this study, following treatment with 50
mg/kg apocynin every 12 h for 3 days, the mean values in the
inclined plane test and BBB scores were significantly increased
compared with those in the SCI group. Following the successful
establishment of the SCI model, apocynin promoted the recovery of
locomotor function in rats following SCI, and exhibited
neuroprotective effects on the spinal cord.
In conclusion, the results of the present study
demonstrate that the protective mechanism associated with apocynin
against acute SCI may partly depend on anti-apoptotic and
anti-inflammatory signaling pathways, downregulation of MPO and
MDA, and upregulation of GSH, SOD activity, which together inhibit
secondary oxidation following SCI. However, it is unclear whether
or not the current level and route of administration of apocynin
provides the maximal neuroprotective benefits, and further detailed
investigations into the underlying mechanisms are required.
References
|
1
|
Grigorean VT, Sandu AM, Popescu M,
Iacobini MA, Stoian R, Neascu C, Strambu V and Popa F: Cardiac
dysfunctions following spinal cord injury. J Med Life. 2:133–145.
2009.PubMed/NCBI
|
|
2
|
Kwon BK, Tetzlaff W, Grauer JN, Beiner J
and Vaccaro AR: Pathophysiology and pharmacologic treatment of
acute spinal cord injury. Spine J. 4:451–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu D, Xu GY, Pan E and McAdoo DJ:
Neurotoxicity of glutamate at the concentration released upon
spinal cord injury. Neuroscience. 93:1383–1389. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hausmann ON: Post-traumatic inflammation
following spinal cord injury. Spinal Cord. 41:369–378. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walker CL, Walker MJ, Liu NK, Risberg EC,
Gao X, Chen J and Xu XM: Systemic bisperoxovanadium activates
Akt/mTOR, reduces autophagy and enhances recovery following
cervical spinal cord injury. PLoS One. 7:e300122012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu J, Ashwell KW and Waite P: Advances in
secondary spinal cord injury: Role of apoptosis. Spine (Phila Pa
1976). 25:1859–1866. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiong Y, Rabchevsky AG and Hall ED: Role
of peroxynitrite in secondary oxidative damage after spinal cord
injury. J Neurochem. 100:639–649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khayrullina G, Bermudez S and Byrnes KR:
Inhibition of NOX2 reduces locomotor impairment, inflammation, and
oxidative stress after spinal cord injury. J Neuroinflammation.
12:1722015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo W, Bravo T, Jadhav V, Titova E, Zhang
JH and Tang J: NADPH oxidase inhibition improves neurological
outcomes in surgically-induced brain injury. Neurosci Lett.
414:228–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White CN, Figtree GA, Liu CC, Garcia A,
Hamilton EJ, Chia KK and Rasmussen HH: Angiotensin II inhibits the
Na+-K+ pump via PKC-dependent activation of NADPH oxidase. Am J
Physiol Cell Physiol. 296:C693–C700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi BY, Jang BG, Kim JH, Lee BE, Sohn M,
Song HK and Suh SW: Prevention of traumatic brain injury-induced
neuronal death by inhibition of NADPH oxidase activation. Brain
Res. 1481:49–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Song YS and Chan PH: Inhibition of
NADPH oxidase is neuroprotective after ischemia-reperfusion. J
Cereb Blood Flow Metab. 7:1262–1272. 2009. View Article : Google Scholar
|
|
14
|
Impellizzeri D, Mazzon E, Esposito E,
Paterniti I, Bramanti P and Cuzzocrea S: Effect of Apocynin, an
inhibitor of NADPH oxidase, in the inflammatory process induced by
an experimental model of spinal cord injury. Free Radic Res.
