Introduction
Heart failure involves complex clinical symptoms and
is a severe stage of various heart diseases, which has a high
incidence and mortality. Incidence rate of adult is 1–2%; mortality
rates as high as 50% for five years. All diseases that cause
chronic heart failure can lead to acute heart failure. As a result
of the increasing number of patients with chronic heart failure,
acute decompensated heart failure has become the predominant
adverse event in patients with heart failure (1).
Acute decompensated heart failure is characterized
by the first presentation or worsening of signs and symptoms of
heart failure, leading to death or readmission. Biomarkers may
support the diagnosis and prognosis of patients and may aid in
their management. Previous studies have suggested that the
N-terminal B-type natriuretic peptide (NT-proBNP) concentration
increased in patients with heart failure, and that this may provide
reliable diagnostic and prognostic information (2–5).
It is well established that neuroendocrine
activation has a central role in the pathophysiology of heart
failure, is extremely hazardous and results in myocardial injury in
patients with heart failure if activation is sustained (6,7). Other
candidate peptides for the treatment of patients with heart failure
should also be considered. One such peptide is arginine vasopressin
(AVP), which is released from the hypovolaemia (8). AVP plasma values are elevated in
patients with heart failure, and are associated with the severity
of the disease (9). Copeptin, a
peptide consisting of 39 amino acids, is a fragment of
pre-pro-vasopressin that is synthesized and secreted in equimolar
amounts to vasopressin (8). In
contrast to AVP, copeptin is stable and can be easily measured
(10,11). A previous study revealed that
copeptin is a strong prognostic biomarker for patients with chronic
heart failure (12) and acute
decompensated heart failure (13,14).
The aim of the present study was to perform a
head-to-head comparative evaluation of copeptin and NT-proBNP as
predictors of 90-day mortality or recurrence in patients with
severe acute decompensated heart failure.
Materials and methods
Study population
Between August 2011 and February 2012, the present
study prospectively enrolled consecutive patients who had
previously been referred to the Department of Cardiology of the
First Affiliated Hospital of Chongqing Medical University
(Chongqing, China) with acute heart failure. The Framingham Risk
Score (15) for heart failure was
used for the diagnosis of acute heart failure. The exclusion
criteria were as follows: Patients below the age of 18 years or
with a history of mental illness, coma, chronic renal
insufficiency, acute renal insufficiency or currently receiving
hemodialysis, previous tumors, the presence of acute myocardial
infarction within the previous 3 months, significant pulmonary
infectious diseases, liver disease or liver function abnormalities
(increase of ammonia enzyme levels to twice the normal limit or
above) or those who did not provide informed consent. The New York
Heart Association (NYHA) functional classification was used to
classify the level of cardiac function.
Only patients with severe acute decompensated heart
failure, who were confirmed to have NYHA functional class III or IV
by two senior cardiologists simultaneously, and those who had
completed at least a 90-day period of follow-up were enrolled. A
total of 129 patients with severe acute decompensated heart failure
were analyzed in the present study.
The study was conducted according to the Declaration
of Helsinki and was approved by the Chongqing Medical University
Review Board (Chongqing, China). All participants provided written
informed consent.
Follow-up and study end-points
Clinical follow-up was usually performed either
during outpatient clinic visits each month or by telephone
interviews. The pre-defined end-point of the follow-up
investigation was defined as cardiovascular death or
re-hospitalization due to decompensated heart failure. Mortality
and re-hospitalization data were obtained from hospital medical
records and/or by telephone contact with the patients' relatives.
All study participants were followed up for a minimum of 90 days
from the time-point they were diagnosed at our department.
Clinical and laboratory
evaluation
Demographic and clinical data, including the NYHA
functional class, were obtained. During the initial patient
examination, blood samples were taken from an antecubital vein for
the measurement of NT-proBNP concentrations and were analyzed
within 4 h using a commercially available assay (Roche Elecsys
proBNP Immunoassay; Roche Diagnostics, Basel, Switzerland).
Aliquots of the collected blood samples (0.6 TIU/ml) were loaded
into plastic tubes containing EDTA and aprotinin. The tubes were
placed on ice prior to centrifugation at 1,600 × g for 15
min at 4°C to collect the plasma, which was stored at −70°C until
it was used in assays in 1 batch at ~9 months after collection.
Researchers were blinded to the clinical features and biochemical
data during assays. No sample was thawed >2 times. Copeptin was
detected with a novel commercial chemiluminescence assay [EK-065-32
Copeptin (Human); Phoenix Pharmaceuticals Inc., Burlingame, CA,
USA]. After a fasting period of at least 12 h, blood samples were
drawn to examine blood counts, lipid parameters, high sensitive
C-reactive protein (hs-CRP), renal function parameters and hepatic
function.
Statistical analysis
The normal distribution of continuous variables was
expressed as the mean ± standard deviation. Skewed distribution
variables were expressed as medians and interquartile ranges, or
sums and percentages. Differences between groups for normally
distributed continuous variables were analyzed using an
independent-samples t-test. The Mann-Whitney U test was employed
when continuous variables were not normally distributed.
Categorical variables were tested using Pearson's Chi-square test.
Spearman's rank correlation was used to assess the association
between variables. To evaluate the prediction accuracy of adverse
events, receiver operating characteristic (ROC) plots were
constructed and areas under the curves (AUCs) were calculated for
plasma NT-proBNP, copeptin and their combination. AUCs were
compared according to the method by Hanley and McNeil (16). The entire study population (n=129)
was then stratified on the basis of cut-off concentrations for the
two analytes. Cut-off concentrations were determined according to
the ‘Youden index’ derived directly from the ROC curves.
