Introduction
Stroke is a major life-threatening disease with a
worldwide incidence of approximately two in 1,000 per year, and a
annual 8% mortality rate (1).
Despite the development of shock therapy and the application of
advanced thrombolytic agents and intravascular procedures, clinical
therapy of the debilitating disorder remains unsatisfactory
(2,3). Therefore, neuroprophylaxis against
stroke has received more attention.
Ampelopsin
(2R,3R)-3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one;
AMP; Fig. 1), also referred to as
dihydromyricetin, is the primary bioactive component and one of the
most common flavonoids isolated from the tender stem and leaves of
the Chinese medicinal herb Ampelopsis grossedentata
(Hand-Mazz) W.T. Wang, which is typically used to make an
infusion called Rattan tea (4,5). AMP is
collected from the tender stem and leaves of the species, which
contain >27%, and the cataphylls, which contains >40%
(6,7).
AMP exhibits a number of biological and
pharmacological properties including antimicrobial,
anti-inflammatory, antioxidative, anti-hypertensive,
hepatoprotective and anticarcinogenic activities in addition to
providing cough relief (5,8–13).
Previous studies have demonstrated that AMP not only exhibits
acetylcholinesterase inhibitory activities, suggesting
anti-Alzheimer effects (14), but
also decreases the aggregation of β-amyloid peptide, which is a key
compound in the induction of Alzheimer's disease (15). These results suggest a role of AMP in
protecting against brain cell dysfunction. AMP protects PC12 cells
from H2O2-induced apoptosis by activating the
extracellular-signal related kinase (ERK) and protein kinase B
(Akt) signaling pathways and upregulation of heme oxygenase-1
(12). Additionally, the protective
effects of the leaf and stem of Vitis amurensis against
neuronal injury induced by middle cerebral artery occlusion (MCAO)
followed by reperfusion in rats and against glutamate-induced
excitotoxicity in cultured rat cortical neurons have been reported
(10,12). AMP is reportedly one of the active
components contributing to the neuroprotective effect of V.
amurensis against glutamate-induced neurotoxicity (10). However, based on the previous studies
described, it is not clear whether AMP has a direct or indirect
protective effect against cerebral ischemia reperfusion injury.
Upregulation of cerebral inflammatory cytokines,
disruption of the blood-brain barrier (BBB), activation of systemic
lymphocytes, local microglia and astrocytes substantially
contribute to ischemic damage (16–19).
Rattan tea extraction has been indicated to reduce
carrageenan-induced acute inflammation in vivo (20) and AMP inhibited the production of
nitric oxide in lipopolysaccharide (LPS)-challenged RAW264.7
macrophages (21). Previous studies
have confirmed that AMP reduced LPS/toll-like receptor 4-mediated
inflammation characterized by nitric oxide biosynthesis and
pro-inflammatory cytokines [interleukin (IL)-1β, tumor necrosis
factor-α (TNF-α) and IL-6] production in RAW264.7 macrophages,
mediated by the reactive oxygen species (ROS), Akt, inhibitor of
nuclear factor-κB (IKK) and nuclear factor κB (NF-κB) signaling
pathways (9). However, whether AMP
inhibits inflammatory responses such as the generation of
inflammatory cytokines and destruction of the BBB remains
unknown.
The aims of the current study were to systematically
evaluate whether AMP protected against acute brain injury following
focal cerebral ischemia in rats and to elucidate the mechanisms
underlying AMP activity. The results of the present study provide
experimental evidence to support the development of AMP as an
effective and safe candidate for the prevention and/or therapy of
cerebral ischemia. The current study used the cysteinyl leukotriene
receptor 1 (CysLT1R)-selective antagonist, pranlukast,
an anti-inflammatory agent as a positive control, as it has been
reported to protect against cerebral ischemia (17,22–24).
Materials and methods
Animals
A total of 92 male Sprague-Dawley rats weighing
250–300 g and 10–12 weeks old were supplied by the Experimental
Animal Center, Zhejiang Academy of Medicine Sciences (Hangzhou,
China; Certificate no. SCXK (Zhe) 2014-0001). The animals were
housed in a controlled temperature of 20–24°C, under a 12-h
light/dark cycle with ad libitum access to food and water
with the exception of preoperative fasting. Procedures involving
animals and their care were performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals. All experimental protocols were approved by the
Ethics Committee of Laboratory Animal Care and Welfare at the
School of Medicine, Zhejiang University (Hangzhou, China). Every
effort was made to minimize the number of animals used and their
suffering.
Chemicals
AMP with 95% purity was donated by Dr Jiye-Zhang
(College of Pharmacy. Xi'an Jiaotong University, Xi'an, China),
3,5-triphenyltetrazolium chloride (TTC) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Fluoro-Jade B was
purchased from Merck Millipore (Merck KGaA). Pranlukast was a kind
gift from Dr. Masami Tsuboshima (Ono Pharmaceutical Co. Ltd, Osaka,
Japan). Paraformaldehyde, sucrose, cresyl violet and chloral
hydrate were purchased from Sigma-Aldrich; Merck KGaG (Darmstadt,
Germany). Biotinylated anti-rat IgG antibody (catalogue no.
20141021) and streptavidin horse radish peroxidase (catalogue no.
20140815) were from Vector laboratories, Inc., (Burlingame, CA,
USA), repacked and sold by Zhongshan Goldenbridge Biotechnology
Co., Ltd (Beijing, China).
Transient focal cerebral ischemia
induction
Transient focal cerebral ischemia was induced using
a modified method of MCAO (25)
according to a previously reported method (26). The 92 rats were anaesthetized with an
intraperitoneal injection of chloral hydrate (400 mg/kg) and placed
in dorsal recumbency. The left common carotid artery, external
carotid artery (ECA) and internal carotid artery (ICA) were
isolated. A 4–0 monofilament nylon suture (Beijing Sunbio Biotech
Co., Ltd., Beijing, China) with a round poly-L-lysine coated tip
was inserted from the ECA into the ICA 18–19 mm until a slight
resistance was felt, which indicated that the suture had blocked
the origin of the left middle cerebral artery (MCA). The MCA was
occluded for 60 min and the suture was withdrawn to allow
reperfusion. The ECA was ligated and the incision was closed.
Sham-operated rats (n=12) were manipulated similarly; the
monofilament was inserted to a depth of 1.0–2.0 mm without
occluding the MCA. Body temperature was maintained at 36–37°C with
a warming platform (Kent Scientific Services, West Malling, UK).
Following surgery, rats were maintained for ~2 h in a warm box
heated by lamps to maintain body temperature.
The rats were randomized into six groups: Sham
(n=12), saline (n=17), AMP [40 (n=16), 80 (n=15) or 160 (n=15)
mg/kg, 1 ml per 100 g bodyweight per os (p.o.) (12)] and pranlukast (n=17, 0.1 mg/kg
intraperitoneally (i.p.)). Due to a 20–30% mortality during the
surgical procedure, the final number of each surgery group was
n=12. AMP and pranlukast were administered to the rats (n=12) 30
min prior to and 30 min following MCAO. Pranlukast was used as a
positive control. The injuries were evaluated 24 h following
reperfusion. An equal volume (10 ml/kg) of saline was administered
orally as the control.