45:221–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rivlin A and Tator CH: Effect of duration
of acute spinal cord compression in a new acute injury model in the
rat. Surg Neurol. 10:38–43. 1978.PubMed/NCBI
|
|
16
|
Hillegass LM, Griswold DE, Brickson B and
Albrightson- Winslow C: Assessment of myeloperoxidase activity in
whole rat kidney. J Pharmacol Methods. 24:285–295. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beuge JA and Aust SD: Microsomal lipid
peroxidation. Methods Enzymol. 52:302–310. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oyanagui Y: Revaluation of assay methods
and establishment of kit for superoxide dismutase activity. Anal
Biochem. 142:290–296. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Tu J, Zhang Q, Lu D, Zhu Y, Zhang
W, Yang F, Brann DW and Wang R: Delayed ischemic postconditioning
protects hippocampal CA1 neurons by preserving mitochondrial
integrity via Akt/GSK3β signaling. Neurochem Int. 59:749–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Donnelly DJ, Gensel JC, Ankeny DP, van
Rooijen N and Popovich PG: An efficient and reproducible method for
quantifying macrophages in different experimental models of central
nervous system pathology. J Neurosci Methods. 181:36–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rivlin AS and Tator CH: Objective clinical
assessment of motor function after experimental spinal cord injury
in the rat. J Neurosurg. 47:577–581. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blight AR: Morphometric analysis of blood
vessels in chronic experimental spinal cord injury:
Hypervascularity and recovery of function. J Neurol Sci. 2:158–174.
1991. View Article : Google Scholar
|
|
24
|
Song Y, Liu J, Zhang F, Zhang J, Shi T and
Zeng Z: Antioxidant effect of quercetin against acute spinal cord
injury in rats and its correlation with the p38MAPK/iNOS signaling
pathway. Life Sci. 92:1215–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Juurlink BH and Paterson PG: Review of
oxidative stress in brain and spinal cord injury: Suggestions for
pharmacological and nutritional management strategies. J Spinal
Cord Med. 21:309–334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hernandes MS, D'Avila JC, Trevelin SC,
Reis PA, Kinjo ER, Lopes LR, Castro-Faria-Neto HC, Cunha FQ, Britto
LR and Bozza FA: The role of Nox2-derived ROS in the development of
cognitive impairment after sepsis. J Neuroinflammation. 11:362014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keirstead HS, Nistor G, Bernal G, Totoiu
M, Cloutier F, Sharp K and Steward O: Human embryonic stem
cell-derived oligodendrocyte progenitor cell transplants
remyelinate and restore locomotion after spinal cord injury. J
Neurosci. 19:4694–4705. 2005. View Article : Google Scholar
|
|
28
|
Zhang Z, Huang Z, Dai H, Wei L, Sun S and
Gao F: Comparison of methylprednisolone and calpain inhibitor for
the protection of ischemiareperfusion spinal cord injury in rats.
Ortho J Chin. 19:1026–1029. 2011.
|
|
29
|
Fehlings MG and Nguyen DH: Immunoglobulin
G: A potential treatment to attenuate neuroinflammation following
spinal cord injury. J Clin Immunol. 30:(Suppl 1). S109–S112. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Faulkner JR, Herrmann JE, Woo MJ, Tansey
KE, Doan NB and Sofroniew MV: Reactive astrocytes protect tissue
and preserve function after spinal cord injury. J Neurosci.
24:2143–2155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Impellizzeri D, Esposito E, Mazzon E,
Paterniti I, Di Paola R, Bramanti P, Morittu VM, Procopio A, Perri
E, Britti D and Cuzzocrea S: The effects of a polyphenol present in
olive oil, oleuropein aglycone, in an experimental model of spinal
cord injury in mice. Biochem Pharmacol. 83:1413–1426. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rong W, Wang J, Liu X, Jiang L, Wei F, Hu
X, Han X and Liu Z: Naringin treatment improves functional recovery
by increasing BDNF and VEGF expression, inhibiting neuronal
apoptosis after spinal cord injury. Neurochem Res. 37:1615–1623.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei H, Teng H, Huan W, Zhang S, Fu H, Chen
F, Wang J, Wu C and Zhao J: An upregulation of SENP3 after spinal
cord injury: Implications for neuronal apoptosis. Neurochem Res.
37:2758–2766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dlugosz PJ, Billen LP, Annis MG, Zhu W,
Zhang Z, Lin J, Leber B and Andrews DW: Bcl-2 changes conformation
to inhibit Bax oligomerization. EMBO J. 25:2287–2296. 2006.
View Article : Google Scholar : PubMed/NCBI
|