Kaplan-Meier estimates of the distribution of times from baseline
to the end-point were computed and log-rank tests to determine
trends were performed to compare the survival curves between the
groups. Furthermore, Cox proportional hazards regression was used
to analyze the effect of several confounding risk factors on
survival. Initially, univariate Cox-regression analysis was
performed to estimate the impact of risk factors on the survival of
patients. Subsequently a multivariate Cox regression analysis was
performed using the forwards conditional method, which was adjusted
to the patients' confounding risk factors. All data were analyzed
using SPSS statistical software (version 17.0; SPSS, Inc., Chicago,
IL, USA), and the MedCalc package (version 11.5.0.0; MedCalc
Software bvba, Ostend, Belgium). All probabilities were two-tailed
and P<0.05 was considered to indicate a significant
difference.
Results
A total of 129 patients were included in the present
study, and etiological classifications of the patients are
presented in Table I. The median age
was 73 years (range, 64.5–79.5 years), the number of males was 54
(41.9%), 44 patients (34.1%) had a history of smoking, 76 (58.9%)
had hypertension, 37 (28.7%) were diabetics, 19 (14.7%) had a
history of chronic obstructive pulmonary disease and 59 (45.7%) had
NYHA functional class IV status. Of the 129 patients who completed
the 90-day follow-up, 82 (63.6%) were in a stable condition and 47
(36.4%) reached an adverse end-point within 90 days from the time
they were enrolled.
 | Table I.Etiological classification of
patients. |
Table I.
Etiological classification of
patients.
| Etiological
classification | Total patients, n
(%) | Patients with Stable
condition, n (%) | Patients reaching
adverse end-point, n (%) |
|---|
| Coronary
arteriosclerosis | 41 (31.8) | 26 (20.2) | 15 (11.6) |
| Hypertensive heart
disease | 40 (31.0) | 30 (23.2) | 10 (7.8) |
| Dilated
cardiomyopathy | 25 (19.4) | 14 (10.9) | 11 (8.5) |
| Rheumatic heart
disease | 12 (9.3) | 7 (5.4) | 5 (3.9) |
| Senile valvular
disease | 4 (3.1) | 3 (2.3) | 1 (0.7) |
| Hyperthyroid
cardiomyopathy | 3 (2.3) | 2 (1.6) | 1 (0.7) |
| Pulmonary heart
disease | 2 (1.6) | 0 (0) | 2 (2.6) |
| Unknown causes | 2 (1.6) | 0 (0) | 2 (1.6) |
Table II shows the
baseline demographics and clinical characteristics according to the
90-day follow-up result categories. Patients who reached an adverse
end-point showed a higher NYHA III/IV status, NT-proBNP and
copeptin levels compared with patients in a stable condition, while
there were no significant differences between the two groups in
terms of length of hospital stay, left ventricular ejection
fraction and general demographic characteristics. The results of
correlation analyses for the plasma copeptin levels and other
factors are displayed in Table
III. It was shown that copeptin concentration was positively
correlated with the age of the patients and the grading of NYHA
classification.
 | Table II.Demographics and clinical
characteristics of patients at follow-up. |
Table II.
Demographics and clinical
characteristics of patients at follow-up.
| Variable | Patients in a stable
condition (n=82) | Patients reaching the
adverse end-point (n=47) | P-value |
|---|
| Demographics |
|
|
|
| Age
(years) | 73 (66–79) | 74 (58–82) | 0.743 |
| Male
gender | 33 (40.2) | 21 (44.7) | 0.624 |
|
Smoking | 30 (36.6) | 14 (29.8) | 0.435 |
| BMI
(kg/m2) | 22.7±3.0 | 21.9±2.9 | 0.129 |
| Co-morbidities |
|
|
|
|
Hypertension | 52 (63.4) | 24 (51.1) | 0.172 |
|
Diabetes | 27 (32.9) | 10 (21.3) | 0.161 |
| COPD | 14 (17.1) | 5 (10.6) | 0.323 |
| NYHA class |
|
|
|
| NYHA
III | 51 (62.2) | 19 (40.4) | 0.017 |
| NYHA
IV | 31 (37.8) | 28 (59.6) |
|
| LVEF | 52.0 (39.8–62.0) | 49.5 (39.8–57.8) | 0.354 |
| Length of hospital
stay (days) |
|
|
|
|
Total | 8 (6–13) | 9 (4–14) | 0.763 |
| In
ICU | 0 (0–2) | 1 (0–3) | 0.103 |
| Biochemical
parameters |
|
|
|
| WBC
(x109) | 6.8 (5.4–8.2) | 6.6 (4.7–8.5) | 0.676 |
|
Hemoglobin (g/l) | 126.5±21.7 | 119.2±19.8 | 0.067 |
| LDL-C
(mmol/l) | 2.1 (1.6–2.8) | 1.9 (1.5–2.2) | 0.063 |
| TG
(mmol/l) | 1.0 (0.8–1.6) | 1.1 (0.8–1.3) | 0.292 |
| hs-CRP
(mg/l) | 6.4 (1.6–14.5) | 8.6 (2.6–19.6) | 0.209 |
| Serum
sodium (mmol/l) | 139.0
(136.0–141.0) | 139.0
(136.0–141.0) | 0.994 |
| hs-cTNT
(pg/ml) | 24.0
(10.0–41.0) | 29.0
(16.0–59.5) | 0.118 |
| MYO
(ng/ml) | 53.0
(34.8–82.3) | 46.6
(29.0–66.7) | 0.631 |
| CK-MB
(ng/ml) | 2.7 (1.9–3.6) | 2.8 (2.2–3.5) | 0.470 |
| Biomarkers
values |
|
|
|
|
NT-proBNP (pg/ml) | 3275
(1008.8–8827.0) | 6656
(2919.0–15701.0) | 0.003 |
|
Copeptin (ng/ml) | 0.79±0.30 | 0.92±0.31 | 0.022 |
 | Table III.Correlation between plasma copeptin
levels and other factors in the entire study sample (n=129). |
Table III.