Cerebrospinal fluid (CSF)
collection
CSF was collected as previously described (27,28) and
modified as follows: Animals were anesthetized with chloral hydrate
(400 mg/kg) via i.p. injection. The fur on the neck region of the
rat was removed using an oster clipper. The anesthetized rat was
placed in a stereotaxic frame (Stoelting Inc., Wood Dale, IL, USA)
and secured with ear bars. The position of the animal's head was
maintained downward at ~45° using straps and clamps. Using tweezers
and scissors, the atlanto-occipital membrane between the occipital
protuberance and the spine of the atlas was exposed. A needle
connected to a draw syringe was inserted horizontally and centrally
into the cisterna magna for CSF collection without making any
incision at this region. Once a change in resistance was felt along
the direction of insertion, the colorless CSF was gently aspirated
through the needle into the syringe. The color of the CSF was
closely observed to avoid any blood contamination. In the process
of sampling the CSF, the pumping strength was controlled to avoid
blood contamination. CSF was ejected into 0.5 ml Eppendorf tubes
and frozen at −80°C. The volume of CSF ranged from 60–150 µl in all
the samples.
Behavioral assessments
Neurological deficit scores were evaluated 24 h
following MCAO, as previously described (22): 0, no deficit; 1, failure to extend
left forepaw fully; 2, circling to the left; 3, falling to the
left; and 4, no spontaneous walking with a depressed level of
consciousness. An inclined board test was used to assess balance
and coordination based on a modified method (29). Rats were placed on a board (50×30 cm)
and stabilized. The board was then inclined from the horizontal to
a vertical plane. The degree at which the animal fell from the
board (holding angle) was recorded. The assessment was repeated
three times and the average degree was used. An examiner blinded to
the experiment groups completed all behavioral assessments.
Infarct volume and brain edema
Following behavioral assessments, 36 rats were
sacrificed by decapitation, following anesthetic with chloral
hydrate (400 mg/kg) via i.p. injection; the brains were removed
rapidly and stored at −20°C for 10 min. The brain was sliced into
2-mm coronal sections and stained with 0.5% TTC solution at 37°C
for 20 min in the dark, then fixed at 25°C in 4% buffered formalin
overnight. Images of the stained slices were obtained using a
FinePix S602 Zoom digital camera (Fujifilm, Tokyo, Japan). The
total infarction volume was measured as the sum of the infarcts of
each of the six sections. Infarct areas of all sections were added
to obtain the total infarct area, which was multiplied by the
thickness of the brain sections. Edema was evaluated indirectly as
the percentage increase of ischemic vs. non-ischemic hemisphere
volume. To partially compensate for the effects of edema, the
corrected infarct area was determined as previously described
(30) Corrected infarct area =
Measured infarct area × (1 - [(ipsilateral hemisphere area -
contralateral hemisphere area) / contralateral hemisphere
area]).
Histopathology and
immunohistochemistry
From a second group, 36 rats were anesthetized with
chloral hydrate (400 mg/kg) via i.p. injection 24 h following MCAO
and perfused transcardially with 4% paraformaldehyde following a
pre-wash with saline prior to being sacrificed by decapitation. The
brain was then removed, fixed at 25°C in 4% paraformaldehyde
overnight and transferred to 30% sucrose and incubated for 5 days
at 25°C. The whole brain was imaged using a FinePix S602 Zoom
digital camera to record the changes on the surface. Subsequently,
frozen coronal sections were sliced into 10-µm sections using a CM
1900 cryomicrotome (Leica Microsystems GmbH, Wetzlar, Germany). The
sections were stained with cresyl violet and Fluoro-Jade B prior to
use in immunohistochemical examination.
Cresyl violet staining
Cresyl violet is a basic aniline dye used to stain
RNA blue in order to highlight important structural features of
neurons, specifically in brain and spinal cord tissue. The brain
sections were deparaffinized by soaking in a 1:1 100% alcohol/100%
chloroform mixture for 15 min followed by 15 min in 100% alcohol
and 95% alcohol/5% deionized H2O mixture for
rehydration. The sections were transferred to 0.1% cresyl violet
solution for 10 min, rinsed quickly in distilled water and
differentiated in 95% ethyl alcohol for 30 min to allow for the
best contrast. The sections were dehydrated in 100% alcohol,
immersed in xylene and cover-slipped. The stained sections were
observed under a BX-51 fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The neurons in the cortex, both the
ischemic core and the boundary zone, were counted using ImageJ
software (National Institutes of Health, Bethesda, MA, USA).
Fluoro-Jade B staining
Fluoro-Jade B has high affinity for degenerating
apoptotic and necrotic neurons, exhibiting a green fluorescence.
Fluoro-Jade B staining was performed according to a modified
protocol (31). Briefly, the brain
sections were air-dried on a slide warmer at 50°C and immersed in
1% sodium hydroxide in 80% alcohol for 5 min followed by 2 min in
70% alcohol and 2 min in distilled water. The sections were then
transferred to 0.06% potassium permanganate for 15 min, followed by
2 min in distilled water. The sections were transferred to 0.0004%
Fluoro-Jade staining solution for 30 min and rinsed with distilled
water for 2 min. Following drying, the sections were immersed in
xylene, cover-slipped with mounting medium (Dako, Glostrup,
Denmark) and examined using a BX-51 fluorescence microscope. ImageJ
was used to count the degenerating neurons.
Endogenous immunoglobulin (Ig)G
immunostaining
Endogenous IgG immunostaining was performed to
detect the permeability of the BBB (32). Sections (1.8–2.0 mm caudal from
bregma) were incubated with biotinylated anti-rat IgG antibody
(#20141021; 1:200) for 2 h at 37°C, and with streptavidin horse
radish peroxidase (#20140815; 1:200; Vector laboratories, Inc.,
Burlingame, CA, USA) for 2 h at 37°C. Finally, the sections were
exposed for 2 min to 0.05% 3,3′-diaminobenzidine and 0.03%
H2O2. The sections were then digitized, the
optical gray scales in the immunostained sections were detected
with ImageJ 1.37c (National Institutes of Health, Bethesda, MD,
USA). IgG exudation was evaluated by determining the stained area
and as percentage increase of the gray scales of the ischemic
hemisphere: (Gi-G0)/G0xl00%, where Gi is the grayscale intensity of
the ischemic hemisphere and G0 is the grayscale intensity of the
contralateral uninjured hemisphere. The total IgG extravasation
area of all coronal sections was evaluated using the same image
analysis program.
Cytokine assay by ELISA
The serum and CSF were collected 24 h following MCAO
and were frozen at −80°C until analysis. The cytokines IL-1β and
TNF-α in the medium were measured using commercial ELISA kits for
IL-1β (Rat IL-1β ELISA kit, #140609) and TNF-α (Rat TNF-alpha ELISA
kit, #140528; Changzhou Lianke Chemical Technology Co., Ltd.,
Hangzhou, China) according to the manufacturers' protocol.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using one-way
analysis of variance, followed by Newman-Keuls post-hoc multiple
comparison and nonparametric Kruskal-Wallis test for neurological
deficit scores using Prism software, version 4.02 (GraphPad
Software Inc., La Jolla, CA, USA). P<0.05 was considered to
represent a statistically significant difference.
