Introduction
Despite advances in pharmacology, interventional
cardiology techniques, and devices and surgery, heart failure (HF)
remains the leading cause of mortality and hospitalization
worldwide (1). Cardiac remodeling is
generally accepted as a determinant of the clinical course of HF.
Following myocardial infarction (MI), cardiomyocyte loss and
increased load trigger gene expression changes, result in
molecular, cellular, and interstitial changes that manifest
clinically in changes in size, shape, and function of the heart
(2,3). Preventing or attenuating the signaling
pathways leading to changes in heart size is an important goal of
anti-remodeling therapy (4).
A target recently identified in the heart remodeling
pathway is integrin-linked kinase (ILK), a serine/threonine protein
kinase widely expressed in mammalian tissues that plays important
roles in transducing cell-matrix interaction-induced biomechanical
signals, including cytoskeleton remodeling, angiogenesis, cell
growth, proliferation, survival, and differentiation (5,6). Indeed,
mutations in ILK have been reported in human patients with dilated
cardiomyopathy (DCM) (7) and
targeted ILK deletion in the murine heart causes spontaneous DCM
and HF (8). In addition, ILK
controls the recruitment of endothelial progenitor cells to the
ischemic tissue (9) and ILK gene
therapy improves cardiac remodeling and function in rats after MI
(5).
The promising results in rodents led us to conduct
further investigation on the therapeutic effects of ILK in a large
animal model that is closer to approximate human physiology and
anatomy. In the present study, we investigated whether heart
transfection of ILK following acute MI in swine showed the same
therapeutic effects as those described in mice and rats. The
present study focused on the mechanisms of ILK action and the
safety of ILK intervention.
Materials and methods
Recombinant adenovirus
construction
Recombinant adenoviral vector carrying human
wild-type ILK together with humanized recombinant green fluorescent
protein (hrGFP) cDNA (ad-ILK) or only hrGFP (ad-null) under the
control of the CMV promoter was prepared as previously described
(10).
Animal model of MI and adenoviral
vector delivery
Animal experiments were performed following the
guidelines in the Guide for the Care and Use of Laboratory Animals
published by the National Institutes of Health (National Institutes
of Health publication No. 85–23, revised 1985). The procedures were
approved by the Institutional Animal Care and Use Committee of
Nanjing Drum Tower Hospital.
MI was induced as described previously
(11)
Briefly, 20 mini pigs (15–17.5 kg) were randomly
divided into two groups that received ad-ILK (n=10) or ad-null
(n=10). The mini pigs were pre-medicated using intramuscular
ketamine. After the intravenous line was placed, mini pigs were
intubated and ventilated with 100% oxygen (oxygen flow was 3
l/min). Electrocardiograms were monitored continuously during the
operation. A 7F femoral artery sheath was then advanced to the left
coronary artery ostium. After confirming catheter positioning by
coronary angiogram, a coronary angioplasty balloon was placed in
the proximal left anterior descending coronary artery (LAD) to the
first diagonal branch and pre-conditioned for 30 sec for three
times followed by 90 min reperfusion (the balloon was inflated to 4
atm). Then, 2×109 viral particles of ad-ILK or ad-null
were delivered by anterograde intracoronary infusion over 10 min.
The MI model was confirmed by observing a substantial ST-segment
elevation and by serum cTnT levels six hours after surgery.
Ultrasonic cardiogram
Transthoracic echocardiography was assessed before
inducing MI and four weeks after gene delivery. After general
anesthesia, all the measurements were performed under repeated,
short end-expiratory breath holds. Transthoracic 2D
echocardiography was performed with a dynamic focused 3.5–55 MHz
probe and using SONO 5500 ultrasound system (Philips, Eindhoven,
The Netherlands). Left ventricular (LV) end-systolic diameter, LV
end-diastolic diameter, interventricular septal thickness in
diastole (IVSD), LV posterior wall thickness (LVPW), and LV
ejection fraction (LVEF) were measured.
Positron emission tomography
(PET)
Regional myocardial PET was evaluated four weeks
after gene transfer as previously described with minor
modifications. Before PET scans, pigs were maintained under general
anesthesia and blood glucose ranged from 5 to 6 mmol/l. Then the
animals were placed in the dorsal (supine) position, and PET scans
were performed 60 min after intravenous injections of 2.65 MBq of
18F-fluorodeoxyglucose per kilogram. PET images were acquired in 2D
mode. Transaxial cardiac images were then reconstructed into
horizontal short-axis, as well as horizontal and vertical long-axis
with a 4 µm thickness. Delayed PET scans were performed if
necessary. Three axis views were analyzed further.
Single-photon emission computed
tomography (SPECT)
Four weeks after gene transfer, 99 mTc-sestamibi of
1 MBq (10 mCi) per kilogram was delivered to the animals. After 40
min, the heart images were collected with a dual-head γ camera
(Skylight, Philips) in step-and-shoot mode. Then, 64 images (64×64
matrix) were acquired, 40 sec each and throughout a 180° arc. The
images were reconstructed along the short, horizontal long, and
vertical long axes of the heart. Quantitative analysis of perfusion
defects was performed according to the AHA procedural guidelines
for myocardial perfusion imaging in nuclear cardiology (12).