Correlation between plasma copeptin
levels and other factors in the entire study sample (n=129).
|
| Copeptin | NT-proBNP |
|---|
|
|
|
|
|---|
| Parameter | r | P-value | r | P-value |
|---|
| Age | 0.183 | 0.038 | −0.121 |
0.172 |
| NYHA
classification | 0.283 | 0.001 | 0.497 | <0.001 |
| LVEF | 0.024 | 0.803 | −0.572 | <0.001 |
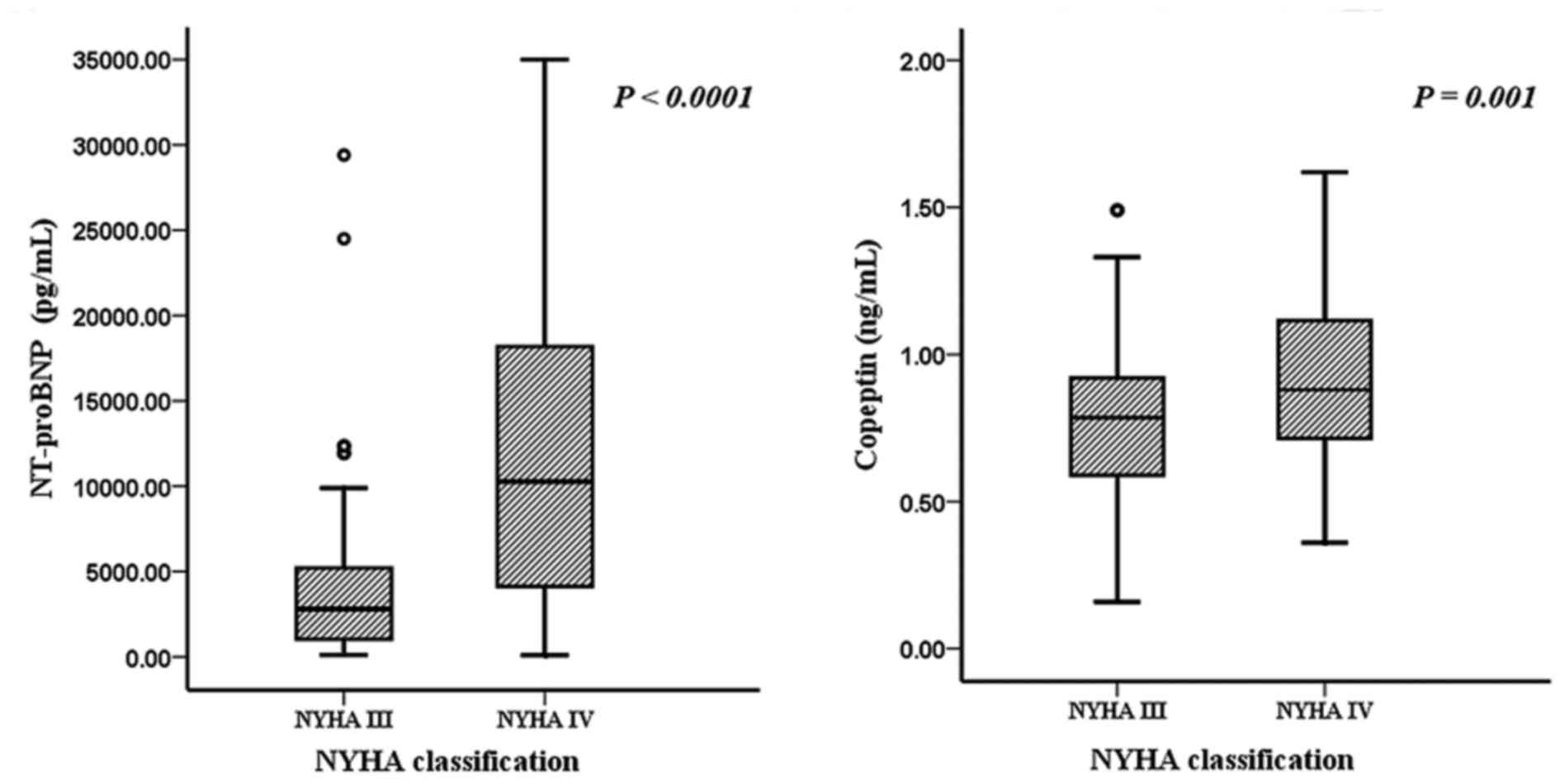
Copeptin and NT-proBNP levels, stratified by NYHA
classification, are shown in Fig. 1.
In patients with NYHA class III, the two markers were significantly
lower compared with those in patients with NYHA class IV
(P<0.01).