Results
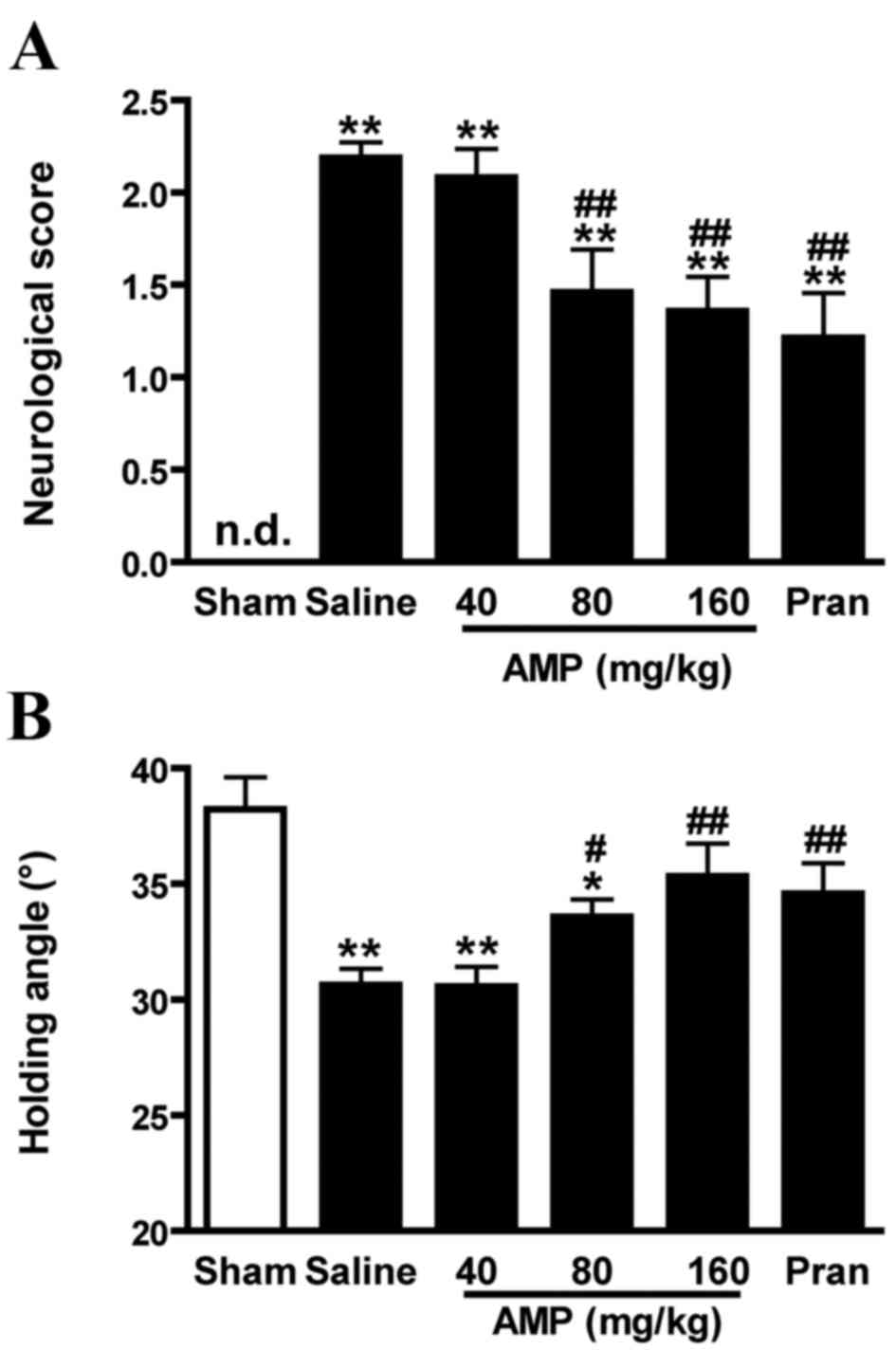
AMP improves neurological score and
holding angle in MCAO-injured rats
No mortality was observed in sham-operated rats. The
mortality of ischemic rats was ~22% in each group (n=12) 24 h
following ischemia. No significant difference in mortality rates
was identified between the groups.
Sham-operated rats demonstrated no neurological
deficit throughout the observation period. Saline-treated ischemic
rats exhibited significant neurological deficits 24 h following
MCAO (P<0.01) compared with the saline control. In rats treated
with 80 and 160 mg/kg AMP and the pranlukast (0.1 mg/kg) groups of
rats, neurological deficits were significantly reduced (P<0.01;
Fig. 2A).
In the inclined board test, the inclination
significantly decreased 24 h following MCAO in saline-treated
ischemic rats compared with sham control (P<0.01). Exposure to
AMP (80 and 160 mg/kg) and pranlukast significantly increased the
holding angles in the inclined board test (P<0.01 and P<0.05,
respectively; Fig. 2B).
AMP reduces MAOC-induced infarct
volume and mitigates the increase in ischemic hemisphere
volume
Ischemia induced brain lesion and edema 24 h
following MCAO. TTC-stained coronal slices exhibited clear lesion
or infarct areas in the ischemic hemispheres (Fig. 3A). AMP (80 and 160 mg/kg) reduced the
lesion volume by 30.7 and 44.6%, respectively, compared with saline
group, 24 h following MCAO (Fig.
3B). There was a reduction in the ratio of the left/right
hemispheric ratio (edema) at 24 h following MCAO of 43.2 and 54.1%,
following treatment with 80 and 160 mg/kg AMP, respectively.
Pranlukast exerted similar effects (Fig.
3C).