Histology
At the end of the study, pigs were euthanized by
injecting 20 mmol potassium and their hearts were sectioned into
six sections. Hearts, liver, and kidneys were fixed with formalin,
embedded in paraffin for subsequent histological,
immunohistochemical, and TUNEL examination. Tissue samples
collected for western blot analysis were snap-frozen with liquid
nitrogen.
Immunohistochemistry
To differentiate between the border and infarct
area, the frozen heart tissue was first stained with hematoxylin
and eosin (H&E). To measure microvessel density, the frozen
sections (5 µm) were stained with anti-rat vWF antibody (1:200, BD
Laboratory), followed by goat anti-rabbit secondary antibody
conjugated with EnVision HRP/DAB (Dako, Carpinteria, CA, USA).
Microvessel density was calculated as previously reported (13).
Transgene expression and TUNEL
assay
The sections were incubated with mouse anti-rabbit
sarcomeric actin (1:50; Abcam, Cambridge, MA, USA) and rabbit
anti-GFP (1:100; Beyotime Institute of Biotechonolgy, Beijing,
China). Alexa Fluor 488 goat anti-mouse secondary antibody (1:200)
and Alexa Fluor 555 goat anti-rabbit secondary antibody (1:250)
(both from Molecular Probes, Inc., Eugene, OR, USA) were applied
for subsequent detection. Apoptosis was assessed by TUNEL assay on
slides of myocardium using the DeadEnd Fluorimetric TUNEL System
(Promega Corp., Madison, WI, USA). The apoptotic cells were
measured by counting the TUNEL-positive nuclei relative to the
total number of nuclei counterstained with
4′,6-diamidino-2-phenylindole (Sigma, St. Louis, MO, USA).
Western blot analysis
Western blotting was conducted as previously
described (14). Rabbit monoclonal
ILK antibody (dilution, 1:500; cat. no. ab52480; Abcam, Cambridge,
MA, USA), rabbit monoclonal GFP antibody (dilution, 1:1,000; cat.
no. 2037S) and rabbit monoclonal GAPDH antibody (dilution, 1:1,000;
cat. no. 3683) (both from CST, Beverly, MA, USA) were used. Protein