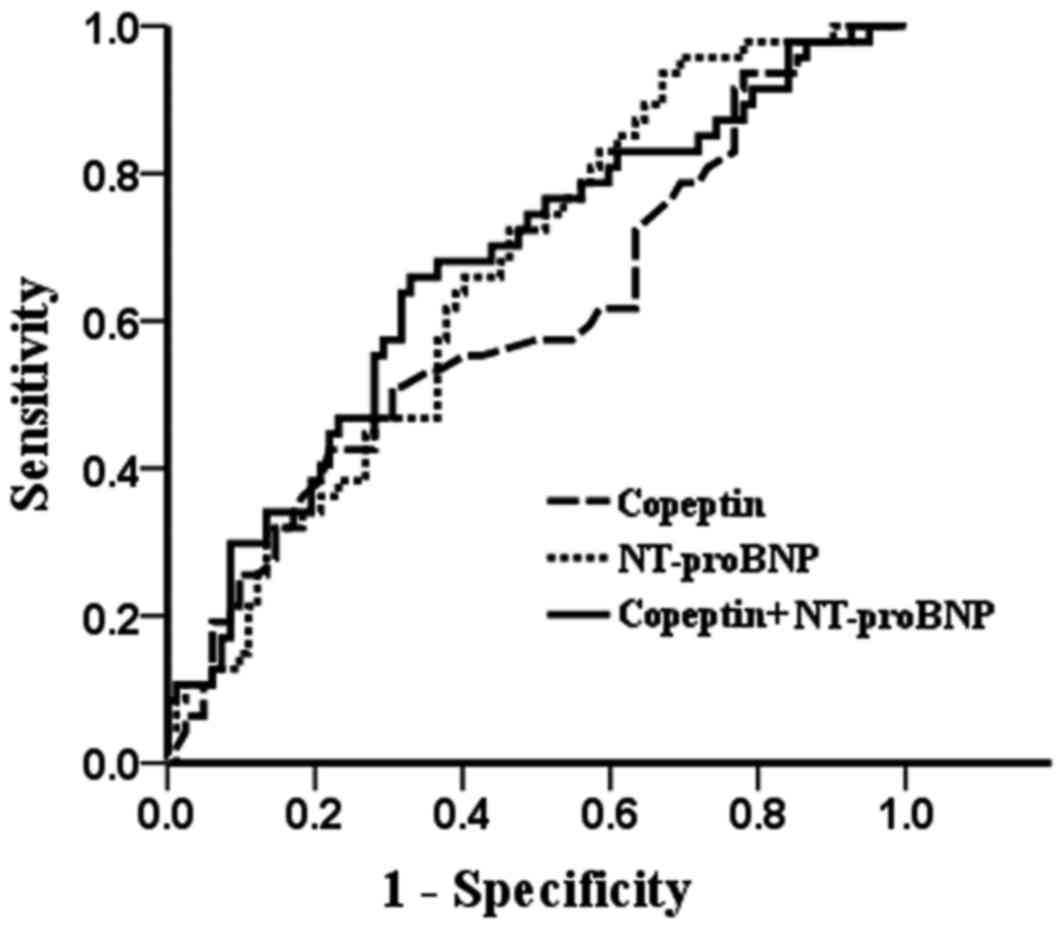
The ROC curves are shown in Fig. 2. With regard to patients who reached
the end-point and those who were in a stable condition, the AUC for
copeptin was 0.602±0.052 (95% CI, 0.499–0.705), that for NT-proBNP
was 0.659±0.048 (95% CI, 0.565–0.753) and that of their combination
was 0.670±0.050 (95% CI, 0.573–0.767). Comparison of the ROC curves
revealed no significant differences between the AUCs for NT-proBNP
and copeptin (P=0.414). Therefore, with regard to the prediction of
90-day survival, the combination of copeptin and NT-proBNP was not
superior to each single marker alone (P>0.05).
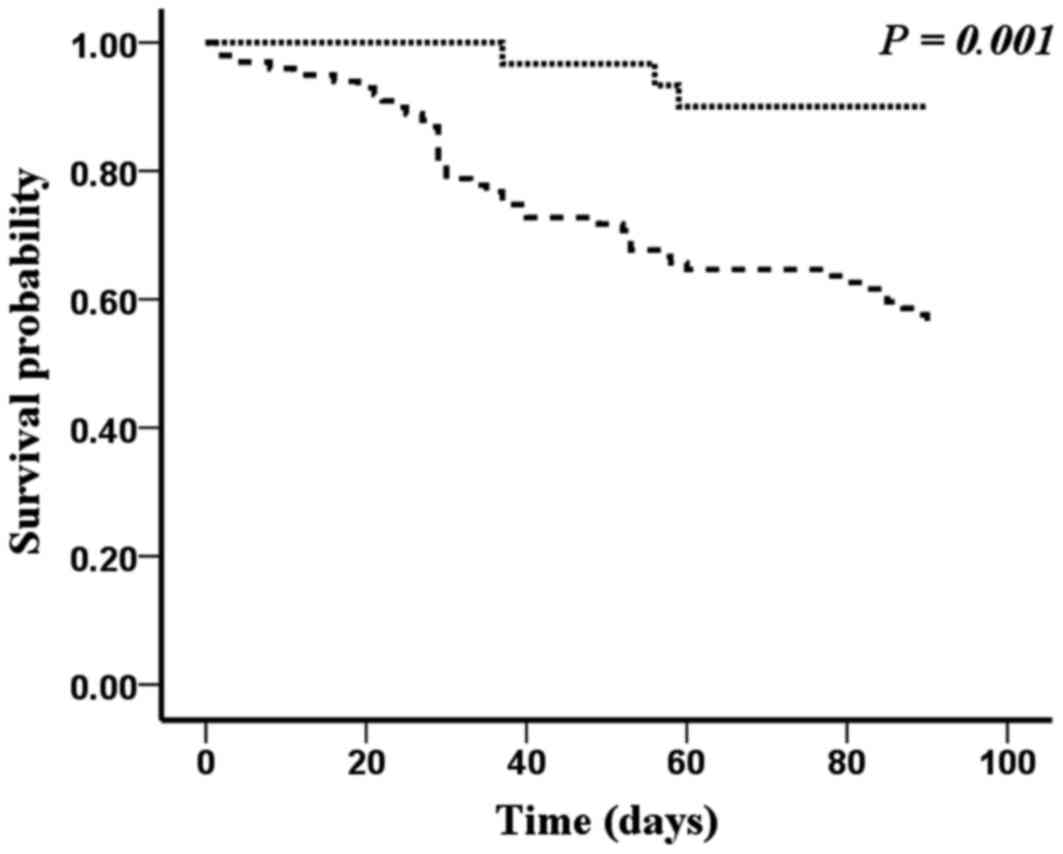
Table IV depicts the
specificity, sensitivity and prognostic value of NT-proBNP and
copeptin regarding 90-day adverse end-points above their cutoff
concentrations for the study's sample (n=129). Figs. 3 and 4
show the Kaplan-Meier curves for the 129 patients with severe acute
decompensated heart failure who were stratified into two groups
according to the cutoff concentrations of NT-proBNP and copeptin.