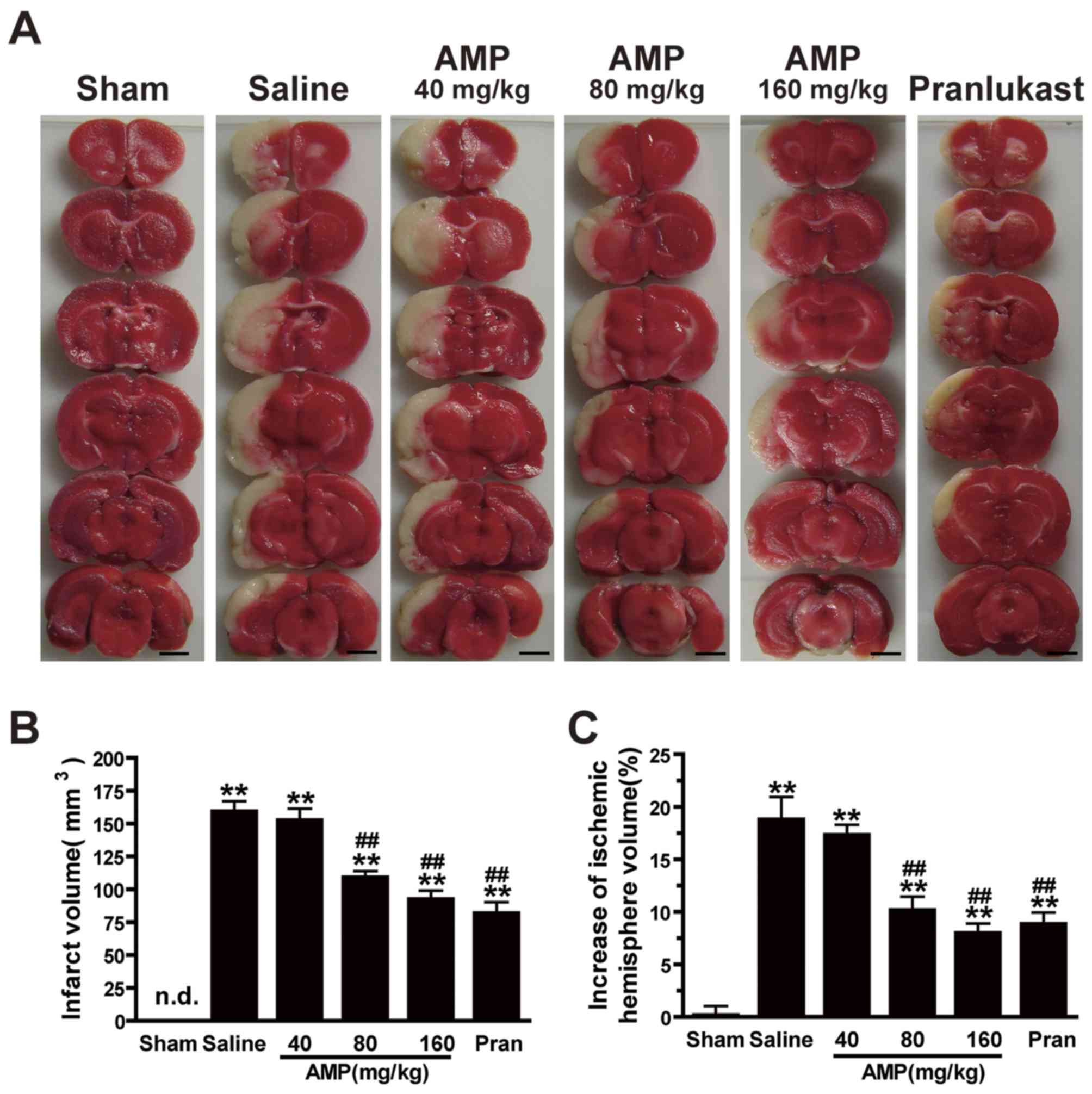 | Figure 3.Effects of ampelopsin and pran on
infarct volume and brain edema 24 h following MCAO in rats. (A)
Images of 3,5-triphenyltetrazolium chloride-stained coronal slices
indicate brain lesions 24 h following MCAO. (B) Infarct volume and
(C) percent increase in ischemic hemisphere volume were reduced
dose-dependently by AMP (80 and 160 mg/kg, p.o.) and pran (0.1
mg/kg, i.p.) treatment (n=6). *P<0.05 and **P<0.01 vs. sham,
#P<0.05 and ##P<0.01 vs. ischemic
control (vehicle), analyzed by one-way analysis of variance. Scale
bar, 4 mm. AMP, ampelopsin; Pran, pranlukast; MCAO, middle cerebral
artery occlusion; n.d., not detectable. |
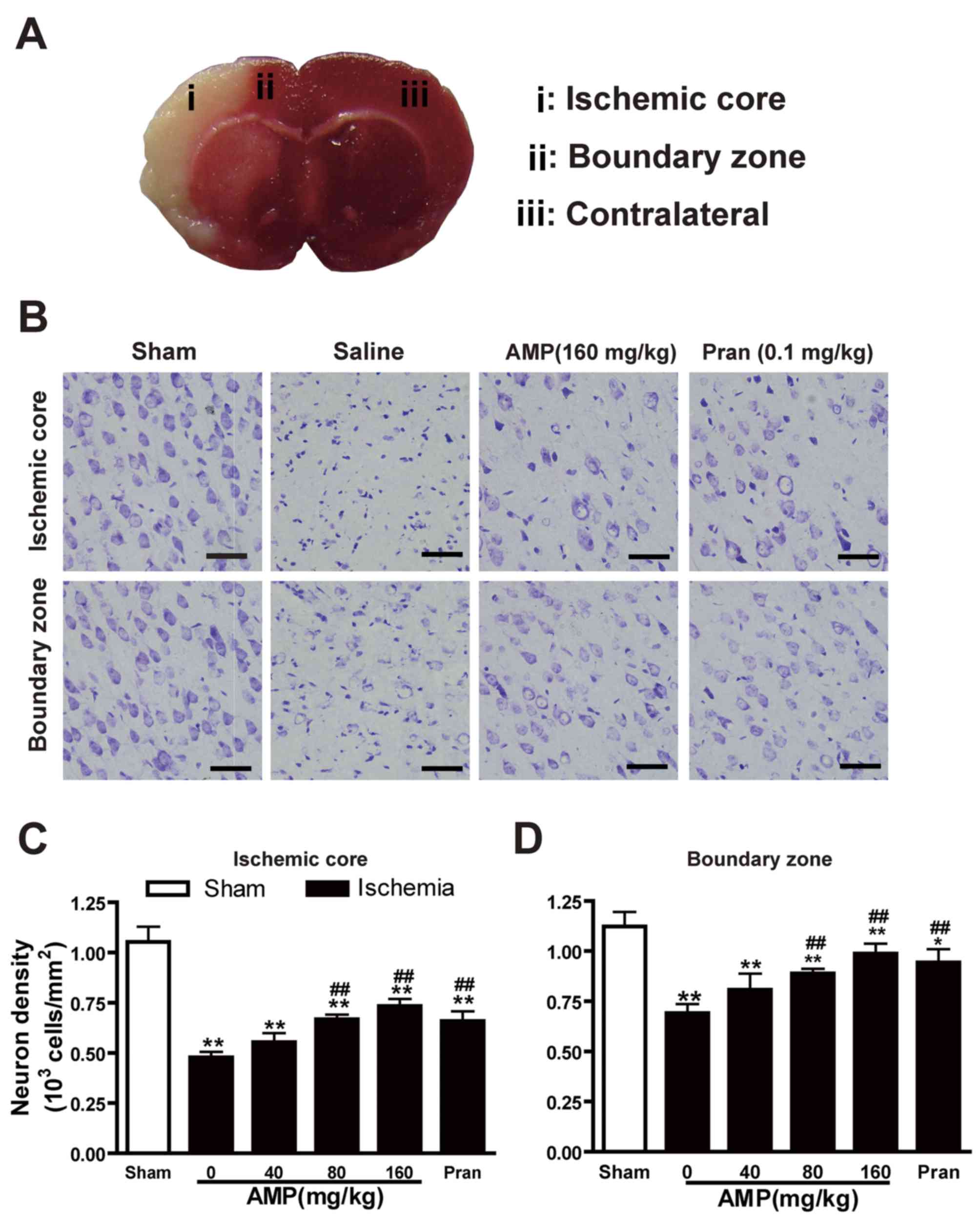
AMP inhibited MCAO-induced neuronal
loss in the ischemic core
Cresyl violet staining of coronal slices indicated
neuronal damage in the temporoparietal cortex of ischemic
hemispheres (Fig. 4A), as a
reduction in nissl bodies, shrunken and deep-stained cell bodies
(Fig. 4B). Neuronal dendrites were
significantly decreased 24 h following reperfusion (P<0.05). AMP
(80 and 160 mg/kg) in addition to pranlukast (1 mg/kg, i.p.)
ameliorated neuronal loss in the ischemic core (Fig. 4C) and the boundary zone (Fig. 4D) adjacent to the infarcted area.