band densities and were calculated by ImageJ followed by
normalization using GAPDH as the internal control.
Statistical analysis
Continuous variables were reported as mean ±
standard error of the mean. Data analysis was performed using SPSS
20 (IBM SPSS, Armonk, NY, USA). The unpaired Student's t-test was
used between group differences if normal distribution was assumed
or Mann-Whitney U test when distributions were not normal. ANOVA
was used to test for differences among at least three groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
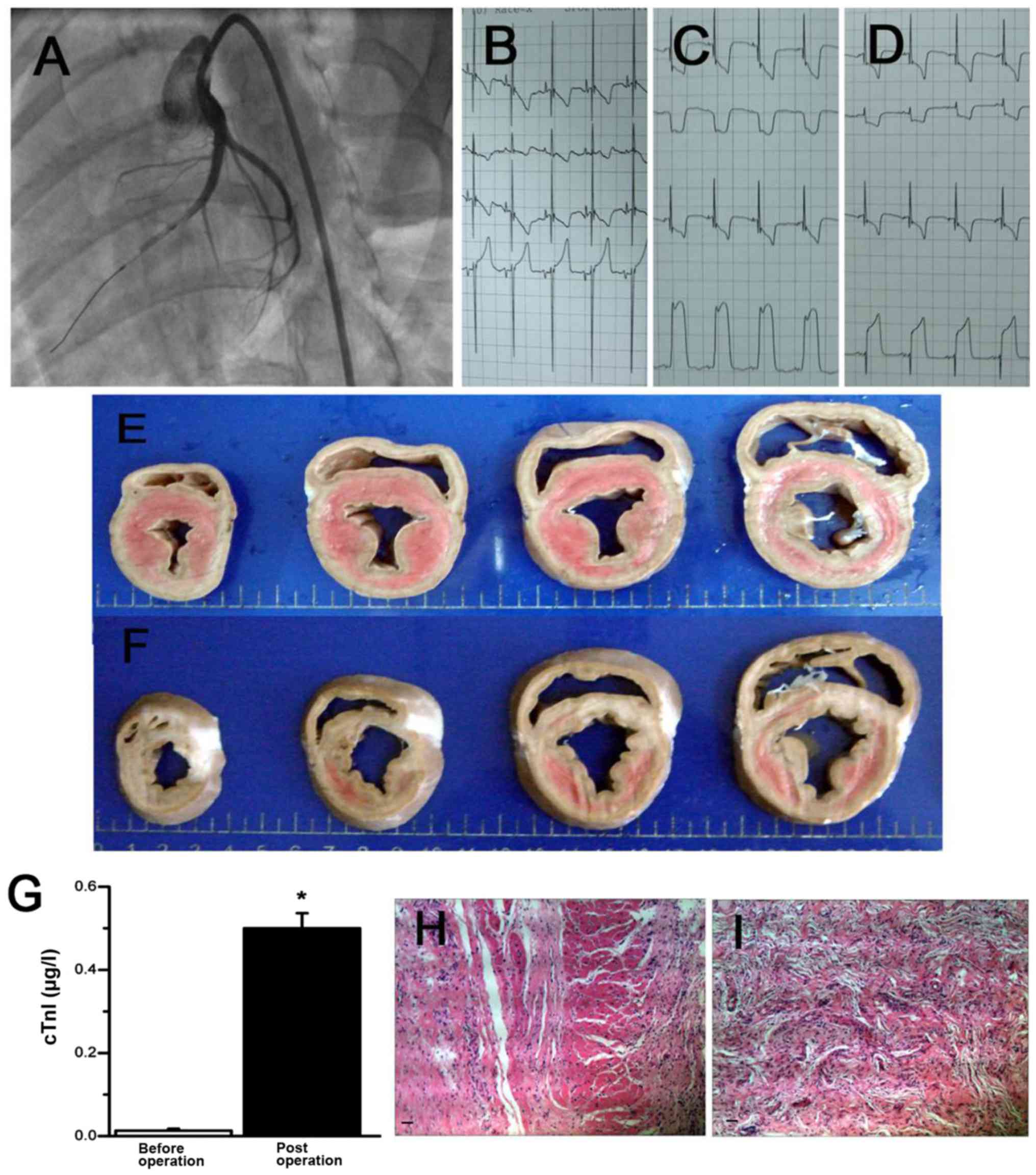
Large-animal model of MI
Percutaneous, catheter-based intermittent balloon
occlusion of the proximal LAD (Fig.
1A) resulted in reproducible perfusion defects of the anterior
wall and MI. The infarcted pigs were confirmed by a substantial
ST-segment elevation (Fig. 1B-D),
the detection of serum cTnT 6 h after surgery (Fig. 1G), and pathological H&E staining
after sacrificing the animals (Fig. 1H
and I). These results supported the successful induction of MI
in mini pig that we can use to study the therapeutic effects of
ILK.
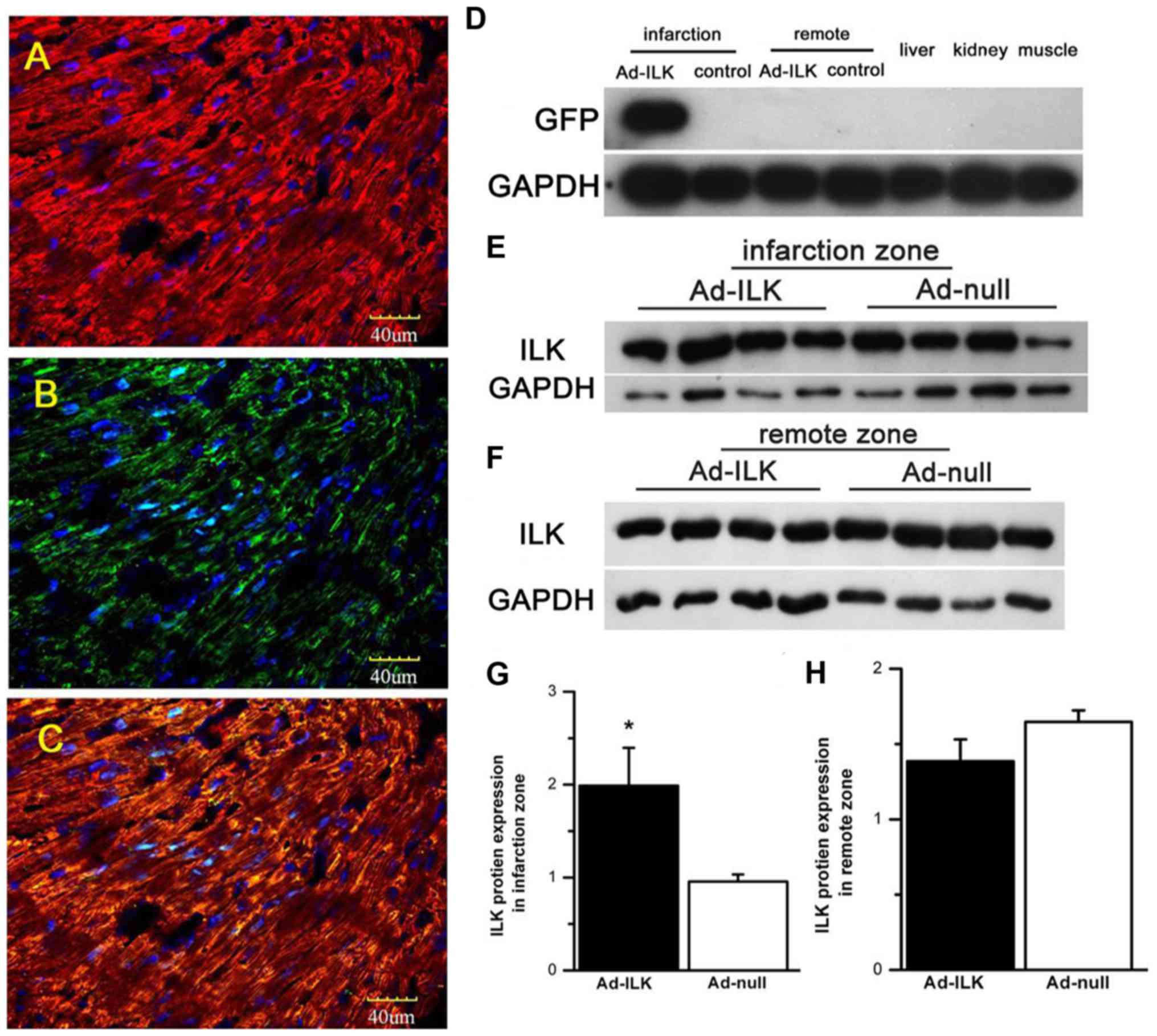
ILK gene therapy increases cardiac ILK
expression
Following the injection of adenoviral particles,
gene transfer was confirmed by detecting the expression of ILK
and/or hrGFP. First, we detected in situ transgene
expression derived from adeno-virus. For this, we stained heart
sections from MI animals four weeks after transfection for
α-sarcomeric actin, a marker of cardiomyocytes (Fig. 2A), and hrGFP. GFP-positive cells were
found in the infarction zone (Fig.
2B). In addition, the majority of hrGFP-positive cells were
overlaid with α-sarcomeric actin (Fig.
2C), indicating successful ad-ILK transfection in
cardiomyocyte, although some cardiac fibroblasts appeared
hrGFP-positive.
For quantitative studies, we detected the exogenous
expression of transfected ILK using western blot analysis. Four