The survival rate was significantly lower in patients with
increased baseline plasma NT-proBNP and copeptin
concentrations.
 | Table IV.Diagnostic information for the
prediction of adverse events within 90 days by NT-proBNP and
copeptin in the entire study sample (n=129). |
Table IV.
Diagnostic information for the
prediction of adverse events within 90 days by NT-proBNP and
copeptin in the entire study sample (n=129).
| Cutoff
concentration (pg/ml) | Sensitivity, % (95%
CI) | Specificity, % (95%
CI) | PPV, % (95%
CI) | NPV, % (95%
CI) |
|---|
| NT-proBNP:
1471.5 | 93.6
(82.5–98.7) | 32.9
(22.9–44.2) | 44.4
(34.5–54.8) | 90.0
(73.1–98.0) |
| Copeptin:
890.0 | 46.8
(32.1–61.9) | 69.5
(58.4–79.2) | 46.8
(32.1–61.9) | 69.5
(58.3–79.3) |
The results of the Cox proportional-hazards
regression analysis are shown in Table
V. According to univariate analyses, NT-proBNP and copeptin
were associated with a significant risk with the presence of NYHA
class III/IV at baseline being a possible confounding factor.
Significant predictors for the adverse end-point of severe acute
decompensated heart failure are summarized in Table V. Multivariate Cox proportional
hazards regression analysis indicated that the copeptin
concentration [risk ratio (RR), 1.956; 95% CI, 1.048–3.648;
P=0.035] and the NT-proBNP concentration (RR, 4.415; 95% CI,
1.357–14.358; P=0.014) were independently associated with adverse
end-points.
 | Table V.Results of univariate Cox
proportional-hazards regression analyzing the effect of baseline
variables on adverse end-points. |
Table V.
Results of univariate Cox
proportional-hazards regression analyzing the effect of baseline
variables on adverse end-points.
|
| Univariate
analysis | Mutivariate
analysis |
|---|
|
|
|
|
|---|
| Baseline
variable | Risk ratio (95%
CI) | P-value | Risk ratio (95%
CI) | P-value |
|---|
| Copeptin (≥0.89
ng/ml) | 1.800
(1.015–3.190) | 0.044 | 1.956
(1.048–3.648) | 0.035 |