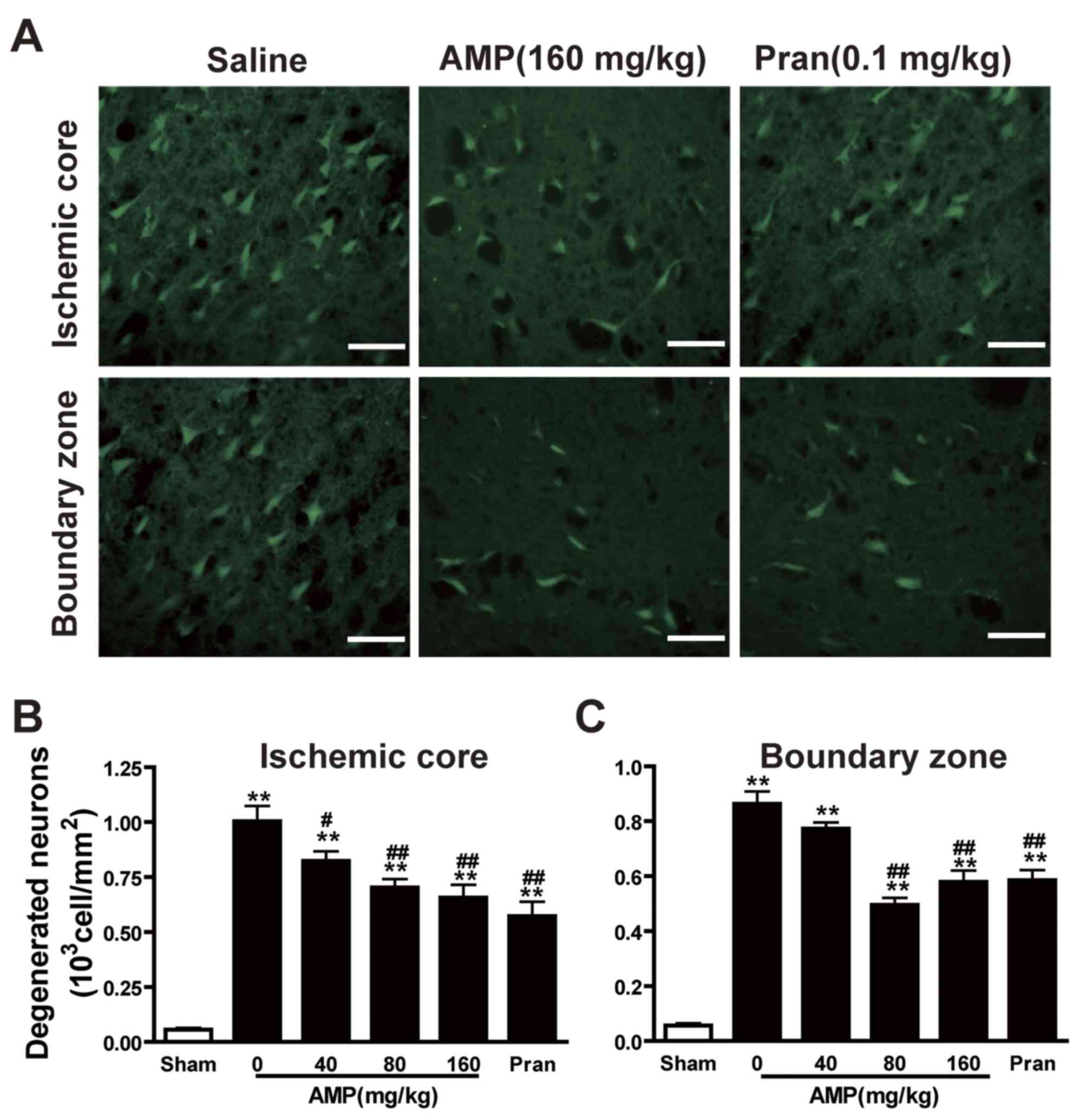
AMP mitigates MCAO-induced neuronal
degeneration in the ischemic core and boundary zone of the rat
brain
The density of Fluoro-Jade B-positive degenerating
neurons in the cortex III and IV layers of the infarcted hemisphere
increased gradually 24 h following MCAO, specifically in the
ischemic core. A small number of degenerating neurons were observed
in the sham-operated rats (Fig. 5A).
In addition to AMP (80 and 160 mg/kg), pranlukast decreased the
number of degenerating neurons in the ischemic core (Fig. 5B) and boundary zone (Fig. 5C) 24 h following MCAO. However, the
most marked reduction in the number of degenerating neurons was
induced by 80 mg/kg AMP, while 160 mg/mg AMP and 0.1 mg/kg
pranlukast treatments had a similar and less marked effect.
AMP decreases MCAO-induced elevation
of IgG exudation
The BBB was disrupted 24 h following reperfusion, as
IgG exudation was detected in the ischemic hemispheres however, in
the sham operated rats the level of IgG exudation was almost
undetectable (Fig. 6A). IgG
exudation was significantly increased 24 h following reperfusion in
the 0 mg/kg AMP control group compared with the sham group
(P<0.01; Fig. 6B). AMP (40, 80
and 160 mg/kg) and pranlukast significantly inhibited the increase
of IgG exudation at 24 h following reperfusion by 33.4, 48.1, 55.6
and 46.7%, respectively (P<0.01; Fig.
6B), compared with the 0 mg/kg AMP control group, indicating an
inhibitory effect on BBB disruption.
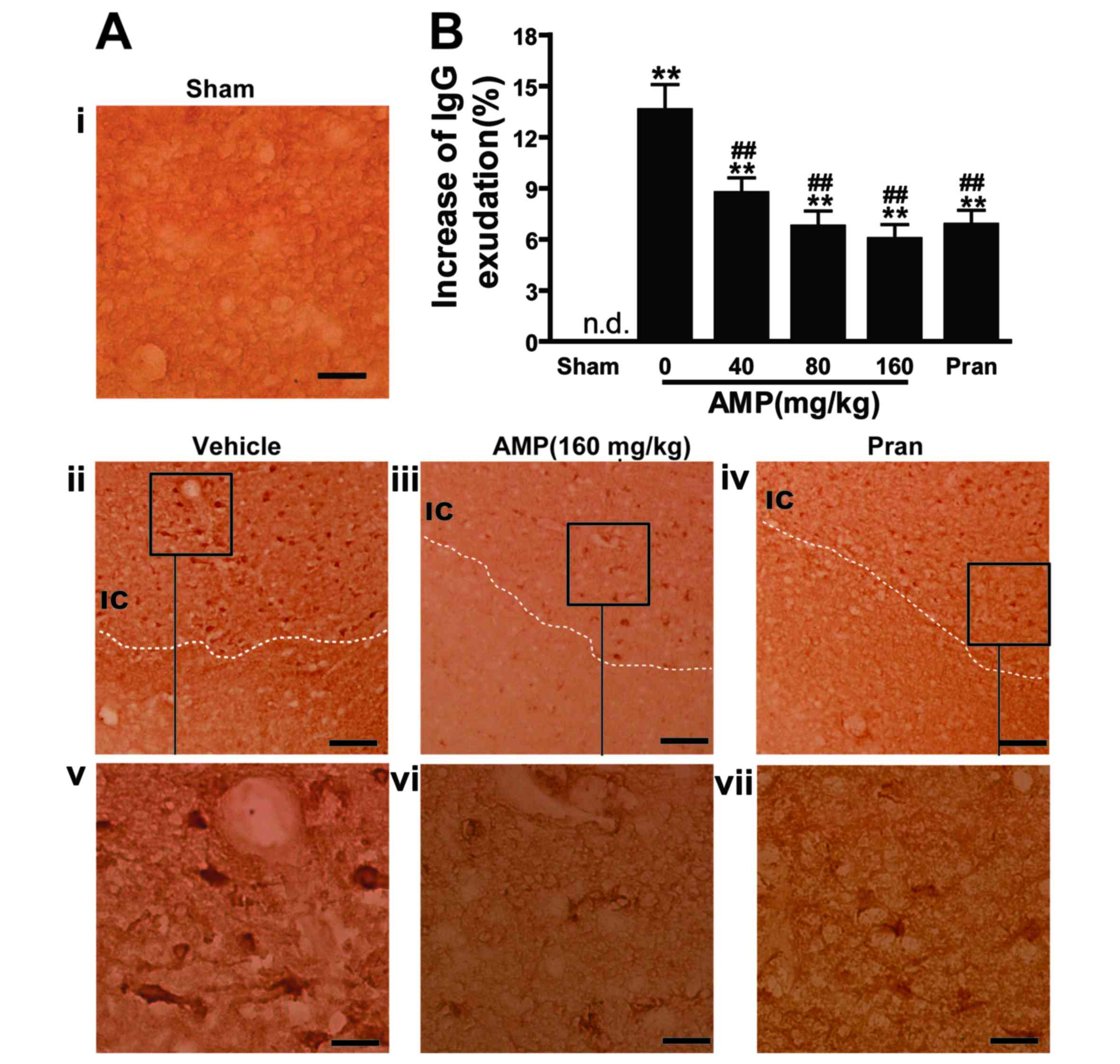 | Figure 6.Effect of AMP and pran on IgG
exudation in ischemic hemisphere 24 h following middle cerebral
artery occlusion in rats. (A) Representative images of
IgG-immunostained positive cells demonstrated that IgG exudation 24
h following reperfusion was attenuated by AMP (160 mg/kg, p.o.) and
pran (0.1 mg/kg, i.p.). Scale bars, 100 µm (i-iv) and, 25 µm
(v-vii). (B) Percentage increase of gray-scales of the injured
hemisphere. AMP (80 and 160 mg/kg, p.o.) and pran (0.1 mg/kg, i.p.)