weeks after adenoviral delivery, the expression of ILK in the
infarcted zone was significantly elevated in the ad-ILK group
relative to the ad-null controls by western blot analysis (Fig. 2D, E and G; P<0.05). However, we
found no differences in ILK levels in the infarction remote area
(Fig. 2F-H; P>0.05). These
results confirmed that the expression of ILK from the viral vector
was localized to the infarcted site.
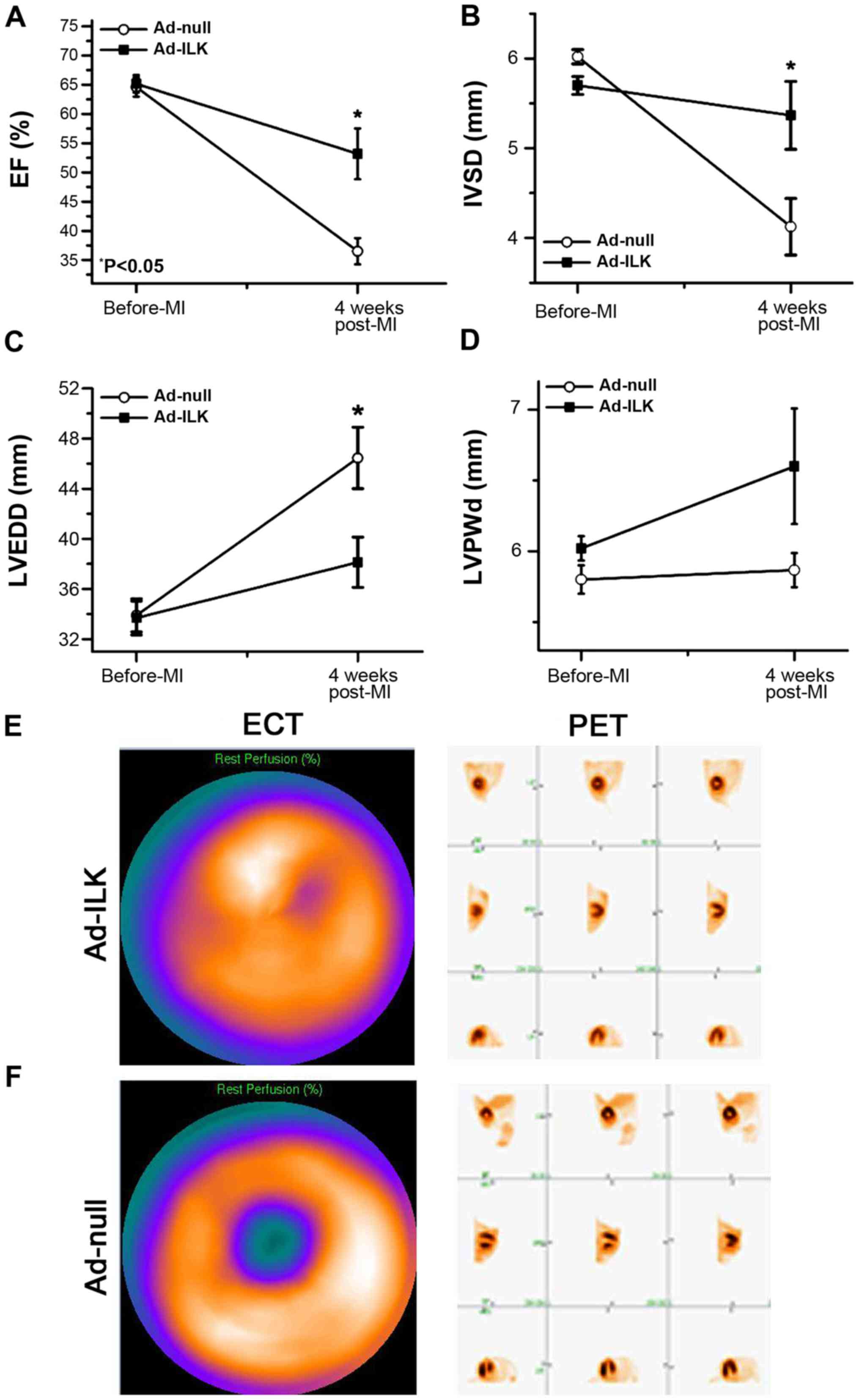
ILK gene rescues cardiac function and
preserves myocardial perfusion
After detecting positive expression of ILK from the
viral transfection, we performed cardiac functional studies with
echocardiography (Fig. 3A-D). Four
weeks after MI, two cases of apical aneurysm occurred in ad-null
group, whereas no cases occurred in the ad-ILK group. ILK
overexpression exhibited a further improvement in percentage of EF
(56.03±3.19%) compared to controls (36.70±2.68%, P<0.05)
(Fig. 3A). Moreover,
interventricular septum thickness (IVSd) improved in the ad-ILK
group (5.48±0.87 mm) compared to the ad-null group (4.17±0.74 mm,
P<0.05) (Fig. 3B). Percent
fractional shortening (% FS) also demonstrated significant
improvement in the ad-ILK group (46.23±2.35 mm) compared to the
ad-null group (37.31±1.98 mm, P<0.05) (Fig. 3C). By contrast, the LVPW showed no
significant difference between the two groups. These data suggested
improved cardiac function four weeks after adenoviral delivery of
ILK.
SPECT is a commonly available direct method for
assessing myocardial cell viability (15). We used SPECT to determine whether
ad-ILK delivery can attenuate the severity of myocardial ischemia.
The extent of rest/stress perfusion defect was markedly smaller
when ILK was overexpressed compared with the control (SRS 8.25±5.7
vs. 14±3.12, P<0.05) (Fig. 3E and
F), further supporting the beneficial effect of ILK.
Compared to SPEPCT, PET-CT possesses the advantage