| NT-proBNP (≥1471.50
pg/ml) | 5.611
(1.741–18.080) | 0.004 | 4.415
(1.357–14.358) | 0.014 |
| NYHA classes
III/IV | 2.042
(1.140–3.658) | 0.016 |
| NS |
| Advanced age | 1.496
(0.810–2.762) | 0.198 |
|
|
| Systolic
dysfunction | 1.027
(0.502–2.102) | 0.941 |
|
|
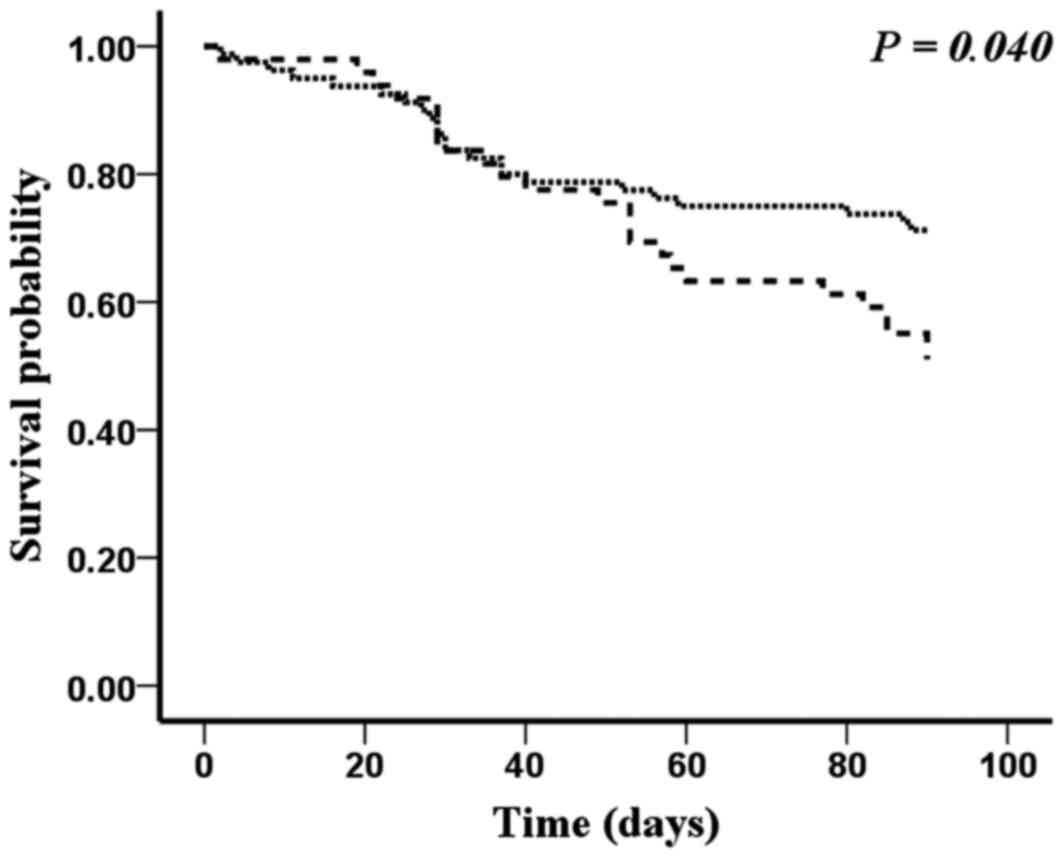
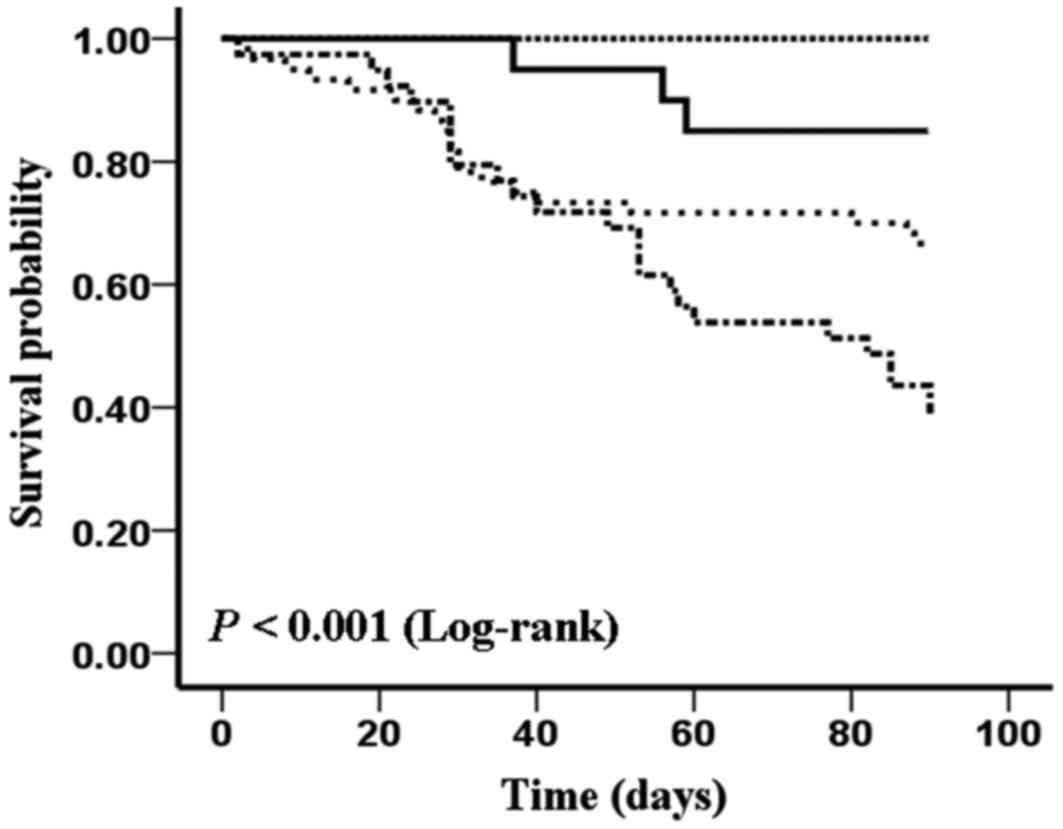
The survival probability of patients with different
serum concentration level of NT-proBNP and copeptin is shown in
Fig. 5. As shown in Fig. 5, patients with the lower level of
NT-proBNP (<1,471.50 pg/ml) and copeptin (<0.89 ng/ml) had
the highest survival probability. Neither a higher level of
NT-proBNP or copeptin could lead to the increase of mortality.
Furthermore, patients with the higher level of NT-proBNP
(>1,471.50 pg/ml) and copeptin (>0.89 ng/ml) reach the
highest mortality in the four groups at the end of follow-up time
(log rank test, P<0.05).
Discussion
The the major conclusion of the present study is
that increased concentrations of copeptin and NT-proBNP determined
in plasma samples drawn from patients with severe acute
decompensated heart failure at the time of initial presentation
indicate an increased risk of mortality and re-hospitalization.
Patients with high plasma concentrations of copeptin and NT-proBNP
exhibited higher rates of poor outcome independent of other
confounders, including advanced age, systolic dysfunction and NYHA
class III/IV at baseline in the sample investigated. For patients
in the severe stage of heart failure, copeptin was an independent
predictive factor; however, it was not superior to NT-proBNP.
The majority of single-variable markers are
characterized by the unsatisfactory discrimination of patients with
and without increased mortality due to heart failure. By contrast,
NT-proBNP has been shown to be a useful marker for risk
stratification and prognosis in the setting of heart failure, and
the present study showed similar results (17,18).