inhibited IgG exudation (n=6). **P<0.01 vs. sham,
##P<0.01 vs. ischemic control, analyzed by one-way
analysis of variance. AMP, ampelopsin; Pran, pranlukast; IgG,
immunoglobulin G; n.d. not detectable. |
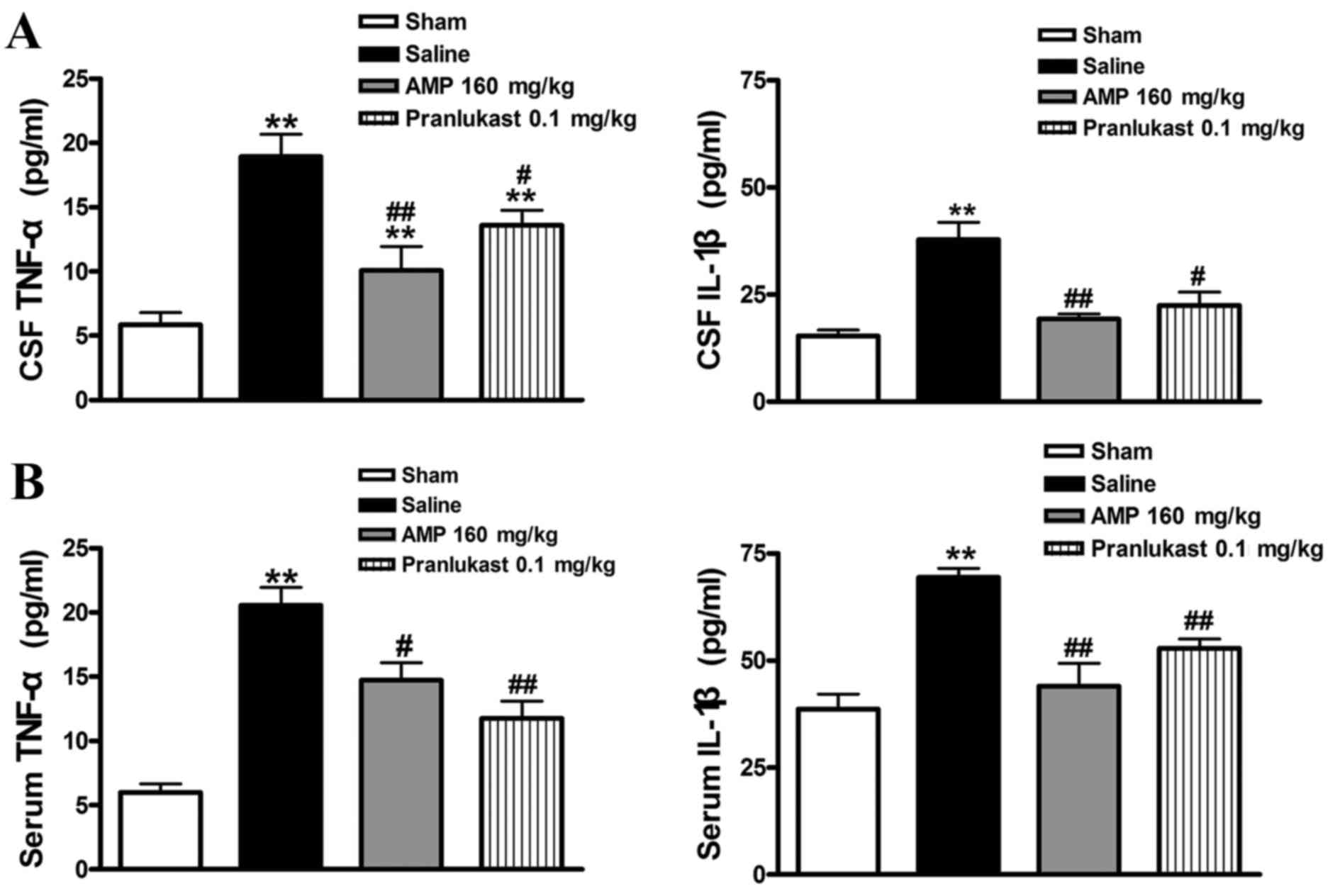
AMP reduces the MCAO-induced
expression of IL-1β and TNF-α in rat serum and CSF
Furthermore, the increased serum level of
MCAO-induced IL-1β and TNF-α 24 h following reperfusion was
significantly decreased following treatment with AMP (160 mg/kg).
Pranlukast (0.1 mg/kg) similarly reduced the concentration of IL-1β
and TNF-α in the rat serum and CSF (Fig.
7).
Discussion
The present study, to the best of our knowledge, is
the first to indicate the neuroprotective effect of AMP on acute
brain injury following focal cerebral ischemia in rats. AMP
attenuated the neurological deficits and neuronal loss in addition
to reducing the lesion volume, edema, neuronal degeneration, IgG
exudation and the release of TNF and IL-1β following MCAO. This
protective effect was similar to that of the CysLT1R
antagonist pranlukast, an anti-inflammatory agent (17,22–24).
These findings support the hypothesis that AMP reduced ischemic
injury in the brain, at least in the acute phase.
The severity of neurological deficits is associated
with the size of brain infarct and edema. The recovery of
neurological function is the primary determinant in the treatment
of cerebral ischemia/reperfusion injury (33). Following 60 min of MCAO and 24 h
reperfusion, rats exhibited serious neurological dysfunction
including increased neurological deficit scores and decreased
holding angles in the inclined board test (34). AMP administration improved the
behavioral assessment in a dose-dependent fashion, suggesting a
neuroprotective effect.