measuring cardiomyocytes metabolism quantitatively (16). In the present study, PET images
obtained four weeks after ILK treatment displayed improved levels
of myocardial metabolism compared to control. Then, we combined the
PET and SPECT images and the infarcted zones were determined
according to the calculation of DS scores, a sensitive index that
discriminates the vitality of the ischemic myocardium. The DS
scores for the ad-ILK group were highly significant, indicating the
improved vitality of myocardium when treating with ad-ILK (Fig. 3E and F).
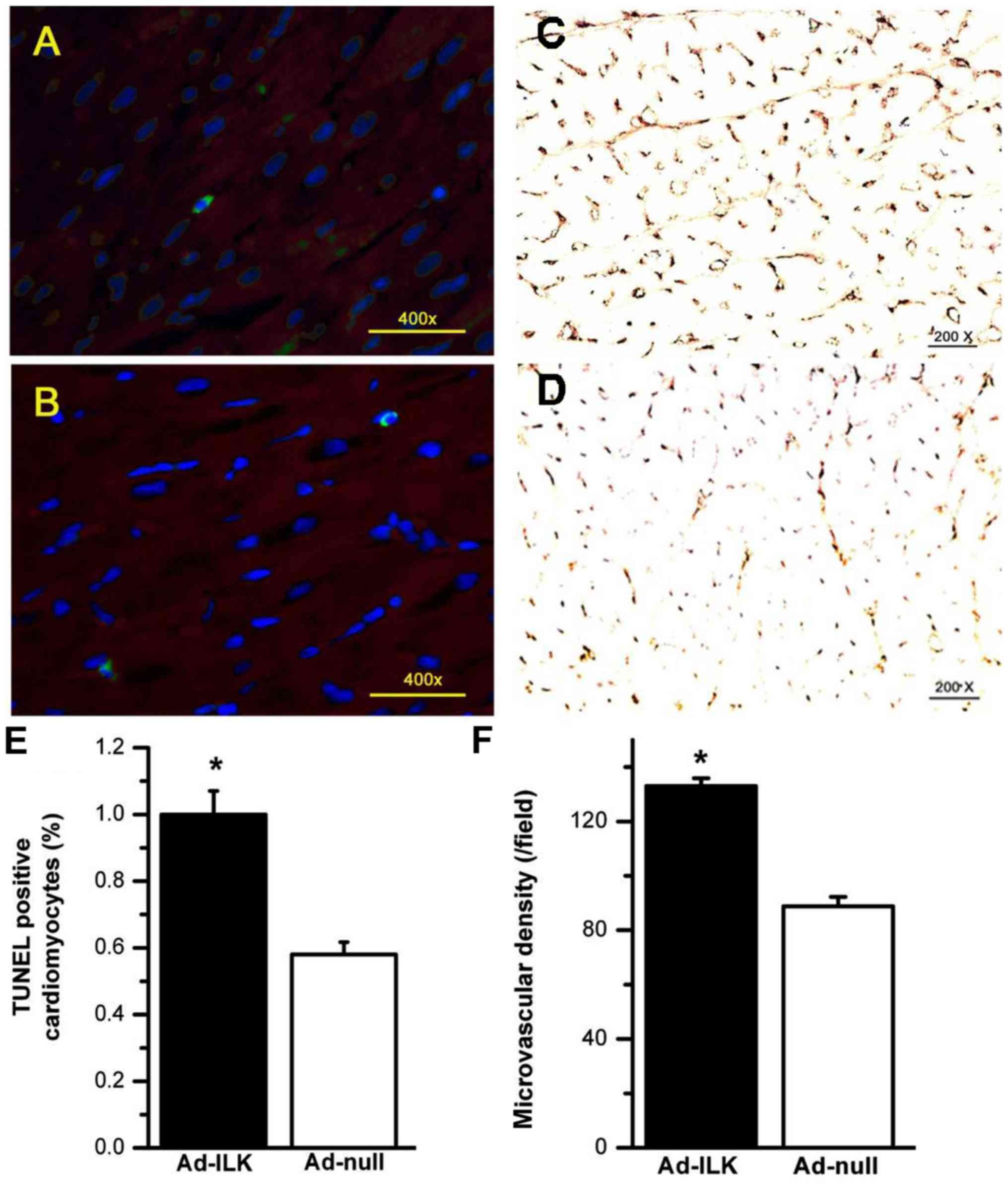
ILK gene therapy increases microvessel density and
reduces apoptosis in the infarct zone. vWF staining four weeks
after MI demonstrated that ad-ILK delivery increased microvessel
density in the peri-infarct myocardium (136.02±6.32) compared to
the ad-null group (91.56±7.81, P<0.01) (Fig. 4C and D). TUNEL assay was conducted to
quantify cardiomyocyte apoptosis following the infarction. The
percentage of TUNEL-positive cardiomyocytes was markedly reduced in
the ad-ILK group (0.57±0.37%) compared to the ad-null group
(1.01±0.93%, P<0.01) in the infarct zone four weeks post-MI
(Fig. 4A and B). These studies
confirmed the protective effect of ILK by promoting microvessel
density and preventing cardiomyocyte cell death.
Discussion
The present preclinical study provides evidence for
the benefits for ILK transfer on cardiac function after MI caused
by a transient coronary occlusion in mini pigs. Four weeks after
gene transfer, the ILK treatment group exhibited reduced infarct
scar size, preserved LV geometry, enhanced angiogenesis, decreased
apoptosis, and increased cardiomyocyte proliferation. These results
support previous work in rodent models on the protective activity
of ILK during MI.
The argument of gene transfection efficiency arises
due to the paradox of time selection for gene transfer.
Post-infarction remodeling has been arbitrarily divided into an