In the present study, copeptin levels were
significantly higher in patients with higher NYHA classes, as well
as in patients who reached the adverse end-point. However, the
stimuli resulting in increased secretion of vasopressin in patients
with heart failure have yet to be elucidated. Several previous
studies have assessed the utility of copeptin as a prognostic
biomarker for a variety of other indications, including hemorrhagic
and septic shock (10),
exacerbations of chronic obstructive pulmonary disease (19), certain respiratory tract infections
(16), stroke (20,21) and
traumatic brain injury (22). It may
be hypothesized that the body responds to emergency and severe
diseases by the instantaneous release of immediate AVP and
copeptin. The use of copeptin as a biomarker appears to be
indicative of the patients' stress levels (23). For patients with severe-stage heart
failure (NYHA functional classes III and IV), copeptin provided
independent information; however, it was not the marker with the
highest predictive value and was inferior to NT-proBNP. An
explanation for this finding may be that the ability of biomarkers
to predict outcomes is not static, but rather varies over time
(24). Previous studies have shown
that copeptin had initially strong prognostic abilities, but became
slightly less accurate (24). By
contrast, natriuretic peptides, which were initially poor acute
prognostic markers, showed improved performance during the
follow-up period, suggesting that markers reflecting the
hemodynamic status or myocardial necrosis have superior short-term
prognostic value, which however deteriorates following the
resolution of the acute insult.
While the present study was not an interventional
trial, the results demonstrated that the patients who reached the
adverse end-point did not have an increased length of hospital
stay. The period spent in hospital (in days) showed no significant
difference with regard to stays in a regular ward or intensive care
unit, suggesting that in future clinical practice, high-risk
patients should receive additional care. It is important to note
that, while elevated copeptin or NT-proBNP levels are associated
with the risk of adverse end-points, severe acute decompensated
heart failure treatment should not solely depend on biomarkers, as
these are hemodynamic markers that are not specific to heart
failure (25). However, a high
concentration of either of these markers appears to be an
indication of an increased risk of adverse events and poor
outcome.
The present study had several potential limitations.
One major limitation was that the study only assessed a relatively
small number of patients and was single-centered. Thus, the results
may not accurately represent the general demographics of patients
with severe acute decompensated heart failure in other
institutions. Therefore, the reproduction of the results of the
present study in other centers or by multicenter studies may
provide a stronger argument for their validity. A further
limitation is that during follow-up, due to personal economic
problems, the treatment of certain patients was not in accordance
with best clinical practice or in strict accordance with
guidelines. Thus, the treatment modalities during follow-up may
have affected the results. Furthermore, a number of parameters
[blood pressure and renal function, which have consistently
demonstrated prognostic value (26)]
have not been included in the analysis of the present study.
Finally, copeptin was measured separately, using different
standardized evaluations. Although reflective of real-world
clinical practice, comparison to other studies may indicate a
limitation in the copeptin portion of this analysis.
In conclusion, the results of the present study
suggested that NT-proBNP and copeptin measurements may have similar
predictive value regarding adverse events occurring within 90 days
of severe acute decompensated heart failure. However, copeptin may
provide inferior 90-day prediction compared with NT-proBNP. Future
studies evaluating the role of these biomarkers are warranted and
may further improve the prediction of clinical outcome.
Acknowledgements
The content of the present study was presented at
the 24th Great Wall International Congress of Cardiology/Asia
Pacific Heart Congress/International Congress of Cardiovascular
Prevention and Rehabilitation in Beijing, P.R. China,
10–13.10.2013, pp E218-E219. The present study was supported by
National Natural Science Fund (grant no. 81570212) and the Natural
Science Foundation Project of CQ CSTC (grant no. CSTC,
2011jjA10008). Chongqing Municipal Health Bureau fund (grant nos.
010-1-07, 2012-2-125 and ZY20132124) and the National key Clinical
Specialties Construction Program of China (grant no. 2011-170).
References
|
1
|
Cowie MR, Mosterd A, Wood DA, Deckers JW,
Poole-Wilson PA, Sutton GC and Grobbee DE: The epidemiology of
heart failure. Eur Heart J. 18:208–225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsutamoto T, Wada A, Maeda K, Hisanaga T,
Maeda Y, Fukai D, Ohnishi M, Sugimoto Y and Kinoshita M:
Attenuation of compensation of endogenous cardiac natriuretic
peptide system in chronic heart failure: Prognostic role of plasma
brain natriuretic peptide concentration in patients with chronic
symptomatic left ventricular dysfunction. Circulation. 96:509–516.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Januzzi JL, van Kimmenade R, Lainchbury J,
Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM and
Richards M: NT-proBNP testing for diagnosis and short-term
prognosis in acute destabilized heart failure: An international
pooled analysis of 1256 patients: The international collaborative
of NT-proBNP study. Eur Heart J. 27:330–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moe GW, Howlett J, Januzzi JL and Zowall
H: Canadian Multicenter Improved Management of Patients With
Congestive Heart Failure (IMPROVE-CHF) Study Investigators:
N-terminal pro-B-type natriuretic peptide testing improves the
management of patients with suspected acute heart failure: Primary
results of the Canadian prospective randomized multicenter
IMPROVE-CHF study. Circulation. 115:3103–3110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maisel A, Mueller C, Adams K Jr, Anker SD,
Aspromonte N, Cleland JG, Cohen-Solal A, Dahlstrom U, DeMaria A, Di
Somma S, et al: State of the art: Using natriuretic peptide levels
in clinical practice. Eur J Heart Fail. 10:824–839. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braunwald E: Biomarkers in heart failure.