Neuronal cells are particularly vulnerable to
cerebral ischemia. Therefore, in acute ischemia, neuronal injury is
predominantly attributed to factors including inflammation,
oxidative stress, energy failure and excitotoxicity (18,35).
Neuronal degeneration and necrosis have been identified to be
associated with behavioral deficits (36,37). The
present study demonstrated that not only neuronal loss and
degeneration but also the behavior assessment was improved
following treatment with AMP. The neuroprotective effect of AMP was
apparent in the ischemic core and the boundary zone. The results of
the current study were consistent with AMP protection of
neuron-like PC12 cells against H2O2-induced
cytotoxicity via ERK and Akt signaling pathways (12).
BBB breakdown is a common feature in cerebral
ischemia and is known to occur due to vascular damage, within 3–5 h
and persists for a number of days (38). Therefore, rapid protection against
BBB dysfunction is critical in any therapeutic intervention to
minimize neuronal injury. The data of the current study
demonstrates that AMP reduces cerebral IgG extravasation following
focal cerebral ischemia. This suggests that AMP improves cerebral
ischemia injury by attenuating BBB breakdown, by decreasing the
cerebral edema and neuronal loss and degeneration.
Inflammation is a significant contributing factor to
the pathology of brain damage following ischemic insult mediated by
the activation of microglia and astrocytes, BBB disruption,
production of various cytokines such as TNF-α and IL-1β (16,18).
Systemic administration of anti-inflammatory agents reduces brain
edema and infarct sizes, improves neurological deficit and
regulates cytokine expression in the cortex (17,22–24). In
a prior study, AMP decreased the expression of LPS-induced
pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and pro-inflammation
mediator nitric oxide via the direct downregulation of
intracellular ROS levels and Akt phosphorylation indirectly, which
subsequently results in suppression of NF-κB activation in RAW264.7
macrophages (9). Furthermore, Rattan
tea extract has been suggested to reduce carrageenan-induced acute
inflammation in vivo (20).
AMP prevented oxidative stress in vivo due to ROS generated
by macrophages following the effect of D-galactosamine (9,21). In
PC12 cells, AMP inhibits H2O2-induced
apoptosis via ERK and Akt signaling pathways, which are markedly
involved in inflammatory cascades (9). The present study confirms that AMP
inhibited the increase of MCAO-induced IL-1β and TNF-α in serum and
CSF, while decreasing IgG exudation. TNF-α is a key inflammatory
mediator in the altered permeability of BBB during reperfusion
(39). TNF-α caused cramps in the
microartery and increased the permeability and IL-1β caused an
inflammatory reaction by promoting the adherence of leukocytes to
endothelial cells, resulting in BBB injury (40,41).
Therefore, the current study concluded that AMP exhibited
neuroprotective effects via its anti-inflammatory activity and
increased resistance to BBB damage.
Ischemic stroke triggers a highly interconnected and
complex cascade of cellular and molecular events in three phases:
Acute (min to h), subacute (h to days) and chronic (days to months)
(42,43). In the acute phase, neuronal injury is
predominantly due to ion imbalance, oxidative stress, energy
failure, excitotoxicity and BBB dysfunction. In the late
(subacute/chronic) phases, reactive astrocytosis and glial scar
formation are critical responses, and are associated with
beneficial effects including, restoration of astrocyte reactivity,
de novo expression of glial proteins and process enlarging and
detrimental effects, including disrupted planar cell polarity and
slower ventricular movement (42–44). In
the present study, AMP was demonstrated to attenuate neuronal
injury by improving BBB breakdown in the acute phase. It was
previously indicated that AMP attenuated oxygen-glucose
deprivation/recovery injury in rat astrocytes (data not published),
which suggests that AMP may protect rats against chronic ischemic
brain injury. Future studies are required to elucidate the effects
of AMP in the late phase of ischemic injury, along with the
underlying mechanisms.
In conclusion, the present study demonstrated that
oral administration of AMP mitigates acute brain injury following
focal cerebral ischemia in rats, similar to the effect of the
anti-inflammatory agent pranlukast. The results of the current
study suggest that AMP may represent a novel class of therapeutic
agent in the treatment of ischemic stroke. However, the
neuroprotective effects of AMP were only demonstrated in rats, and
therefore the detailed mechanisms underlying the effects of AMP in
ischemic brain injury require further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31301933 and
81401566), the Zhejiang Provincial Natural Science Foundation of
China (grant no. Q15H090023), the Science and Technology Planning
Project in Zhejiang Province (grant no. 2014C37011) and the Medical
Science and Technology Planning Project Zhejiang Province (grant
no. 201477310).
References
|
1
|
Tsai CF, Thomas B and Sudlow CL:
Epidemiology of stroke and its subtypes in Chinese vs white
populations: A systematic review. Neurology. 81:264–272. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pereira VM, Yilmaz H, Pellaton A, Slater
LA, Krings T and Lovblad KO: Current status of mechanical
thrombectomy for acute stroke treatment. J Neuroradiol. 42:12–20.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pierot L, Soize S, Benaissa A and Wakhloo
AK: Techniques for endovascular treatment of acute ischemic stroke:
From intra-arterial fibrinolytics to stent-retrievers. Stroke.
46:909–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kou X and Chen N: Pharmacological
potential of ampelopsin in Rattan tea. Food Sci Human Wellness.
1:14–18. 2012. View Article : Google Scholar
|
|
5
|
Murakami T, Miyakoshi M, Araho D, Mizutani
K, Kambara T, Ikeda T, Chou WH, Inukai M, Takenaka A and Igarashi
K: Hepatoprotective activity of tocha, the stems and leaves of
Ampelopsis grossedentata, and ampelopsin. Biofactors.
21:175–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tahara S: A journey of twenty-five years
through the ecological biochemistry of flavonoids. Biosci
Biotechnol Biochem. 71:1387–1404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klotter F and Studer A: Total synthesis of
resveratrol-based natural products using a palladium-catalyzed
decarboxylative arylation and an oxidative Heck reaction. Angew
Chem Int Ed Engl. 53:2473–2476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pflieger A, Teguo Waffo P, Papastamoulis
Y, Chaignepain S, Subra F, Munir S, Delelis O, Lesbats P, Calmels
C, Andreola ML, et al: Natural stilbenoids isolated from grapevine
exhibiting inhibitory effects against HIV-1 integrase and eukaryote
MOS1 transposase in vitro activities. PLoS One. 8:e811842013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L
and Yin Z: Ampelopsin reduces endotoxic inflammation via repressing
ROS-mediated activation of PI3K/Akt/NF-kB signaling pathways. Int
Immunopharmacol. 12:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JY, Jeong HY, Lee HK, Kim S, Hwang BY,
Bae K and Seong YH: Neuroprotection of the leaf and stem of
Vitis amurensis and their active compounds against ischemic
brain damage in rats and excitotoxicity in cultured neurons.
Phytomedicine. 19:150–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One. 9:e890212014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kou X, Shen K, An Y, Qi S, Dai WX and Yin
Z: Ampelopsin inhibits H2O2-induced apoptosis
by ERK and Akt signaling pathways and up-regulation of heme
oxygenase-1. Phytother Res. 26:988–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Dong S, Cen X, Wang X, Liu X,
Zhang H, Zhao X and Wu Y: Ampelopsin sodium exhibits antitumor
effects against bladder carcinoma in orthotopic xenograft models.