early phase (within 72 h) and a late phase (beyond 72 h) (17). Currently, most preclinical gene
transfer studies have focused on the chronic ischemic heart disease
(a late phase of remodeling) (18).
However, fewer studies address gene transfer on acute MI (an early
phase) model. The early phase is associated with the expansion of
the infarct zone and this time is critical to normalize several key
molecules during the early phase of MI (17). Substantial evidence supports the
effectiveness of emergency percutaneous coronary intervention (PCI)
in acute MI (19). ILK transfer can
be conducted concurrently with emergency PCI, which is available in
clinical practice. Recombinant adenoviruses can express
physiologically significant levels of transgene starting 2–4 h
after delivery (20). Thus, in the
present study, we delivered ILK immediately after inducing MI in
the animal model. Aneurysms form during the early remodeling phase
(17) and we detected them only in
the ad-null group, partly indicating the benefits of early phase
transfer.
The effectiveness of ILK delivery could be
ascertained from the studies using AAV1-mediated SERCA-2a gene
transfer in preclinical (14,21,22)
and clinical studies (23,24), which have shown to boost cardiac
contractility and to preserve cardiac function. Traister et
al (25) initially revealed
increased baseline LV global systolic and diastolic functions in
mice with cardiac-specific overexpression of ILK in a
SERCA-2a/PLN-dependent mechanism. Thus, ILK serves to link
mechanoreception to the dynamic modulation of cardiac contractility
in the SERCA-2a/PLN signaling module. Furthermore, ILK provides
more potential advantages compared to SERCA-2a based on its
SERCA-2a-independent functions. ILK has been confirmed to promote
cardiac contractility in the AKT/GSK-3β, CamKII, SERCA-2a and
ras/raf/MEK pathways. In addition, ILK contributed to angiogenesis
and reduced cardiomyocytes apoptosis (26).