N Engl J Med. 358:2148–2159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fiore G, Suppress P, Triggiani V, Resta F
and Sabba C: Neuroimmune activation in chronic heart failure.
Endocr Metab Immune Disord Drug Targets. 13:68–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Land H, Schütz G, Schmale H and Richter D:
Nucleotide sequence of cloned cDNA encoding bovine arginine
vasopressin-neurophysin II precursor. Nature. 295:299–303. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Friedmann AS, Malott KA, Memoli VA, Pai
SI, Yu XM and North WG: Products of vasopressin gene expression in
small-cell carcinoma of the lung. Br J Cancer. 69:260–263. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morgenthaler NG, Müller B, Struck J,
Bergmann A, Redl H and Christ-Crain M: Copeptin, a stable peptide
of the arginine vasopressin precursor, is elevated in hemorrhagic
and septic shock. Shock. 28:219–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgenthaler NG, Struck J, Alonso C and
Bergmann A: Assay for the measurement of copeptin, a stable peptide
derived from the precursor of vasopressin. Clin Chem. 52:112–119.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stoiser B, Mörtl D, Hülsmann M, Berger R,
Struck J, Morgenthaler NG, Bergmann A and Pacher R: Copeptin, a
fragment of the vasopressin precursor, as a novel predictor of
outcome in heart failure. Eur J Clin Invest. 36:771–778. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gegenhuber A, Struck J, Dieplinger B,
Poelz W, Pacher R, Morgenthaler NG, Bergmann A, Haltmayer M and
Mueller T: Comparative evaluation of B-type natriuretic peptide,
mid-regional pro-A-type natriuretic peptide, mid-regional
pro-adrenomedullin, and Copeptin to predict 1-year mortality in
patients with acute destabilized heart failure. J Card Fail.
13:42–49. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maisel A, Xue Y, Shah K, Mueller C, Nowak
R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, et al:
Increased 90-day mortality in patients with acute heart failure
with elevated copeptin: Secondary results from the Biomarkers in
Acute Heart Failure (BACH) study. Circ Heart Fail. 4:613–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dickstein K, Cohen-Solal A, Filippatos G,
McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van
Veldhuisen DJ, Atar D, Hoes AW, et al: ESC Guidelines for the
diagnosis and treatment of acute and chronic heart failure 2008 Rhe
Task Force for the Diagnosis and Treatment of Acute and Chronic
Heart Failure 2008 of the European Society of Cardiology. Developed
in collaboration with the Heart Failure Association of the ESC
(HFA) and endorsed by the European Society of Intensive Care
Medicine (ESICM). Eur Heart J. 29:2388–2442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Müller B, Morgenthaler N, Stolz D, Schuetz
P, Müller C, Bingisser R, Bergmann A, Tamm M and Christ-Crain M:
Circulating levels of copeptin, a novel biomarker, in lower
respiratory tract infections. Eur J Clin Invest. 37:145–152. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noveanu M, Breidthardt T, Potocki M,
Reichlin T, Twerenbold R, Uthoff H, Socrates T, Arenja N, Reiter M,
Meissner J, et al: Direct comparison of serial B-type natriuretic
peptide and NT-proBNP levels for prediction of short- and long-term
outcome in acute decompensated heart failure. Critical Care.
15:R12011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Metra M, Nodari S, Parrinello G, Specchia
C, Brentana L, Rocca P, Fracassi F, Bordonali T, Milani P, Danesi
R, et al: The role of plasma biomarkers in acute heart failure.
Serial changes and independent prognostic value of NT-proBNP and
cardiac troponin-T. Eur J Heart Fail. 9:776–786. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanley JA and McNeil BJ: A method of
comparing the areas under receiver operating characteristic curves
derived from the same cases. Radiology. 148:839–843. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katan M, Nigro N, Fluri F, Schuetz P,
Morgenthaler NG, Jax F, Meckel S, Gass A, Bingisser R, Steck A, et
al: Stress hormones predict cerebrovascular re-events after
transient ischemic attacks. Neurology. 76:563–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Urwyler SA, Schuetz P, Fluri F,
Morgenthaler NG, Zweifel C, Bergmann A, Bingisser R, Kappos L,
Steck A, Engelter S, et al: Prognostic value of copeptin: One-year
outcome in patients with acute stroke. Stroke. 41:1564–1567. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong XQ, Huang M, Yang SB, Yu WH and Zhang
ZY: Copeptin is associated with mortality in patients with
traumatic brain injury. J Trauma. 71:1194–1198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katan M, Morgenthaler N, Widmer I, Puder
JJ, König C, Müller B and Christ-Crain M: Copeptin, a stable
peptide derived from the vasopressin precursor, correlates with the
individual stress level. Neuro Endocrinol Lett. 29:341–346.
2008.PubMed/NCBI
|
|
24
|
Peacock WF, Nowak R, Christenson R,
DiSomma S, Neath SX, Hartmann O, Mueller C, Ponikowski P, Möckel M,
Hogan C, et al: Short-term mortality risk in emergency department
acute heart failure. Acad Emerg Med. 18:947–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nickel CH, Bingisser R and Morgenthaler
NG: The role of copeptin as a diagnostic and prognostic biomarker
for risk stratification in the emergency department. BMC Medicine.
10:72012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fonarow GC, Adams KF Jr, Abraham WT, Yancy
CW and Boscardin WJ: ADHERE Scientific Advisory Committee, Study
Group, and Investigators: Risk stratification for in-hospital
mortality in acutely decompensated heart failure: Classification
and regression tree analysis. JAMA. 293:572–580. 2005. View Article : Google Scholar : PubMed/NCBI
|