Anticancer Drugs. 23:590–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dung HV, Cuong TD, Chinh NM, Quyen D, Kim
JA, Byeon JS, Woo MH, Choi JS and Min BS: Compounds from the aerial
parts of Piper bavinum and their anti-cholinesterase
activity. Arch Pharm Res. 38:677–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papastamoulis Y, Richard T, Nassra M,
Badoc A, Krisa S, Harakat D, Monti JP, Mérillon JM and Waffo-Teguo
P: Viniphenol A, a complex resveratrol hexamer from Vitis
vinifera stalks: Structural elucidation and protective effects
against amyloid-β-induced toxicity in PC12 cells. J Nat Prod.
77:213–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lambertsen KL, Biber K and Finsen B:
Inflammatory cytokines in experimental and human stroke. J Cereb
Blood Flow Metab. 32:1677–1698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi QJ, Wang H, Liu ZX, Fang SH, Song XM,
Lu YB, Zhang WP, Sa XY, Ying HZ and Wei EQ: HAMI 3379, a CysLT2R
antagonist, dose- and time-dependently attenuates brain injury and
inhibits microglial inflammation after focal cerebral ischemia in
rats. Neuroscience. 291:53–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xing C, Arai K, Lo EH and Hommel M:
Pathophysiologic cascades in ischemic stroke. Int J Stroke.
7:378–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Turner RC, Dodson SC, Rosen CL and Huber
JD: The science of cerebral ischemia and the quest for
neuroprotection: Navigating past failure to future success. J
Neurosurg. 118:1072–1085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HH, Oh MH, Park KJ, Heo JH and Lee MW:
Anti-inflammatory activity of sulfate-containing phenolic compounds
isolated from the leaves of Myrica rubra. Fitoterapia.
92:188–193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ku KT, Huang YL, Huang YJ and Chiou WF:
Miyabenol A inhibits LPS-induced NO production via IKK/IkappaB
inactivation in RAW 264.7 macrophages: Possible involvement of the
p38 and PI3K pathways. J Agric Food Chem. 56:8911–8918. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi QJ, Xiao L, Zhao B, Zhang XY, Wang XR,
Xu DM, Yu SY, Fang SH, Lu YB, Zhang WP, et al:
Intracerebroventricular injection of HAMI 3379, a selective
cysteinyl leukotriene receptor 2 antagonist, protects against acute
brain injury after focal cerebral ischemia in rats. Brain Res.
1484:57–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chu LS, Wei EQ, Yu GL, Fang SH, Zhou Y,
Wang ML and Zhang WP: Pranlukast reduces neutrophil but not
macrophage/microglial accumulation in brain after focal cerebral
ischemia in mice. Acta Pharmacol Sin. 27:282–288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu GL, Wei EQ, Wang ML, Zhang WP, Zhang
SH, Weng JQ, Chu LS, Fang SH, Zhou Y, Chen Z, et al: Pranlukast, a
cysteinyl leukotriene receptor-1 antagonist, protects against
chronic ischemic brain injury and inhibits the glial scar formation
in mice. Brain Res. 1053:116–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang LH and Wei EQ: Neuroprotective
effect of ONO-1078, a leukotriene receptor antagonist, on transient
global cerebral ischemia in rats. Acta Pharmacol Sin. 24:1241–1247.
2003.PubMed/NCBI
|
|
26
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nirogi R, Kandikere V, Mudigonda K,
Bhyrapuneni G, Muddana N, Saralaya R and Benade V: A simple and
rapid method to collect the cerebrospinal fluid of rats and its
application for the assessment of drug penetration into the central
nervous system. J Neurosci Methods. 178:116–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pegg CC, He C, Stroink AR, Kattner KA and
Wang CX: Technique for collection of cerebrospinal fluid from the
cisterna magna in rat. J Neurosci Methods. 187:8–12. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yonemori F, Yamaguchi T, Yamada H and
Tamura A: Evaluation of a motor deficit after chronic focal
cerebral ischemia in rats. J Cereb Blood Flow Metab. 18:1099–1106.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin TN, He YY, Wu G, Khan M and Hsu CY:
Effect of brain edema on infarct volume in a focal cerebral
ischemia model in rats. Stroke. 24:117–121. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmued LC and Hopkins KJ: Fluoro-Jade B:
A high affinity fluorescent marker for the localization of neuronal
degeneration. Brain Res. 874:123–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmidt-Kastner R, Meller D, Bellander BM,
Strömberg I, Olson L and Ingvar M: A one-step immunohistochemical
method for detection of blood-brain barrier disturbances for
immunoglobulins in lesioned rat brain with special reference to
false-positive labelling in immunohistochemistry. J Neurosci
Methods. 46:121–132. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tominaga T and Ohnishi ST:
Interrelationship of brain edema, motor deficits, and memory
impairment in rats exposed to focal ischemia. Stroke. 20:513–518.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang SH, Wei EQ, Zhou Y, Wang ML, Zhang
WP, Yu GL, Chu LS and Chen Z: Increased expression of cysteinyl
leukotriene receptor-1 in the brain mediates neuronal damage and
astrogliosis after focal cerebral ischemia in rats. Neuroscience.
140:969–979. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gürer G, Gursoy-Ozdemir Y, Erdemli E, Can
A and Dalkara T: Astrocytes are more resistant to focal cerebral
ischemia than neurons and die by a delayed necrosis. Brain Pathol.
19:630–641. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jia F, Yin YH, Gao GY, Wang Y, Cen L and
Jiang JY: MMP-9 inhibitor SB-3CT attenuates behavioral impairments
and hippocampal loss after traumatic brain injury in rat. J
Neurotrauma. 31:1225–1234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang Z, Watts LT, Huang S, Shen Q,
Rodriguez P, Chen C, Zhou C and Duong TQ: The effects of methylene
blue on autophagy and apoptosis in MRI-defined normal tissue,
ischemic penumbra and ischemic core. PLoS One. 10:e01319292015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gotoh O, Asano T, Koide T and Takakura K:
Ischemic brain edema following occlusion of the middle cerebral
artery in the rat. I: The time courses of the brain water, sodium
and potassium contents and blood-brain barrier permeability to
125I-albumin. Stroke. 16:101–109. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv S, Song HL, Zhou Y, Li LX, Cui W, Wang
W and Liu P: Tumour necrosis factor-alpha affects blood-brain
barrier permeability and tight junction-associated occludin in
acute liver failure. Liver Int. 30:1198–1210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rodrigues SF and Granger DN: Blood cells
and endothelial barrier function. Tissue Barriers. 3:e9787202015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Willis CL, Brooks TA and Davis TP: Chronic
inflammatory pain and the neurovascular unit: A central role for
glia in maintaining BBB integrity? Curr Pharm Des. 14:1625–1643.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Candelario-Jalil E: Injury and repair
mechanisms in ischemic stroke: Considerations for the development
of novel neurotherapeutics. Curr Opin Investig Drugs. 10:644–654.
2009.PubMed/NCBI
|
|
43
|
Kriz J: Inflammation in ischemic brain
injury: Timing is important. Crit Rev Neurobiol. 18:145–157. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jørgensen HS, Nakayama H, Raaschou HO and
Olsen TS: Progressive apoplexy. Incidence, risk factors and
prognosis-the Copenhagen stroke study. Ugeskr Laeger.
157:3619–3622. 1995.(In Danish). PubMed/NCBI
|