Apart from the benefits on cardiomyocytes, ILK also
protects fibroblasts from apoptosis (27,28),
therefore contributing to the post-infarct healing process by
proliferation, collagen synthesis, transformation into
myofibroblasts, and reducing the ventricular rupture or aneurysm
formation. The present study was consistent with the above
discoveries with no aneurysm found in ILK transfer group compared
with two pigs in the control group.
Several factors may limit the scope of the present
study. First, a more extended period of follow-up would better
reflect the progression of heart remodeling and to better contrast
the advantages of ILK overexpression relative to the ad-null group.
A previous study demonstrated that the improvement in cardiac
function after ad-ILK transfection persists up to at least seven
weeks in a rat MI model (29). In
the present study, although the GFP-positive cells were found in
the infarction zone for weeks after gene transfer, we did not
directly confirm the expression of ILK. Additionally, apart from
detecting ILK and performing histopathology in liver, kidneys, and
spleen from ILK-treated animals (data not shown), serum indexes as
well as cellular and humoral immunogenicity profiles should be
considered to validate the safety of ILK transfer. Furthermore, the
expression of the adenoviral vector used in the current study is
robust but transient. This approach may be appropriate for
short-term, pro-angiogenic responses as in our acute MI model.
However, transgene expression levels exhibited adenoviral vector
peaks within three days and decreases by four weeks (20). In future studies, it may be useful to
examine the effectiveness of alternative vectors expressing ILK for
much longer periods with lower immunity response, such as
recombinant adeno-associated virus.
In conclusion, ILK overexpression by ad-ILK transfer
via intracoronary delivery in an acute MI porcine model can
preserve global LVEF, improve ventricular remodeling and restore
regional perfusion with no signs of toxicology by histopathology
assessment, which paves the way for further clinical studies to
better define safety and efficacy of this promising approach to
ischemic heart disease.
References
|
1
|
Braunwald E: The war against heart
failure: The Lancet lecture. Lancet. 385:812–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gajarsa JJ and Kloner RA: Left ventricular
remodeling in the post-infarction heart: A review of cellular,
molecular mechanisms, and therapeutic modalities. Heart Fail Rev.
16:13–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koitabashi N and Kass DA: Reverse
remodeling in heart failure - mechanisms and therapeutic
opportunities. Nat Rev Cardiol. 9:147–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tilemann L, Ishikawa K, Weber T and Hajjar
RJ: Gene therapy for heart failure. Circ Res. 110:777–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding L, Dong L, Chen X, Zhang L, Xu X,
Ferro A and Xu B: Increased expression of integrin-linked kinase
attenuates left ventricular remodeling and improves cardiac
function after myocardial infarction. Circulation. 120:764–773.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hannigan GE, Leung-Hagesteijn C,
Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC and Dedhar
S: Regulation of cell adhesion and anchorage-dependent growth by a
new beta 1-integrin-linked protein kinase. Nature. 379:91–96. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knöll R, Postel R, Wang J, Krätzner R,
Hennecke G, Vacaru AM, Vakeel P, Schubert C, Murthy K, Rana BK, et
al: Laminin-alpha4 and integrin-linked kinase mutations cause human
cardiomyopathy via simultaneous defects in cardiomyocytes and
endothelial cells. Circulation. 116:515–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White DE, Coutu P, Shi YF, Tardif JC,
Nattel S, St Arnaud R, Dedhar S and Muller WJ: Targeted ablation of
ILK from the murine heart results in dilated cardiomyopathy and
spontaneous heart failure. Genes Dev. 20:2355–2360. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SP, Youn SW, Cho HJ, Li L, Kim TY,
Yook HS, Chung JW, Hur J, Yoon CH, Park KW, et al: Integrin-linked
kinase, a hypoxia-responsive molecule, controls postnatal
vasculogenesis by recruitment of endothelial progenitor cells to
ischemic tissue. Circulation. 114:150–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling L, Bai J, Gu R, Jiang C, Li R, Kang
L, Ferro A and Xu B: Sca-1+ cardiac progenitor cell
therapy with cells overexpressing integrin-linked kinase improves
cardiac function after myocardial infarction. Transplantation.
95:1187–1196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao Q, Lin C, Gao J, Liang X, Gao W, Shen
L, Kang L and Xu B: Mesenchymal stem cells overexpressing
integrin-linked kinase attenuate left ventricular remodeling and
improve cardiac function after myocardial infarction. Mol Cell
Biochem. 397:203–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hendel RC, Berman DS, Di Carli MF,
Heidenreich PA, Henkin RE, Pellikka PA, Pohost GM and Williams KA:
American College of Cardiology Foundation Appropriate Use Criteria
Task Force; American Society of Nuclear Cardiology; American
College of Radiology; American Heart Association; American Society
of Echocardiology; Society of Cardiovascular Computed Tomography;
Society for Cardiovascular Magnetic Resonance; Society of Nuclear
Medicine: ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use
criteria for cardiac radionuclide imaging: A report of the American
College of Cardiology Foundation appropriate use criteria task
force, the American Society of Nuclear Cardiology, the American
College of Radiology, the American Heart Association, the American
Society of Echocardiography, the Society of Cardiovascular Computed
Tomography, the Society for Cardiovascular Magnetic Resonance, and
the Society of Nuclear Medicine. J Am Coll Cardiol. 53:2201–2229.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rutanen J, Rissanen TT, Markkanen JE,
Gruchala M, Silvennoinen P, Kivelä A, Hedman A, Hedman M, Heikura
T, Ordén MR, et al: Adenoviral catheter-mediated intramyocardial
gene transfer using the mature form of vascular endothelial growth
factor-D induces transmural angiogenesis in porcine heart.
Circulation. 109:1029–1035. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi
Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, et al: Reversal
of cardiac dysfunction after long-term expression of SERCA2a by
gene transfer in a pre-clinical model of heart failure. J Am Coll
Cardiol. 51:1112–1119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR and White HD: Writing Group on behalf of the Joint
ESC/ACCF/AHA/WHF Task Force for the Universal Definition of
Myocardial Infarction: Third universal definition of myocardial
infarction. Glob Heart. 7:275–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flotats A, Knuuti J, Gutberlet M, Marcassa
C, Bengel FM, Kaufmann PA, Rees MR and Hesse B: Cardiovascular
Committee of the EANM, the ESCR and the ECNC: Hybrid cardiac
imaging: SPECT/CT and PET/CT. A joint position statement by the
European Association of Nuclear Medicine (EANM), the European
Society of Cardiac Radiology (ESCR) and the European Council of
Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging. 38:201–212.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sutton MG and Sharpe N: Left ventricular
remodeling after myocardial infarction: Pathophysiology and
therapy. Circulation. 101:2981–2988. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scimia MC, Gumpert AM and Koch WJ:
Cardiovascular gene therapy for myocardial infarction. Expert Opin
Biol Ther. 14:183–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Gara PT, Kushner FG, Ascheim DD, Casey
DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM,
Franklin BA, et al: CF/AHA Task Force: 2013 ACCF/AHA guideline for
the management of ST-elevation myocardial infarction: executive
summary: a report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines. Circulation. 127:529–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hajjar RJ: Potential of gene therapy as a
treatment for heart failure. J Clin Invest. 123:53–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vejpongsa P and Yeh ETH: Wrestling with
heart failure: SUMO-1 to the rescue. Circ Res. 114:1561–1563. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tilemann L, Lee A, Ishikawa K, Aguero J,
Rapti K, Santos-Gallego C, Kohlbrenner E, Fish KM, Kho C and Hajjar
RJ: SUMO-1 gene transfer improves cardiac function in a
large-animal model of heart failure. Sci Transl Med.
5:211ra1592013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K,
Greenberg B, Jessup M and Hajjar RJ: Long-term effects of
AAV1/SERCA2a gene transfer in patients with severe heart failure:
Analysis of recurrent cardiovascular events and mortality. Circ
Res. 114:101–108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Greenberg B, Yaroshinsky A, Zsebo KM,
Butler J, Felker GM, Voors AA, Rudy JJ, Wagner K and Hajjar RJ:
Design of a phase 2b trial of intracoronary administration of
AAV1/SERCA2a in patients with advanced heart failure: The CUPID 2
trial (calcium up-regulation by percutaneous administration of gene
therapy in cardiac disease phase 2b). JACC Heart Fail. 2:84–92.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Traister A, Li M, Aafaqi S, Lu M, Arab S,
Radisic M, Gross G, Guido F, Sherret J, Verma S, et al:
Integrin-linked kinase mediates force transduction in
cardiomyocytes by modulating SERCA2a/PLN function. Nat Commun.
5:45332014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao Q, Lin CX, Liang XL, Gao JS and Xu B:
Mesenchymal stem cells overexpressing integrin-linked kinase
attenuate cardiac fibroblast proliferation and collagen synthesis
through paracrine actions. Mol Med Rep. 7:1617–1623.
2013.PubMed/NCBI
|
|
28
|
Nho RS, Xia H, Kahm J, Kleidon J, Diebold
D and Henke CA: Role of integrin-linked kinase in regulating
phosphorylation of Akt and fibroblast survival in type I collagen
matrices through a beta1 integrin viability signaling pathway. J
Biol Chem. 280:26630–26639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding L, Dong L, Chen X, Zhang L, Xu X,
Ferro A and Xu B: Increased expression of integrin-linked kinase
attenuates left ventricular remodeling and improves cardiac
function after myocardial infarction. Circulation. 120:764–773.
2009. View Article : Google Scholar : PubMed/NCBI
|